Abstract
Background
Selection of colorectal cancer patients with concomitant peritoneal (PM) and liver metastases (LM) for radical treatment with cytoreductive surgery (CRS), including liver resection and hyperthermic intraperitoneal chemotherapy (HIPEC), needs improvement. This retrospective, monocentric study was designed to evaluate the predictive factors for early recurrence, disease-free survival (DFS), and overall survival (OS) in such patients treated in a referral center.
Methods
Consecutive colorectal cancer patients with concomitant LM and PM treated with curative intent with perioperative systemic chemotherapy, simultaneous complete CRS, liver resection, and HIPEC in 2011–2022 were included. Clinical, radiological (before and after preoperative chemotherapy), surgical, and pathological data were investigated, along with long-term oncologic outcomes. A multivariate analysis was performed to identify predictive factors associated with early recurrence (diagnosed <6 months after surgery), DFS, and OS.
Results
Of more than 61 patients included, 31 (47.1%) had pT4 and 27 (40.9%) had pN2 primary tumors. Before preoperative chemotherapy, the median number of LM was 2 (1–4). The median surgical PCI (peritoneal carcinomatosis index) was 3 (5–8.5). The median DFS and OS were 8.15 (95% confidence interval [CI] 5.5–10.1) and 34.1 months (95% CI 28.1–53.5), respectively. In multivariate analysis, pT4 (odds ratio [OR] = 4.14 [1.2–16.78], p = 0.032]) and pN2 (OR = 3.7 [1.08–13.86], p = 0.042) status were independently associated with an early recurrence, whereas retroperitoneal lymph node metastasis (hazard ratio [HR] = 39 [8.67–175.44], p < 0.001) was independently associated with poor OS.
Conclusions
In colorectal cancer patients with concomitant PM and LM, an advanced primary tumor (pT4 and/or pN2) was associated with a higher risk of early recurrence following a radical multimodal treatment, whereas RLN metastases was strongly detrimental for OS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Colorectal cancer ranks third in terms of incidence worldwide and second in terms of mortality.1 Half of these patients present with metastases at diagnosis, sometimes multifocal.2,3 Their location impacts survival; peritoneum is associated with the worst prognosis.4 The advent of effective systemic chemotherapy regimens, the development of minimally invasive ablative therapies, and the improvement of imaging, surgical techniques, and perioperative management extended the boundaries of resecability while improving the control of the residual microscopic disease, leading to prolonged survival.5,6,7,8,9,10 These strategies, based on complete surgical resection, have been reinforced by the locoregional administration of chemotherapy with techniques, such as hepatic intra-arterial infusion and hyperthermic intraperitoneal chemotherapy (HIPEC).11,12 This strategy, developed to treat oligometastatic colorectal cancer patients with curative-intent, has been detailed in the ESMO recommendations.13 Briefly, patients with a limited number of metastases according to precise workup, responding to systemic chemotherapy, requiring a resection with predicted low rate of severe morbidity and managed in an expert center could be considered for a radical strategy prone to improve long-term outcomes. Thus, patient selection is crucial.14
The existence of both peritoneal and liver metastases (PM and LM) from colorectal cancer has long been seen as a contraindication to curative-intent treatment.15,16 Later, the safety of the synchronous treatment of PM and LM, combining complete peritoneal cytoreductive surgery (CRS), liver resection, and HIPEC, was compared to the outcomes of patients treated for PM only, with contradictory results.17,18,19,20 Nevertheless, this strategy appeared feasible in a selected population, because median overall survival (OS) could range from of 13 to 36 months and severe postoperative complication rates from 27 to 56%.21,22 These results compared favorably with nonoperative treatment strategy, based on systemic treatments only, whose OS was evaluated at 12.3 months.4 However, no randomized trials directly compared these two approaches.21,22 Although these results are encouraging, the recurrence rate (67–73%) following complete resection remains high.22 Of note, besides the postoperative risk, a primary concern is the risk of an early recurrence (occurring in less than 6 months). It is associated with a significant negative psychological impact, because patients may perceive it as treatment futility.23 Predicting patients at high risk of early recurrence is thus a crucial stake in the management of multifocal metastatic disease. There is a lack of accurate criteria in the literature as some studies had few patients, short-term follow-up, or inclusion of patients treated before 2000.18,24,25,26
The purpose of this retrospective, monocentric study was to evaluate clinical, radiological, surgical, and histopathologic risk factors for early recurrence in a selected cohort of patients treated simultaneously with curative intent for PM and LM by CRS, including liver resection, HIPEC, and perioperative systemic chemotherapy.
Material and Methods
This study was performed according to the ethical standards of the World Medical Association Declaration of Helsinki and approved by the Institutional Review Board of the Hospices Civils de Lyon. The informed consent requirement was waived by the ethics committees based on the nature of this retrospective study, in which patient data were kept confidential.
