Abstract
Background
Prognostic nomograms for patients with resected extremity soft tissue sarcoma (STS) include the Sarculator and Memorial Sloan Kettering (MSKCC) nomograms. We sought to validate these two nomograms within a large, modern, multi-institutional cohort of resected primary extremity STS patients.
Methods
Resected primary extremity STS patients from 2000 to 2017 were identified across nine high-volume U.S. institutions. Predicted 5- and 10-year overall survival (OS) and distant metastases cumulative incidence (DMCI), and 4-, 8-, and 12-year disease-specific survival (DSS) were calculated with Sarculator and MSKCC nomograms, respectively. Predicted survival probabilities stratified in quintiles were compared in calibration plots to observed survival assessed by Kaplan–Meier estimates. Cumulative incidence was estimated for DMCI. Harrell’s concordance index (C-index) assessed discriminative ability of nomograms.
Results
A total of 1326 patients underwent resection of primary extremity STS. Common histologies included: undifferentiated pleomorphic sarcoma (35%), fibrosarcoma (13%), and leiomyosarcoma (9%). Median tumor size was 8.0 cm (IQR 4.5–13.0). Tumor grade distribution was: Grade 1 (13%), Grade 2 (9%), Grade 3 (78%). Median OS was 172 months, with estimated 5- and 10-year OS of 70% and 58%. C-indices for 5- and 10-year OS (Sarculator) were 0.72 (95% CI 0.70–0.75) and 0.73 (95% CI 0.70–0.75), and 0.72 (95% CI 0.69–0.75) for 5- and 10-year DMCI. C-indices for 4-, 8-, and 12-year DSS (MSKCC) were 0.71 (95% CI 0.68–0.75). Calibration plots showed good prognostication across all outcomes.
Conclusions
Sarculator and MSKCC nomograms demonstrated good prognostic ability for survival and recurrence outcomes in a modern, multi-institutional validation cohort of resected primary extremity STS patients. External validation of these nomograms supports their ongoing incorporation into clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Soft tissue sarcomas (STS) encompass a rare and heterogeneous family of malignancies.1 The significant variability observed across more than 100 distinct STS histologic subtypes, has made traditional TNM classification challenging and has historically hampered the prognostic applicability of the American Joint Committee on Cancer (AJCC) staging systems for STS.2,3,4 The most recent 8th edition AJCC staging for STS does account for the importance of anatomic site, such that staging for sarcomas of the extremities and trunk is now distinctly different from that of sarcomas arising in retroperitoneal, head and neck, or intra-abdominal locations.5 Additional modifications were made to the T classification, with tumor size now stratified into four categories.5,6 Whether these modifications to the AJCC staging system translate to enhanced prognostication and discrimination, however, remains controversial.7,8,9
As a result of the inherent limitations of the AJCC staging systems for STS, several prognostic nomograms accounting for individual patient clinicopathologic features have been proposed. Two of the most widely adopted nomograms for patients with extremity STS are the Sarculator nomogram10 and the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram.11,12 In the contemporary era of personalized cancer care, these prognostic nomograms are becoming increasingly incorporated into the clinical management of extremity STS patients.13,14 While these models have been proposed as tools to counsel patients regarding their prognosis and to guide clinical decisions regarding intensity and duration of postoperative surveillance and clinical trial eligibility, external validation of these nomograms for extremity STS has been somewhat limited.10,12,15,16,17,18 The purpose of the current study was to evaluate and validate the Sarculator and MSKCC prognostic nomograms in a diverse, modern, multi-institutional cohort of patients undergoing resection of primary extremity STS.
