Abstract
Co(II) and Ni(II) are two common toxic heavy metals, and may simultaneously exist in contaminated water, soil, and sediment systems in Earth’s surface environment. Under this circumstance, competitive adsorption between the two metals may influence their migration, toxicity, and bioavailability. In this research, the competitive sorption of Co(II) and Ni(II) on γ-Al2O3 was studied using both macroscopic sorption experiments and extended X-ray absorption fine structure (EXAFS) spectroscopy. Results suggest that Ni(II) reduced the amount of Co(II) sorption in a binary-solute system at pH 6.0. This is because both Co(II) and Ni(II) form inner-sphere surface complexes during sorption on γ-Al2O3 and compete for the surface reactive sites. However, Co(II) exhibited a negligible influence on sorption amount of Ni(II) under the same conditions, which suggests Ni(II) has a stronger affinity to alumina surface. At pH 7.5, Co(II) and Ni(II) sorption density were much higher than that at pH 6.0, but there no mutual competitive effect was observed. EXAFS analysis further revealed that formation of layered double-hydrated precipitates was the dominant sorption mechanism for both Co(II) and Ni(II) at pH 7.5. Because this type of sorption does not rely on surface reactive sites, there was no competition between Co(II) and Ni(II). This finding sheds light on risk assessment and remediation of Ni/Co pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Sorption of heavy metals on clay minerals (or oxides) affects their migration, transformation, and bioavailability in the Earth surface environment (Li et al. 2012). Sorption mechanisms for heavy metals include outer-sphere complexation, inner-sphere complexation, surface-induced precipitation, diffusion, and isomorphic substitution with the mineral structure (Sparks 2005). In the natural environment, Co(II) and Ni(II) adsorption may significantly affect their partitioning behavior and fate. Therefore, it is of great importance to gain a molecular-scale understanding of the competitive sorption behavior and underlying mechanisms of Co(II) and Ni(II) at environmental interfaces. This study reports competitive sorption of Co(II) and Ni(II) on γ-Al2O3 at pH 6.0 and 7.5 through macroscopic batch sorption experiments conducted with advanced extended X-ray absorption fine structure (EXAFS) spectroscopy.
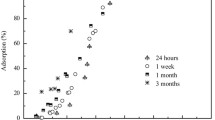
In the macroscopic sorption experiments, competitive adsorption between Co(II) and Ni(II) was observed at pH 6.0. The sorption amount of Co(II) at 48 h in a binary-solute system was about 18.6 μmol g−1, significantly lower than the 66.5 μmol g−1 observed in the single-solute system at pH 6.0 (Fig. 1). Previous studies (Thompson et al. 1999; Voegelin and Kretzschmar 2005) have suggested inner-sphere complexation is the driving force for Co(II) and Ni(II) sorption on γ-Al2O3 in an acidic environment. Ni(II)—with electronegativity of 1.91—is expected to be a stronger competitor for the similar sites on γ-Al2O3 than Co(II), with electronegativity of 1.88 (Li and Xue 2006).