Population
All patients treated for PM and LM were reviewed. The inclusion criteria were patients with synchronous or metachronous PM and LM from colorectal cancer treated surgically in our center between January 2011 and January 2022 by synchronous CRS, liver-directed treatment, HIPEC, and perioperative chemotherapy. These surgical strategies had been validated by a multidisciplinary meeting using clinical, biological, and radiological data. The exclusion criteria were: patients treated consecutively for PM and LM, concomitant extralymphatic, extraperitoneal and extrahepatic metastases, those with prophylactic HIPEC, with CRS without HIPEC, with previous CRS (with or without HIPEC), and patients with nonavailable imaging (performed >1 month before initiation of neoadjuvant chemotherapy or >1 month before curative surgery), or uninterpretable examination because of poor image quality.
Data Screening
Pathologic and molecular data of the primary tumor and details of pre- and postoperative chemotherapy were collected. The synchronous or metachronous status of liver and peritoneal metastases and the existence of retroperitoneal lymph nodes suspected to be invaded at diagnosis were reviewed. A metastasis was considered synchronous if it was present or appeared within 6 months after the discovery of the primary cancer.
Imaging Screening
A thoraco-abdomino-pelvic CT at the portal phase was performed at baseline, before chemotherapy and during follow-up. A board-certified radiologist with 15 years of experience in gastrointestinal oncology imaging (PR) and a board-certified radiologist with 5 years of experience (RG) analysed the images by consensus (Centricity Universal Viewer, General Electric Healthcare). Liver MRI scans were not performed routinely, only in select cases of doubt regarding the liver CT. These MRI scans were not analysed for this study.
The total number of LM and of involved liver segments and the size of the largest LM were evaluated at baseline (before) and after preoperative chemotherapy by cross-sectional imaging. The size of the largest LM was measured in the axial plane in millimetres at the portal phase. The RECIST 1.1 guidelines were used to assess the response of LM to chemotherapy, between baseline and preoperative CT scans.27 The burden score was calculated as previously reported.28 Disappearing LM following preoperative chemotherapy also were recorded. Regarding PM, the radiological peritoneal carcinomatosis index (PCI) was calculated at baseline and after preoperative chemotherapy by using a free dedicated software PROMISE.29
Surgery
The surgical treatment of LM and PM was performed simultaneously for all patients. Liver resection was considered major if it involved three or more hepatic segments. The PM extension was quantified using to the PCI.30 Intraoperative thermal ablation was used as an adjunct in patients where complete resection leaving adequate remnant liver was impossible or when maximal parenchymal sparing surgery was desired. When retroperitoneal lymph node (RLN) invasion was suspected, based on a small lymph node axis >10 mm on CT and/or hypermetabolism on the PET scanner, radical para-aortic lymphadenectomy was performed.
The PM were resected with the intention of complete removal of macroscopic lesions by combining peritonectomies and organ resections. The CRS radicality was defined according to the completeness of cytoreduction (CC) score: CC-0, no macroscopic residual tumor; CC-1, residual tumor <2.5 mm, residual tumor between 3.5 mm and 25 mm; CC-3, residual tumor >25 mm.31 HIPEC using the closed abdomen technique was performed intraoperatively with a continuous flow at 800–1200 mL/min at 41–43 °C and 10–15 mmHg of intraperitoneal pressure. Main protocols were: mitomycin C 35 mg/m2 in 3 fractions for 90 min, cisplatin 100 mg/m2 in 3 fractions for 90 min, and oxaliplatin 360–460 mg/m2 during 30 min with concomitant intravenous 5-FU.
The postoperative complications were graded according to the Clavien-Dindo Classification.32 Complications graded ≥III were considered as major complications. Postoperative complications and death were defined as occurring within 90 days after surgery or until hospital discharge.
Pathologic Analysis
For LM, resection margins were reported as microscopically >1 mm (R0) or ≤1 mm (R1). A complete pathological response was considered when no evidence of residual tumor was found in the surgical specimen. Major response was considered in LM and PM if necrosis and/or fibrosis predominated over tumoral tissue, and minor response was considered if tumor predominated over necrosis and/or fibrosis (≥50%).
Follow-up
Patients were followed by using clinical examination, serum tumor marker measurements, abdomino-pelvic, and chest CT scan every 3 months during the first 2 years and then every 6 months for the 3 years thereafter. If recurrence was suspected, positron emission tomography and/or liver biopsy were performed. The disease-free survival (DFS) was defined as the time from surgery to the first disease recurrence. The sites of first recurrence were recorded and classified as liver, peritoneum, lung, lymph node, and/or bone. The date of last follow-up and the date of death also were recorded. The OS was defined as the time from the date of surgery to the date of death or last follow-up.
Statistics
Patient and disease characteristics were described by using categorical and continuous variables. Categorical variables were reported as number with corresponding percentage and compared with the chi-test or Fisher’s test, as appropriate. Continuous variables were reported as median with corresponding interquartile range and compared with Wilcoxon test. Factors associated with an early recurrence were identified through uni- and multivariate analyses using a logistic regression model with odds ratio (OR) and 95% confidence intervals (95% CI). Survivals were estimated by using the Kaplan-Meier method and compared with the log-rank test. Corresponding uni- and multivariate analyses were performed by using a Cox’s model regression with hazard ratio (HR) and 95% confidence interval (CI). Statistical significance was considered for p < 0.05. Statistical analyses were performed by using R software (4.2.2 version). Kaplan-Meier curves were used with GraphPad software (8.4.2 version).