Methods
We queried the U.S. Sarcoma Collaborative (USSC) database, which encompasses patients treated from 2000 to 2017 at nine high-volume academic institutions across the United States to identify all patients who underwent resection of primary extremity STS. Histologies excluded from the original Sarculator nomogram development study were also excluded in the current analysis: desmoid fibromatosis, peripheral primitive neuroectodermal tumor (PPNET), alveolar or embryonal rhabdomyosarcoma, dermatofibrosarcoma protuberans, and well-differentiated liposarcoma.10 Patients aged 18 years or older who underwent curative intent resection of primary extremity STS were included. Patients with metastatic or recurrent disease were excluded. Tumor grade was classified according to the French Federation of Cancer Centers (FNCLCC) system.19 Per the original nomogram development studies, patients with gross residual macroscopic disease were excluded.10,12 Margin status was classified as R0 (microscopically negative) or R1 (microscopically positive; tumor <1 mm from margin).10
Clinicopathologic features were retrospectively analyzed and compared with those reported for the Sarculator nomogram development cohort10 and the MSKCC nomogram validation cohort.12 Comparisons were made with Chi-squared test or one-sample exact test comparing USSC distribution versus Sarculator or MSKCC median (included as a null value) for categorical or continuous variables, respectively. The Sarculator extremity nomogram covariates include patient age, tumor size, tumor grade, and histologic subtype. The MSKCC nomogram covariates include patient age, tumor size (<5 cm, 5–10 cm, >10 cm), tumor depth (superficial vs. deep), site, grade, and histologic subtype. Pathologic variables were derived from the final resection specimen. As in the original validation of the MSKCC nomogram by Mariani et al., FNCLCC Grade 2 and 3 tumors were classified as high grade for the MSKCC nomogram, whereas FNCLCC Grade 1 tumors were classified as low grade for the MSKCC nomogram system.12 For comparative analysis, given the evolution of histology nomenclature between studies, the histologic category defined by the MSKCC nomogram as “malignant fibrous histiocytoma (MFH)/undifferentiated pleomorphic sarcoma (UPS)” was classified as the Sarculator histologic category of “UPS” and the MSKCC nomogram histologic category of “fibrosarcoma” was classified as the Sarculator histologic category of “myxofibrosarcoma.” Perioperative chemotherapy and radiation data also were collected. Predicted 5- and 10-year overall survival (OS) and 5- and 10-year distant metastases cumulative incidence (DM CI) were calculated using the Sarculator nomogram, whereas predicted 4-, 8-, and 12-year disease-specific survival (DSS) were calculated using the MSKCC model.
The primary endpoints of the study were OS, DM CI, and DSS. OS and DSS were estimated using the Kaplan–Meier method and cumulative incidence was estimated for distant metastases. To evaluate the calibration of each nomogram, nomogram-predicted survival probabilities were stratified into quintiles and compared to observed patient outcomes, generating calibration plots for each endpoint. Predicted DMCI was similarly plotted against observed DMCI to generate calibration plots. The 45-degree reference line on the calibration plot indicates perfect calibration. Harrell’s concordance index (C-index) was calculated as a quantitative measure of the discriminative ability of each nomogram.20 C-indices are interpreted whereby a value of 0.5 equals random chance and a value of 1.0 equals perfect discriminative ability of the nomogram.
Univariate and multivariable Cox regression analyses were performed to assess associations between clinicopathologic features and OS and DSS. The Fine and Gray model in the competing risks framework was used to analyze the associations between clinicopathologic features and distant metastases, whereby distant metastasis with or without synchronous local recurrence was the event, and death without recurrence and isolated local recurrence were regarded as competing events. For each model, variables significant on univariate analysis were included in the multivariable model. Backwards selection was used to construct the final multivariable regression model, using a p-value threshold of <0.10 for remaining in the model. Additionally, the variables selected for the multivariable model of DM CI based on the Fine and Gray model were included in a Cox model in order to obtain hazard ratio estimates.
Institutional review board (IRB) approval at each participating institution was obtained prior to study initiation. Statistical analyses were performed using SAS software (SAS Institute, Cary, NC), with p-values < 0.05 considered statistically significant.
Results
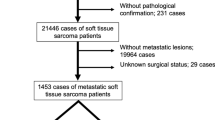
A cohort of 1326 patients who underwent resection of primary extremity STS was identified from the USSC database. Basic demographics and clinicopathologic features of the USSC cohort are summarized in Tables 1 and 2. The most common histologies represented included undifferentiated pleomorphic sarcoma (UPS; 35%), fibrosarcoma (13%), leiomyosarcoma (9%), and synovial sarcoma (9%). Median tumor size was 8.0 cm (interquartile range [IQR] 4.5–13.0). Tumor grade distribution was: Grade 1 (14%), Grade 2 (14%), Grade 3 (72%). Perioperative chemotherapy was delivered to 24% (n = 313) of patients, and 53% (n = 700) of patients received perioperative radiation. Median follow-up time was 34 months. Median OS was 173 months (IQR, 128 months-MNR), with estimated 5- and 10-year OS of 70% and 58%, respectively. The 5-year and 10-year DM CI were 29% and 33%, respectively.