However, no competition effect was observed at pH 7.5, as evidenced by the negligible difference between the sorption amounts of Co(II) and Ni(II) in single- and binary-solute systems. To further interpret the competitive sorption behavior of Co(II) and Ni(II), EXAFS analysis was performed in the Shanghai Synchrotron Radiation Facility (SSRF) and the Beijing Synchrotron Radiation Facility (BSRF). The k 3-weighted EXAFS spectra were collected for the sorption samples prepared at pH 7.5 and for several model compounds (Fig. 2). EXAFS spectra for the Ni single sorption sample showed a weak splitting at 3.7 Å−1, a shoulder at 5.2 Å−1, and a distinctive beat pattern at ~8.0 Å−1, all of which correspond to the characteristics for a layered double hydroxide (LDH) model compound (Aimoz et al. 2012). Fourier transform of the EXAFS data resolved two main peaks, corresponding to the nearest shell of oxygen atoms (first shell) and metal–metal (Me–Me) backscattering pairs (second shell). Shell-by-shell fits yielded a Ni–O distance of 2.06 Å, with coordination number (CN) of 6 for the first shell. The second shell was fitted with 3.9 ± 0.4 Ni and 1.4 ± 0.6 Al at the same RMe–Me distance of 3.05–3.07 Å. Very similar fitting results were obtained for Co EXAFS analysis (Table 1). The fitting data are in agreement with previous research (Thompson et al. 1999; Voegelin and Kretzschmar 2005) and suggest the formation of LDH surface precipitates under alkaline conditions. Distinct from surface complexation reaction, surface precipitation of LDH does not rely on surface sorption sites, therefore resulting in rather weak competitive sorption of Co(II) or Ni(II) in binary-solute systems at pH 7.5. However, further study is needed to ascertain whether Ni and Co co-precipitated in one LDH phase or formed through separate LDH phases.
The k-edge EXAFS results of Co and Ni sorption samples and model compounds prepared in single- and binary-solute systems at pH 7.5. The k3-weighted EXAFS spectra of Co (a) and Ni (c) and their Fourier transforms without phase-shift corrected of Co (b) and Ni (d). Colored lines represent experimental data and dark gray lines the spectral fits
To conclude, the macroscopic and spectroscopic findings together suggest that the competitive behavior and the corresponding microstructures of Co(II) and Ni(II) on γ-Al2O3 are dominantly controlled by solution pH. This provides a crucial scientific basis to understand the potential risk of Co(II) and Ni(II) in the aqueous environment. Specifically, in this work, the sorption amount of Co(II) was significantly reduced by the simultaneous presence of Ni(II) at pH 6.0, implying that the competition of Ni(II) increases environmental contamination risk of Co(II) in an acidic environment, such as acid mine drainage or tailings disposal systems.
References
Aimoz L, Taviotguého C, Churakov SV, Chukalina M, Dähn R, Curti E, Bordet P, Vespa M (2012) Anion and cation order in iodide-bearing Mg/Zn–Al layered double hydroxides. J Phys Chem C 116(9):5460–5475
Li K, Xue D (2006) Estimation of electronegativity values of elements in different valence states. J Phys Chem A 110(39):11332–11337
Li W, Livi KJ, Xu W, Siebecker MG, Wang Y, Phillips BL, Spark DL (2012) Formation of crystalline Zn–Al layered double hydroxide precipitates on γ-alumina: the role of mineral dissolution. Environ Sci Technol 46(21):11670–11677
Sparks DL (2005) Toxic metals in the environment: the role of surfaces. Elements 1(4):193–197
Thompson HA, Parks GA, Brown GE (1999) Dynamic interactions of dissolution, surface adsorption, and precipitation in an aging cobalt(II)-clay-water system. Geochim Cosmochim Acta 63(11–12):1767–1779
Voegelin A, Kretzschmar R (2005) Formation and dissolution of single and mixed Zn and Ni precipitates in soil: evidence from column experiments and extended X-ray absorption fine structure spectroscopy. Environ Sci Technol 39(14):5311–5318
Acknowledgements
This study was co-funded by the National Natural Science Foundation of China (No. 41473084), the Project of China Geological Survey (No. 12120114092001), the 1000 Youth Talent program. We are also grateful to the Beijing Synchrotron Radiation Facility (SSRF) and Shanghai Synchrotron Radiation Facility (SSRF) for use of the synchrotron radiation facilities at beamline 1W1B and 14W, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
The 11th International Symposium on Geochemistry of the Earth’s Surface.
Rights and permissions
About this article
Cite this article
Gou, W., Ji, J. & Li, W. An EXAFS investigation of the mechanism of competitive sorption between Co(II) and Ni(II) at γ-alumina/solution interface. Acta Geochim 36, 462–464 (2017). https://doi.org/10.1007/s11631-017-0196-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-017-0196-9