Results
Patient Characteristics
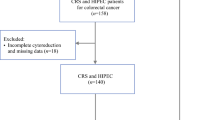
Patients characteristics are detailed in Table 1. Between January 2011 and January 2022, from 91 patients screened for inclusion, 66 matched the selection criteria (Fig. 1). The median age was 59.7 (range 52.8–69.6) years, and 17 (25.8%) patients had a right-sided colon cancer. The primary tumors were treated in emergency in 13 (19.4%) patients; 31 (47.1%) primary were pT4 and 27 (40.9%) were pN2. The KRAS, BRAF, and MMR status were available in 65 (97%), 62 (94%), and 48 (82.7%) patients, respectively. Among them, 33/66 (50%) patients had KRAS mutation, 7/66 (10.6%) had BRAF mutation, and 1/66 (1.5%) patients had microsatellite instability (MSI)-high status.
Regarding preoperative chemotherapy, oxaliplatin-based regimen was chosen in 27/66 (40.9%) patients, in combination with targeted therapy in 43/66 (65.2%) patients. The median number of preoperative chemotherapy cycles was 5 (range 4–6).
Imaging
Imaging characteristics are detailed in Table 2. The LM involvement was bilobar in 21/66 (31.3%) patients. The median number of LM was 2 (range 1–4) in a median of 2 (range 1–4) liver segments. The median diameter of the largest lesion was 22 (range 12–31) mm. The median tumor burden score was 3.3 (range 2.4–5.2).
After preoperative chemotherapy, the median number of LM was 2 (range 1–3.5), and the median diameter of the largest lesion was 14 (range 9–25) mm (Sup. Fig. 1). Median tumor burden score was 2.7 (range 2.1–4.5). Two (3.0%) patients had a complete response, 33(50.0%) patients had a partial response, and 27(40.9%) patients had stable disease, whereas 4(6.1%) patients exhibited LM progression. At least one LM disappeared in 17/66 (25.8%) patients after preoperative chemotherapy. At baseline, the median radiological PCI was 4 (range 2–6) and 3 (range 2–4.5) after preoperative chemotherapy.
Surgery
Patient’s operative details are presented in Table 3. Major hepatectomy was performed in four (6.1%) patients, 17 (25.8%) patients had simultaneous thermal ablation during liver surgery, and one (1.5%) patient had thermal ablation alone. The median surgical PCI was 3 (range 5–8). The Spearman’s correlation between surgical and the radiological preoperative PCI was 0.83 (range 0.73–0.89). The median duration of procedure was 360 (range 273–390) min, and the median blood loss was 300 (range 100–800) mL. The CRS was rated CC-0 and CC-1 respectively in 63 (95.4%) and three (4.6%) patients. There were no in-hospital deaths.
Complications occurred in 46 (69.7%) patients; 17 (25.8%) had major complications (Table 3). Regarding the HIPEC regimen used, 30.6%, 0, and 23.1% of the patients treated with mitomycin, cisplatin, or oxaliplatin-HIPEC presented severe complications, respectively. Of note, 12 of the 19 patients who had severe complications (70.6%) started adjuvant chemotherapy (4 following oxaliplatin-HIPEC and 8 mitomycin-HIPEC), whereas 36/49 (73.5%) patients with no severe postoperative events did.
Regarding histopathologic liver analysis, seven (10.8%) resections were considered R1. Complete response was seen in eight (12.3%) patients regarding LM, 19 (28.8%) patients regarding PM, and four (6.1%) patients regarding both LM and PM. Of the 23 (34.8%) patients with lymph node dissection, five had infrarenal retroperitoneal RLN metastatic invasion.
Oncologic Outcomes
After a median follow-up time of 32.5 (range 18.5–48.5) months, 56 (84.8%) patients had recurrence. The first recurrence location was liver (n = 30), lung (n = 24), peritoneum (n = 21), bone (n = 7), and lymph node (n = 4). Twenty-five (37.9%) experienced a recurrence in multiple sites. The median DFS was 8.2 months (95% CI 5.5–10.1), and the median OS was 34.1 months (95% CI 28.1–53.5) (Fig. 2). The 1-year, 3-year, and 5-year OS rates were 84.5%, 49.7%, and 29.3%, respectively.
Kaplan-Meier curve for overall (A) and disease-free (B) survival of the study population (N = 66). The median OS was 34.1 (95% confidence interval 28.1–53.5) months, and the median DFS of the study population was 8.2 (95% confidence interval 5.5–10.1) months. DFS disease-free survival; OS overall survival
Twenty-three (34.8%) patients had early recurrence (<6 months), in which the median time to recurrence was four (range 2–5) months. Ten (15.1%) patients did not have recurrence with a median follow-up time of 49 months. All five patients with RLN metastasis had an early recurrence (<6 months) and died within 12 months after surgery.
Prognostic Factors
Prognostic factors were analysed in Table 4. There was no difference regarding OS, DFS and early recurrence for age >70, ASA score ≥2, gender, emergency treatment of primary, KRAS mutation, synchronous, mucinous tumor, previous liver surgery, objective response according to RECIST, bilobar involvement, postoperative chemotherapy, and length of intensive care unit stay >7 days.