Significant differences were found in the clinicopathologic features of patients in the current USSC study cohort compared with both the Sarculator cohort (Table 1) and the MSKCC cohort (Table 2). The current study cohort included a greater proportion of Grade 3 tumors compared with both the Sarculator (72% vs. 47%, p < 0.01) and MSKCC cohorts (72% vs. 46% p < 0.01). Differences also were observed with regard to age, tumor size, tumor depth, surgical margins, and distribution of histologic subtypes (all p < 0.01).
The calibration plots showed good predictability for 5- and 10-year OS using the Sarculator nomogram (Fig. 1a, b). Calibration plots for 5- and 10-year DM CI using the Sarculator nomogram showed similarly good predictability (Fig. 1c–d). The C-indices for 5- and 10-year OS were 0.72 (95% CI: 0.70–0.75) and 0.73 (95% CI: 0.70–0.75); the C-index was 0.72 (95% CI: 0.69–0.75) for both 5- and 10-year DM CI. The calibration plots for DSS demonstrated similarly good calibration using the MSKCC nomogram, with C-indices for 4-, 8-, and 12-year of 0.71 (95% CI: 0.68–0.75) (Fig. 2a–c) .
Calibration plots of actual versus predicted outcomes using the Sarculator Prognostic Nomogram. Five- and ten-year overall survival (a, b) and Distant Metastasis-Cumulative Incidence (c, d) were assessed. The nomogram predicted probabilities were stratified into quintiles. For each quintile, the average nomogram-predicted probability (x-axis) was plotted against the observed Kaplan–Meier probability or cumulative incidence for that time point, with 95% confidence intervals indicated. The 45-degree dotted line indicates the reference line where a perfect nomogram would lie. DMCI Distant Metastasis-Cumulative Incidence. N = 1326
Calibration Plots of Actual versus Predicted Disease-Specific Survival Outcomes using the MSKCC Prognostic Nomogram. Four (a)-, eight (b)-, and twelve-year (c) disease-specific survival were assessed. The nomogram predicted probabilities were stratified into quintiles. For each quintile, the average nomogram-predicted probability (x-axis) was plotted against the observed Kaplan–Meier probability for that time point with 95% confidence intervals indicated. The 45-degree dotted line indicates the reference line where a perfect nomogram would lie. N = 1326
Univariate analysis revealed tumor location and tumor depth were not significantly associated with OS (Table 3). Patient age (p < 0.001), tumor size (p < 0.001), tumor grade (p = 0.002), resection margin status (p < 0.001), and histologic subtype (p < 0.001) all remained significantly associated with OS in the multivariable analysis. The Fine and Gray analysis for DM CI resulted in a multivariable model with tumor size, tumor grade, histologic subtype, and tumor depth (Table 4). Independent risk factors associated with DSS on multivariate analysis (Table 5) included age (p < 0.001), tumor size (p < 0.001), tumor grade (p < 0.001), histologic subtype (p < 0.001), and resection margin status (p = 0.006).
Discussion
The Sarculator and MSKCC prognostic nomograms for extremity STS were developed as tools to assist in clinical decision making by utilizing clinicopathologic patient features. While both nomograms have shown promising clinical utility in assessing survival and recurrence outcomes, external validation has been limited. The present study evaluated the prognostic ability of both nomograms in a modern, multi-institutional U.S. cohort of patients undergoing resection of primary extremity STS across nine academic referral centers. Both the Sarculator and MSKCC nomograms demonstrated good prognostic ability for survival and distant recurrence outcomes in our diverse validation cohort. Despite significant differences in clinicopathologic features between the current validation study cohort and the original cohorts for the Sarculator and MSKCC studies, particularly with regards to the distribution of tumor grade and histologic subtypes, both nomograms displayed good calibration and discriminative ability within our population. Both nomograms have been proposed as a means of risk-stratifying patients for therapeutic decision making, clinical trial eligibility, and duration and intensity of postoperative surveillance.21,22 The results of this external validation study support the ongoing incorporation of both the Sarculator and MSKCC nomograms into the clinical management of patients with extremity STS.