Early Recurrence
Among studied factors, pT4 primary status (OR = 3.16 [range 1.12–9.48], p = 0.033), pN2 status (OR = 3.59 [range 1.27–10.73], p = 0.018), the number of involved liver segments (OR = 3.37 [range 1.1–10.72], p = 0.035), tumor burden score >3 (OR = 4.53 [range 1.42–17.65], p = 0.016), >3 LM at baseline (OR = 5.61 [range 1.83–18.65], p = 0.003), and major complications (OR = 3.69 [range 1.18–14.22], p = 0.036) were associated with early recurrence. In multivariate analysis, pT4 status (OR = 4.14 [range 1.2–16.87], p = 0.032) and pN2 status (OR = 3.7 [range 1.08–13.86], p = 0.042) were independently associated with early recurrence. Patients with pT4N2 status had a significant poorer median DFS compared with other patients (5 vs. 10 months, p < 0.05) (Fig. 3A).
Kaplan-Meier curve for disease-free survival (DFS) for patients with pT4N2 primary and others. (A) Median DFS was statistically different between pT4N2 primary patients and others (5 vs. 10 months, p < 0.05). Kaplan-Meier curve for overall survival (OS) for group A (PCI >12 or LM >3 – blue curve) and group B (PCI ≤12 or LM ≤3 – red curve). (B) Median OS was significantly different between groups A and B (24 vs. 45 months respectively, p < 0.001). DFS disease-free survival; PCI peritoneal carcinomatosis index; LM liver metastasis; OS overall survival; pT pathological tumor stage; pN pathological nodal stage
Disease-free Survival
Synchronous LM (HR = 2.37 [range 1.19–4.72], p = 0.014), synchronous PM (HR = 2 [range 1.12–3.56], p = 0.019), number of involved segments >3 (HR = 1.91 [range 1.04–3.49], p = 0.04), tumor burden score >3 (HR = 2.14 [range 1.18–3.86], p = 0.01), surgical PCI >12 (HR = 2.78 [range 1.15–6.74], p = 0.02), RLN metastasis (HR = 4.87 [range 1.75–13.58], p < 0.001) were associated with poorer DFS. In the multivariate analysis, RLN metastasis (HR = 3.11 [range 1–9.66], p = 0.05) were closed to be independently associated with poorer DFS.
Overall Survival
In univariate analysis, tumor burden score >3 (HR = 2.78 (range 1.39–5.58), p < 0.001), RLN metastases (HR = 26.05 (range 6.62–102.45), p < 0.001), >3 LM at baseline (HR = 2.02 (range 1.06–3.88), p = 0.03) were associated with poor OS. In multivariate analysis, RLN metastasis (HR = 39 [range 8.67–175.44], p < 0.001) were independently associated with poor OS. The 22 (33.3%) patients with PCI >12 and/or LM >3 had an overall median survival of 24 months, whereas the 44 (66.6%) patients with PCI ≤12 and LM ≤3 had a median overall survival of 45 months (p < 0.001) (Fig. 3B).
Discussion
The retrospective analysis of this cohort of metastatic colorectal cancer patients showed that patients with locally advanced primary tumors (pT4 and/or pN2) were significantly associated with an increased risk of early recurrence following a comprehensive radical treatment combining perioperative chemotherapy, complete CRS, liver resection, and HIPEC. In parallel, RLN metastasis was confirmed as a strong predictive factor for poor DFS and OS.
The strategy of treatment of concomitant PM and LM from colorectal cancer is controversial.14,22 Among patients treated with combined CRS, HIPEC and liver resection, in the literature, the median DFS ranged from 5 to 24 months, the median OS from 13 to 36.1 months, and the recurrence rate from 67% to 73%, in line with the present study (8.2 months, 34.1 months, and 84.8%, respectively). Among 14 studies comparing survival outcomes of patients treated for combined PM+LM and those treated for PM, five studies identified LM as a negative independent prognostic factor for OS,16,18,33,34,35 whereas nine studies reported no significant difference between patients with or without LM.36,37,38 Nevertheless, most studies showed a trend toward poorer clinical outcomes for patients treated for PM+LM. The challenge facing clinicians is to select good candidates for radical treatment, because this combined strategy can lead to significant improvements in long-term outcomes compared with patients treated with chemotherapy alone or, conversely, to inappropriate, aggressive treatments with early recurrence and poor OS.39 The identification of factors predictive of such early recurrence and OS is therefore fundamental.
In a previous study, we explored the impact of the number and types of metastatic sites in selected patients with colorectal cancer PM treated by CRS and HIPEC.40 Survival was significantly reduced when patients had three or more metastatic sites and in case of RLN invasion, whereas limited extraperitoneal disease involving one other site did not seem to significantly impair the oncologic outcomes.40 In the present study, 61 (of 66) patients without RLN had a median survival of 38 months, whereas all five patients with RLN metastasis had an early recurrence (<6 months) and died within 12 months following surgery. Moreover, we found a particularly strong association (HR = 39) between RLN and poor OS. RLN constitutes a rare metastatic site, found in 2–6% of colorectal cancer.41,42 Synchronous RLN metastasis from colorectal cancer are associated with poor survival outcomes with a 65.7% 3-year OS and 67% recurrence rate during follow-up.43 Surgical resection of colorectal RLN metastasis is associated with a 59–68% 3-year OS, a median OS of 25–83 months, and a median DFS of 8.6–36 months.42,43,44 The oncological benefit of surgical management of RLNs remains controversial.45,46 Conversely, radiotherapy of RLN metastasis is a safe option, offers an alternative to surgical resection, and can achieve an objective response in >60% of patients with isolated RLN metastasis.47,48 To date, no randomized, controlled trials have been performed to compare strategies for RLN management; existing studies concern isolated RLNs.46,47,48 It is therefore difficult to have a uniform strategy for RLN resection, all the more in cases of multiple metastatic sites. CT scans have a high negative predictive value (96.6%), a high specificity (94.1%), but low positive predictive value (66.7%) and sensitivity (66.7%) to detect RLN metastasis from colorectal cancer in the series of Nakai et al.49 Generally, a short axis >1 cm is necessary to declare RLN as suspicious for metastases.50 However, this cutoff does not allow systematic detection of micrometastases,51 although only 50% of patients with lymph node >1 cm are pathologically metastatic.49 Indeed, inflammatory or infectious intercurrent disease during preoperative chemotherapy can lead to false positives on imaging, creating a false-negative prognostic factor. The addition of PET to CT may be useful to outrule retroperitoneal metastases in lymph node ≤10 mm.49 However, the use of PET may delay patient management. There is currently no conclusive scientific evidence available regarding the role of the (18)F-FDG PET/CT in RLN exploration.52 Finally, some patients have a complete response after preoperative chemotherapy and no residual RLN metastasis, as in the present study with 14/19 patients who had RLN resection without histological invasion.
In line with RLN metastasis, pT4 (OR = 4.14 [range 1.2–16.78], p = 0.032) and pN2 (OR=3.7[range 1.08-13.86] p=0.042) were the main factors of early recurrence in the studied population. The pT4 and pN2 primary status reflect a locally advanced disease with an associated rate of peritoneal recurrence of 55% at 5 years and a poor prognosis.53 Furthermore, primary N1-N2 status is independently associated with a higher risk of peritoneal recurrence after curative colorectal cancer surgery,54 and primary pN2 status is associated with impaired OS in colorectal PM patients treated with CRS and HIPEC.55 Should these patients be treated with curative intent? Despite early recurrence, the impact of pT4 or pN2 status on overall survival was not statistically significant (p > 0.05), in agreement with previous literature.26 Thus, radical treatment of recurrences, in that selected population with CRS/HIPEC, could be considered despite the involvement of two metastatic sites, as long as there are no invaded RLN.56 Complementary systemic chemotherapy is mandatory with objective response. Moreover, liver-directed minimal invasive treatments can help to prolong the locoregional control.57 Nevertheless, a particular importance of close serum tumor markers and postoperative imaging monitoring should be given to these high-risk patients during the postoperative period. This would allow potential iterative radical treatment of these early recurrences. In addition, monitoring with circulating tumor DNA and intensification of adjuvant chemotherapy could be individually discussed in this high-risk population to treat microscopic residual disease and prolong DFS.58
This study showed that having more than three LM on baseline imaging was associated with poor OS in univariate analysis. The number of LM was already established as a prognostic factor in patients treated for LM alone.59 It reflects a disease with an aggressive potential, requiring more systemic preoperative chemotherapy to achieve resectability, more extensive surgery, with more frequent major complications. Downs-Canner et al. concluded in a study comparing 32 patients undergoing combined CRS, HIPEC, and liver resection and 173 patients undergoing CRS and HIPEC alone that simultaneous liver resection should be considered for patients with less than three lesions.60 Elias et al. showed that the number of lesions was an important predictive factor in patients treated with HIPEC and/or colorectal LM and included this variable in a predictive nomogram.61 The surgical PCI is considered the most widely used tool to evaluate disease extent in peritoneal surface malignancies.62 Although radiological PCI may be useful in patient management for surgical strategy, CT commonly underestimates the tumor load, missing small PM or PM with lack of contrast with adjacent structures.63 The OS and the PCI have a strong linear relationship.64 In addition, some authors have shown the negative impact of PCI >12 and have suggested this cutoff to select candidates for CRS+HIPEC and LM resection.34 Alzahrani et al. reported a significantly longer OS for patients with PCI ≤7 and LM ≤3.18 The median PCI 3 (range 5–8) in the present study was relatively low compared with previous retrospective studies, ranging from 7 to 19, for patients treated by combined CRS, HIPEC and liver resection.22 This may explain that the median OS of 34.1 (95% CI 28.1–53.5) months in the present study, compares favorably with the 13 to 36.1 months of OS previously reported.22 The 44 (66.6%) patients with PCI ≤12 and LM ≤3 had a median overall survival of 45 months (p < 0.001). These criteria, together with the evidence of RLN metastases, have the advantage of being easy to use to select the right candidates in clinical practice. The present study has some limitations. This is a single-center, observational, retrospective study, with biases inherent to this type of data recording. The focus on patients engaged in a surgical strategy with limited tumor burden induced a selection bias excluding patients with extensive metastatic disease not selected for a radical approach.