The Sarculator nomogram proposed by Callegaro et al was developed and validated using cohorts from sarcoma centers in Italy, France, Canada, and the United Kingdom.10 To the authors’ knowledge, the current study is the first to externally validate the Sarculator nomogram within a diverse cohort of patients from the United States treated across nine high-volume academic institutions. The Harrell’s C-index of 0.72 for 5-year OS from our current validation cohort compared favorably with the C-indices of 0.70–0.78 for the cohorts reported in the original Sarculator study,10 indicating good discrimination, or the ability to distinguish patients who have experienced an event from those without the event, within our study population. Similarly, the C-index for 5-year DMCI of 0.72 in our validation cohort was comparable to the C-indices of 0.65–0.76 reported for the original study cohorts.10 The calibration plots for both OS and DMCI demonstrated good prognostic accuracy of the Sarculator model within our validation cohort.
The general MSKCC prognostic model for STS was originally developed by Kattan et al.11 and was subsequently externally validated by Mariani et al.,12 specifically for patients with extremity STS using a single institution cohort of patients treated from 1980 to 2000 in Milan, Italy. Analysis using the MSKCC nomogram within our current validation cohort resulted in C-index of 0.71 for 4-, 8-, and 12-year DSS, comparable to the C-indices of 0.77 and 0.76 from the original MSKCC development and validation studies, respectively. Our calibration plots for DSS also demonstrated good prognostic accuracy of the MSKCC nomogram. One of the few prior external validation studies of the MSKCC nomogram, recently published by Bagaria et al., analyzed STS patients from 1988 to 2011 identified from the Surveillance, Epidemiology, and End Results (SEER) cancer registry, and reported an overall C-index of 0.74.16 Their study included patients with STS across all disease sites, however, and 50% of the cohort was made up of patients with nonextremity tumors, limiting the applicability of these results. Given the complexity and evolution of nomenclature for histologic classification of STS subtypes, the reliability of historical registry data, such as SEER or National Cancer Database (NCDB) for sarcoma-specific clinicopathologic variables also is potentially problematic. The granularity of the current USSC database, on the other hand, allowed for more reliable confirmation of histologic subtype and tumor grade among a modern cohort of extremity STS patients.
While both nomograms proved to be accurate prognostic tools within our validation cohort, there are some important considerations when assessing their clinical utility. One notable difference between the two existing nomograms is the analysis of patients with liposarcoma histology. The Sarculator model excludes patients with well-differentiated liposarcoma, while including those with myxoid or dedifferentiated/pleomorphic liposarcoma and treating these two subtypes as discrete histologic entities for the purposes of the nomogram. The MSKCC model, by contrast, not only includes patients with well-differentiated liposarcoma histology but makes no distinction between subclassifications of liposarcoma, and groups all liposarcoma patients into one histologic category. Previous studies have demonstrated that these liposarcoma subtypes display distinctly different biologic behaviors and distinctly different survival and recurrence prognoses, arguing for their treatment as separate entities for nomogram calculations.23,24,25,26 In addition, the Sarculator nomogram incorporates tumor size as a continuous variable, while the MSKCC nomogram analyzes the tumor size covariate as a categorical variable.10 The exact relationship between extremity STS tumor size and recurrence and survival outcomes remains somewhat controversial, particularly at either end of the spectrum; the significance of a 1-cm incremental change in tumor size appears to be nonlinear (i.e., the difference between a 2-cm and 3-cm tumor is not the same as the difference between a 30-cm and 31-cm tumor).3,11,17,27
Interestingly, neither the Sarculator nor MSKCC nomograms include resection margin status as a covariate in their respective models, although the current study and others have suggested that margin status may be an independent risk factor for adverse recurrence and survival outcomes.28,29,30 In the current validation cohort, R1 margin status was an adverse prognostic factor independently associated with worse OS and DSS, but not DMCI.