These analysis highlight the importance to assess the intensity of the systemic disease and its response to preoperative chemotherapy in colorectal cancer patients with multiple metastatic sites. In particular, patients with suspected RLN in addition to LM and PM must be carefully selected for radical treatment. These concurrent metastases should probably lead to contraindicate these patients, unless all the other positive prognostic factors are met: favorable clinical criteria (limited number of LM, limited PCI, objective morphologic response to chemotherapy…) and tumor biology (in particular no BRAF V600E mutation and no P53 co-mutations). The advent of accurate circulating tumor DNA monitoring will help to refine that selection process. Considering the importance of the postoperative systemic treatment in these patients and that severe complications slightly decrease the chance to start the adjuvant chemotherapy (70.6% vs. 73.5% started adjuvant chemotherapy in that study), prehabilitation program should be mandatory if a radical treatment is decided. The role of HIPEC in these patients with intense systemic disease remains relevant to control the locoregional spread of metastases within the peritoneal cavity.
Conclusions
pT4 and/or pN2 stage is associated with a risk of early recurrence in patients treated simultaneously with CRS, liver resection, and HIPEC, not necessarily impairing long-term outcomes, whereas RLN metastases were strongly associated with poor survival. Conversely, patients with PCI ≤12 and LM ≤3 had significantly longer OS. These parameters could help to refine the selection of better candidates among oligometastatic patients for a radical strategy, whereas future studies including circulating tumor DNA will perhaps encompass this issue.
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier A-M. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–9.
Koppe MJ, Boerman OC, Oyen WJG, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: Incidence and current treatment strategies. Ann Surg. 2006;243(2):212–22.
Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–19.
Cremolini C, Antoniotti C, Stein A, et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol. 2020;38(28):3314–24.
Molla M, Fernandez-Plana J, Albiol S, et al. Limited liver or lung colorectal cancer metastases. Systemic treatment, surgery, ablation or SBRT. J Clin Med. 2021;10(10):2131.
Rijsemus CJV, Kok NFM, Aalbers AGJ, et al. Diagnostic performance of MRI for staging peritoneal metastases in patients with colorectal cancer after neoadjuvant chemotherapy. Eur J Radiol. 2022;149:110225.
Kambakamba P, Hoti E, Cremen S, Braun F, Becker T, Linecker M. The evolution of surgery for colorectal liver metastases: A persistent challenge to improve survival. Surgery. 2021;170(6):1732–40.
Hübner M, Kusamura S, Villeneuve L, et al. Guidelines for perioperative care in cytoreductive surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced Recovery After Surgery (ERAS®) Society Recommendations — Part II: Postoperative management and special considerations. Eur J Surg Oncol. 2020;46(12):2311–23.
Kotani D, Oki E, Nakamura Y, et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med. 2023;29(1):127–34.
Buisman FE, Filipe WF, Galjart B, et al. Adjuvant intra-arterial chemotherapy for patients with resected colorectal liver metastases: a systematic review and meta-analysis. HPB. 2022;24(3):299–308.
Arjona-Sánchez A, Espinosa-Redondo E, Gutiérrez-Calvo A, et al. Efficacy and safety of intraoperative hyperthermic intraperitoneal chemotherapy for locally advanced colon cancer: A phase 3 randomized clinical trial. JAMA Surg [Internet] 2023 [cited 2023 May 16];Available from: https://jamanetwork.com/journals/jamasurgery/fullarticle/2804110
Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422.
Di Carlo S, Cavallaro G, La Rovere F, et al. Synchronous liver and peritoneal metastases from colorectal cancer: Is cytoreductive surgery and hyperthermic intraperitoneal chemotherapy combined with liver resection a feasible option? Front Surg. 2022;9:1006591.
Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–50.
Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A Multi-Institutional Study. J Clin Oncol. 2004;22(16):3284–92.
Kianmanesh R, Scaringi S, Sabate J-M, et al. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg. 2007;245(4):597–603.
Alzahrani N, Ung L, Valle SJ, Liauw W, Morris DL. Synchronous liver resection with cytoreductive surgery for the treatment of liver and peritoneal metastases from colon cancer: results from an Australian centre: Synchronous liver resection with CRS. ANZ J Surg. 2017;87(11):E167–72.
Pinto A, Hobeika C, Philis A, Kirzin S, Carrère N, Ghouti L. Synchronous liver metastases and peritoneal carcinomatosis from colorectal cancer: different strategies for curative treatment? Langenbecks Arch Surg. 2019;404(4):477–88.
de Cuba EMV, Kwakman R, Knol DL, Bonjer HJ, Meijer GA, te Velde EA. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases. Cancer Treat Rev. 2013;39(4):321–7.
Flood MP, Das AA, Soucisse ML, et al. Synchronous liver resection, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy for colorectal liver and peritoneal metastases: A systematic review and meta-analysis. Dis Colon Rectum. 2021;64(6):754–64.
Zou Y, Chen X, Zhang X, et al. Clinical outcomes of curative treatment for colorectal liver metastases combined with cytoreductive surgery and intraperitoneal chemotherapy for peritoneal metastases: a systematic review and meta-analysis of current evidence. Int J Hyperthermia. 2020;37(1):944–54.
Andersen BL, Shapiro CL, Farrar WB, Crespin T, Wells-DiGregorio S. Psychological responses to cancer recurrence: A controlled prospective study. Cancer. 2005;104(7):1540–7.
Navez J, Remue C, Leonard D, et al. Surgical treatment of colorectal cancer with peritoneal and liver metastases using combined liver and cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Report from a single-centre experience. Ann Surg Oncol. 2016;23(S5):666–73.
Bacalbasa N, Balescu I, Cretoiu D, et al. Determination of whether HIPEC is beneficial in patients with synchronous peritoneal and liver metastases from colorectal cancer (review). Exp Ther Med. 2021;22(5):1267.
Lo Dico R, Faron M, Yonemura Y, et al. Combined liver resection and cytoreductive surgery with HIPEC for metastatic colorectal cancer: Results of a worldwide analysis of 565 patients from the Peritoneal Surface Oncology Group International (PSOGI). Eur J Surg Oncol. 2021;47(1):89–100.
Schwartz LH, Litière S, deVries E, et al. RECIST 1.1—Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132–7.
Sasaki K, Morioka D, Conci S, et al. The tumor burden score: A new, “metro-ticket” prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg. 2018;267(1):132–41.
Villeneuve L, Thivolet A, Bakrin N, et al. A new internet tool to report peritoneal malignancy extent. PeRitOneal malignancy stage evaluation (PROMISE) application. Eur J Surg Oncol (EJSO). 2016;42(6):877–82.
Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis [Internet]. In: Sugarbaker PH, editor. Peritoneal Carcinomatosis: Principles of Management. Boston, MA: Springer US; 1996 [cited 2023 Apr 16]. pp. 359–74. https://doi.org/10.1007/978-1-4613-1247-5_23
Sugarbaker PH. Peritonectomy procedures [Internet]. In: Ceelen WP (ed). Peritoneal carcinomatosis. Boston, MA: Springer US; 2007 [cited 2023 May 23]. pp. 247–64. https://doi.org/10.1007/978-0-387-48993-3_15
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
Baratti D, Kusamura S, Iusco D, et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum. 2014;57(7):858–68.
Maggiori L, Goéré D, Viana B, et al. Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent? A case-control study. Ann Surg. 2013;258(1):116–21.
Cavaliere F, DeSimone M, Virzì S, et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol (EJSO). 2011;37(2):148–54.
Lorimier G, Linot B, Paillocher N, et al. Curative cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis and synchronous resectable liver metastases arising from colorectal cancer. Eur J Surg Oncol. 2017;43(1):150–8.
Duraj FF, Cashin PH. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal and hepatic metastases: a case-control study. J Gastrointest Oncol. 2013;4(4):388–96.
Delhorme J-B, Dupont-Kazma L, Addeo P, et al. Peritoneal carcinomatosis with synchronous liver metastases from colorectal cancer: Who will benefit from complete cytoreductive surgery? Int J Surg. 2016;25:98–105.
Franco F, Monaco D, Volpi A, Marcato C, Larini P, Rossi C. The role of arterial embolization in blunt splenic injury. Radiol Med. 2011;116(3):454–65.
Schell F, Kefleyesus A, Benzerdjeb N, et al. Influence of extraperitoneal metastases on the curative-intent management of colorectal peritoneal metastases. Ann Surg Oncol. 2023. https://doi.org/10.1245/s10434-023-13279-9.
Japanese Society for Cancer of the Colon and Rectum, Watanabe T, Itabashi M, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17(1):1–29.
Zizzo M, Dorma MPF, Zanelli M, et al. Long-term outcomes of surgical resection of pathologically confirmed isolated para-aortic lymph node metastases in colorectal cancer: A systematic review. Cancers. 2022;14(3):661.
Aylward C, Noori J, Tyrrell J, et al. Survival outcomes after synchronous para-aortic lymph node metastasis in colorectal cancer: A systematic review. J Surg Oncol. 2023;127(4):645–56.
Fadel MG, Ahmed M, Pellino G, et al. Retroperitoneal lymph node dissection in colorectal cancer with lymph node metastasis: A systematic review. Cancers. 2023;15(2):455.
Bae SU, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Which patients with isolated para-aortic lymph node metastasis will truly benefit from extended lymph node dissection for colon cancer? Cancer Res Treat. 2018;50(3):712–9.
Gagnière J, Dupré A, Chabaud S, Peyrat P, Meeus P, Rivoire M. Retroperitoneal nodal metastases from colorectal cancer: Curable metastases with radical retroperitoneal lymphadenectomy in selected patients. Eur J Surg Oncol (EJSO). 2015;41(6):731–7.