Visual inspection of our calibration plots suggests that the prognostic accuracy of both of these static nomograms may be weaker for the later endpoints at 10 and 12 years postresection. The 10-year OS (Fig. 1B) and DMCI (Fig. 1D) plots from the Sarculator nomogram and the 12-year DSS plot (Fig. 2C) from the MSKCC nomogram demonstrated a larger spread around the 45-degree reference line of the model. Other studies have suggested that the effects of covariates on survival and recurrence outcomes may in fact change over time after initial sarcoma resection and dynamic nomograms have been proposed to provide more accurate prognostic information during the post-resection follow-up period.31,32,33 Future studies will attempt to validate these dynamic prognostic nomograms within our multi-institutional cohort.
Limitations of the current validation study include its retrospective, multi-institutional cohort design, spanning nine high-volume academic institutions and 17 years of evolving perioperative care for extremity STS. Although data were collected retrospectively, the current study allowed for analysis of more granular, reliable data, particularly regarding tumor grade and histologic subtype. A multi-institutional analysis inherently encompasses interinstitutional variability in treatment paradigms, such as the role of perioperative systemic therapy and radiation therapy for these patients. The Sarculator and MSKCC prognostic nomograms, however, were purposefully built upon clinicopathologic variables related to an individual’s specific tumor rather than treatment-related variables. In addition, tumor grade was assessed by multiple pathologists across these institutions, potentially introducing inter-institutional variability. While the definition of margin status for extremity soft tissue sarcoma remains somewhat controversial,34 the definition of R1 margin as tumor within 1mm of the inked margin from the Sarculator nomogram was utilized. Evolution in pathologic classification of sarcoma subtypes between the Sarculator and MSKCC nomograms and the current validation study required reclassification of a few specific pathologic subtypes, given the inability to re-review pathology from the original nomogram studies. Given the rarity of extremity STS, multi-institutional analyses are necessary to accrue a large enough cohort to draw meaningful conclusions from the data. Despite the inherent heterogeneity of this validation study cohort and significant differences in clinicopathologic features from the more-uniform, institutional data used to generate the Sarculator and MSKCC nomograms, both models demonstrated good prognostic ability within the current cohort.
In conclusion, the Sarculator and MSKCC nomograms were both found to have good discriminative and prognostic ability within a diverse, modern, multi-institutional U.S. validation cohort of patients undergoing resection of primary extremity STS. Ongoing incorporation of these prognostic nomograms into the clinical management of extremity STS patients appears warranted.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Yoon SS. The New American Joint Commission on cancer staging system for soft tissue sarcomas: splitting versus lumping. Ann Surg Oncol. 2018;25:1101–2.
Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260:416–21 (discussion 421-2).
Fletcher CD. The evolving classification of soft tissue tumours—an update based on the new 2013 WHO classification. Histopathology. 2014;64:2–11.
Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. New York: Springer International Publishing; 2017.
Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–9.
Fisher SB, Chiang YJ, Feig BW, et al. Comparative performance of the 7th and 8th editions of the American Joint Committee on Cancer Staging Systems for soft tissue sarcoma of the trunk and extremities. Ann Surg Oncol. 2018;25:1126–32.
Cates JMM. AJCC eighth edition for soft tissue sarcoma of the extremities and trunk. Ann Oncol. 2018;29:2023.
Cates JMM. The AJCC 8th edition staging system for soft tissue sarcoma of the extremities or trunk: a cohort study of the SEER database. J Natl Compr Cancer Netw. 2018;16:144–52.
Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17:671–80.
Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–6.
Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer. 2005;103:402–8.
Callegaro D, Miceli R, Mariani L, Raut CP, Gronchi A. Soft tissue sarcoma nomograms and their incorporation into practice. Cancer. 2017;123:2802–20.
Tseng WW, Pasquali S, Hu JS, Menendez LR, Gronchi A. Staging systems and nomograms in soft tissue sarcoma: outcome prediction by categorization or personalization? Chin Clin Oncol. 2019;8:S12.
Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70:200–29.
Bagaria SP, Wagie AE, Gray RJ, et al. Validation of a soft tissue sarcoma nomogram using a National Cancer Registry. Ann Surg Oncol. 2015;22(Suppl 3):S398-403.
Eilber FC, Brennan MF, Eilber FR, Dry SM, Singer S, Kattan MW. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer. 2004;101:2270–5.
Eilber FC, Kattan MW. Sarcoma nomogram: validation and a model to evaluate impact of therapy. J Am Coll Surg. 2007;205:S90–5.
Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42.
Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87.
Pasquali S, Colombo C, Pizzamiglio S, et al. High-risk soft tissue sarcomas treated with perioperative chemotherapy: Improving prognostic classification in a randomised clinical trial. Eur J Cancer. 2018;93:28–36.
Pasquali S, Pizzamiglio S, Touati N, et al. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur J Cancer. 2019;109:51–60.
Vos M, Koseła-Paterczyk H, Rutkowski P, et al. Differences in recurrence and survival of extremity liposarcoma subtypes. Eur J Surg Oncol. 2018;44:1391–7.
Bartlett EK, Curtin CE, Seier K, et al. Histologic subtype defines the risk and kinetics of recurrence and death for primary extremity/truncal liposarcoma. Ann Surg. 2019;273:1189–96.
Crago AM, Dickson MA. Liposarcoma: multimodality management and future targeted therapies. Surg Oncol Clin N Am. 2016;25:761–73.
Fiore M, Grosso F, Lo Vullo S, et al. Myxoid/round cell and pleomorphic liposarcomas: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2007;109:2522–31.
Trovik C, Bauer HCF, Styring E, et al. The Scandinavian Sarcoma Group Central Register: 6,000 patients after 25 years of monitoring of referral and treatment of extremity and trunk wall soft-tissue sarcoma. Acta Orthop. 2017;88:341–7.
Cahlon O, Brennan MF, Jia X, Qin LX, Singer S, Alektiar KM. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg. 2012;255:343–7.
Gronchi A, Casali PG, Mariani L, et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: a series of patients treated at a single institution. J Clin Oncol. 2005;23:96–104.
Gronchi A, Lo Vullo S, Colombo C, et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251:506–11.
Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of a dynamic prognostic nomogram for primary extremity soft tissue sarcoma survivors. EClinicalMedicine. 2019;17:100215.
Rueten-Budde AJ, van Praag VM, van de Sande MAJ, Fiocco M, PERSARC studygroup. Dynamic prediction of overall survival for patients with high-grade extremity soft tissue sarcoma. Surg Oncol. 2018;27:695–701.
Rueten-Budde AJ, van Praag VM, van de Sande MAJ, Fiocco M, PERSARC Study Group. External validation and adaptation of a dynamic prediction model for patients with high-grade extremity soft tissue sarcoma. J Surg Oncol. 2021;123:1050–6.
Gundle KR, Kafchinski L, Gupta S, et al. Analysis of margin classification systems for assessing the risk of local recurrence after soft tissue sarcoma resection. J Clin Oncol. 2018;36:704–9.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MHS, CGE, JHB, CJA, MHJ, JCP, JSK, MBL, NLG, JSH JCS, RSP, KC, and JHH contributed to the conception/design of the work; MHS, CGE, JHB, MM, NLG, MN, WA, RSP, RCF, BAK, MB, KV, KC, VG, KKR, JT, GP, and TT contributed to the acquisition of data; MHS, EED, CJA, MHJ, JCP, JSK, SJT, JCS, KC, JHH contributed to the analysis/interpretation of the data; MHS, JHB, EED, JCS, KC, JHH drafted the manuscript; all authors critically revised the manuscript for intellectual content; all authors approved the final version submitted and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Disclosures
The authors have no financial or conflict of interest disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Squires, M.H., Ethun, C.G., Donahue, E.E. et al. Extremity Soft Tissue Sarcoma: A Multi-Institutional Validation of Prognostic Nomograms. Ann Surg Oncol 29, 3291–3301 (2022). https://doi.org/10.1245/s10434-021-11205-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-11205-5