Lee J, Chang JS, Shin SJ, et al. Incorporation of radiotherapy in the multidisciplinary treatment of isolated retroperitoneal lymph node recurrence from colorectal cancer. Ann Surg Oncol. 2015;22(5):1520–6.
Shu P, Ouyang G, Wang F, et al. The role of radiotherapy in the treatment of retroperitoneal lymph node metastases from colorectal cancer. CMAR. 2020;12:8913–21.
Nakai N, Yamaguchi T, Kinugasa Y, et al. Diagnostic value of computed tomography (CT) and positron emission tomography (PET) for paraaortic lymph node metastasis from left-sided colon and rectal cancer. Asian J Surg. 2020;43(6):676–82.
Rosenthal MH, Kim KW, Fuchs CS, Meyerhardt JA, Ramaiya NH. CT predictors of overall survival at initial diagnosis in patients with stage IV colorectal cancer. Abdom Imaging. 2015;40(5):1170–6.
Hale GR, Teplitsky S, Truong H, Gold SA, Bloom JB, Agarwal PK. Lymph node imaging in testicular cancer. Transl Androl Urol. 2018;7(5):864–74.
Kwak JY, Kim JS, Kim HJ, Ha HK, Yu CS, Kim JC. Diagnostic value of FDG-PET/CT for lymph node metastasis of colorectal cancer. World J Surg. 2012;36(8):1898–905.
Klaver CEL, Wasmann KATGM, Verstegen M, et al. Postoperative abdominal infections after resection of T4 colon cancer increase the risk of intra-abdominal recurrence. Eur J Surg Oncol. 2018;44(12):1880–8.
Zhang Y, Qin X, Chen W, et al. Risk factors for developing peritoneal metastases after curative surgery for colorectal cancer: A systematic review and meta-analysis. Colorectal Dis. 2021;23(11):2846–58.
Simkens GA, Van Oudheusden TR, Nieboer D, et al. Development of a prognostic nomogram for patients with peritoneally metastasized colorectal cancer treated with cytoreductive surgery and HIPEC. Ann Surg Oncol. 2016;23(13):4214–21.
Vassos N, Förtsch T, Aladashvili A, Hohenberger W, Croner RS. Repeated cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with recurrent peritoneal carcinomatosis. World J Surg Oncol. 2016;14(1):42.
Elias D, De Baere T, Smayra T, Ouellet JF, Roche A, Lasser P. Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br J Surg. 2002;89(6):752–6.
Malla M, Loree JM, Kasi PM, Parikh AR. Using circulating tumor DNA in colorectal cancer: Current and evolving practices. J Clin Oncol. 2022;40(24):2846–57.
Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases – a population-based study on incidence, management and survival. BMC Cancer. 2018;18(1):78.
Downs-Canner S, Shuai Y, Ramalingam L, et al. Safety and efficacy of combined resection of colorectal peritoneal and liver metastases. J Surg Res. 2017;219:194–201.
Elias D, Faron M, Goéré D, et al. A simple tumor load-based nomogram for surgery in patients with colorectal liver and peritoneal metastases. Ann Surg Oncol. 2014;21(6):2052–8.
Esquivel J, Sticca R, Sugarbaker P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007;14(1):128–33.
Bhatt A, Rousset P, Benzerdjeb N, et al. Prospective correlation of the radiological, surgical and pathological findings in patients undergoing cytoreductive surgery for colorectal peritoneal metastases: implications for the preoperative estimation of the peritoneal cancer index. Colorectal Dis. 2020;22(12):2123–32.
Faron M, Macovei R, Goéré D, Honoré C, Benhaim L, Elias D. Linear relationship of peritoneal cancer index and survival in patients with peritoneal metastases from colorectal cancer. Ann Surg Oncol. 2016;23(1):114–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
OG is consultant for GAMIDA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
A 69-year-old female was referred for synchronous PM and LM from sigmoid pT4N2 adenocarcinoma. Initial CT scan at portal phase revealed 9 LM, the largest of which measured 35 mm (arrow) (A). Initial PCI score was 4, including a 10-mm peritoneal nodule of the left pre-renal fascia (dotted arrow) (B). Preoperative CT scan after 12 cycles of Folfirinox-Cetuximab showed a significant reduction in size of the nine LM (partial response according RECIST 1.1 classification), the largest measuring 25 mm with necrotic modification (arrow) (C). Peritoneal analysis assessed the PCI score at 3, with reduction in size of the nodule, and a thickening of the left pre-renal fascia (dotted arrow) (D). PCI score was assessed at 5 intraoperatively. The patient had a complete resection of the PM (completeness of cytoreduction score at 0) with four hepatic wedge resections combined with five radiofrequency ablations. The patient had a postoperative ileus. The patient presented an early recurrence at 2 months, treated with chemotherapy. The patient was still alive 14 months after surgery (JPG 69 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grange, R., Rousset, P., Williet, N. et al. Metastatic Colorectal Cancer Treated with Combined Liver Resection, Cytoreductive Surgery, and Hyperthermic Intraperitoneal Chemotherapy (HIPEC): Predictive Factors for Early Recurrence. Ann Surg Oncol 31, 2378–2390 (2024). https://doi.org/10.1245/s10434-023-14840-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14840-2