Abstract
Background
This study aimed to investigate the association between pathologic stage and recurrence risk and survival for patients with esophageal squamous cell carcinoma (SCC) after neoadjuvant chemoradiotherapy (CRT).
Methods
This retrospective analysis consisted of two patient cohorts who had esophageal SCC treated with neoadjuvant CRT and esophagectomy at two major academic institutions between 2002 and 2015. The study included 174 patients in the training cohort and 51 patients in the validation cohort. Recurrence pattern, frequency, and survival according to pathologic stage were analyzed.
Results
After surgery, patients in the training cohort had the following pathologic categories: stage 0 (44.8%, n = 78), stage 1 (6.9%, n = 12), stage 2 (35.6%, n = 62), and stage 3 (12.6%, n = 22). During a median follow-up period of 53.9 months, recurrences developed in 59 patients. The recurrence rates were 22.2% for stages 0 and 1, 38.7% for stage 2, and 68.2% for stage 3 (stages 0 and 1 vs. stage 2 [P = 0.028], stages 0 and 1 vs. stage 3 [P < 0.001], and stage 2 vs. stage 3 [P = 0.017]). More than 20% of patients with stages 0 and 1 or 2 disease experienced late relapses after 3 years of follow-up evaluation, whereas all the patients with pathologic stage 3 had recurrences within 2 years. The 5-year recurrence-free survival rate was 74.7% for the patients with pathologic stage 0 or 1, 61.4% for those with stage 2, and 20.9% for those with stage 3 disease (P < 0.001). These major findings were successfully reproduced in the Western validation cohort.
Conclusions
Patients with a higher pathologic stage were associated with a significantly higher risk of recurrences and worse survival. Multicenter and prospective validation is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
As the sixth leading cause of cancer-related death worldwide, esophageal cancer (EC) is an aggressive malignancy with a poor prognosis.1 Based on results from randomized phase 3 trials and meta-analyses, neoadjuvant chemoradiotherapy (CRT) followed by surgery has been the standard of care for locally advanced EC.2,3,4 However, despite improvement in locoregional control and distant failure by neoadjuvant treatment, EC remains associated with rather high recurrence risk, showing recurrence rates of 31–50%.5,6,7,8
It has been well demonstrated that salvage treatment could provide survival benefits for EC patients, irrespective of the recurrence pattern.9,10 Therefore, considering the high risk of recurrences and cost effectiveness of EC after trimodality therapy, an appropriate risk-based surveillance strategy for timely detection of relapses has significant clinical implications. Nevertheless, the current surveillance principles remain controversial and do not take into account histologic type or pathologic tumor-node-metastasis (TNM) stage.11 Previous studies have documented that pathologic stage using the 7th TNM staging system could predict overall survival (OS) and recurrence-free survival (RFS) for patients with esophageal adenocarcinoma after neoadjuvant therapy.12,13,14
Given the differences in etiology, epidemiology, and clinical characteristics between esophageal adenocarcinoma and squamous cell carcinoma (SCC), it is unjustified to generalize the outcomes to another histologic type.15 Moreover, a recent comparative genomic study showed some important disparities of esophageal SCC between Asian and Caucasian patient populations,16 suggesting that the clinical results also may differ between these two populations.
Detailed reports documenting recurrence risk stratification in esophageal SCC after neoadjuvant treatment are scarce in the literature. The primary purpose of this study was to investigate the association of pathologic stage with recurrence risk and survival outcomes for patients with esophageal SCC who received neoadjuvant CRT followed by surgery. We also aimed to assess whether the clinical outcomes of esophageal SCC for Chinese patients could be reproducible in Western populations using an external validation study.
Patients and Methods
Patients
As the training cohort, all consecutive patients with EC who underwent neoadjuvant CRT followed by esophagectomy from the prospectively maintained database at Sun Yat-sen University Cancer Center (SYSUCC) between January 2002 and May 2015 were retrospectively analyzed. All the patients had histologically proven and resectable thoracic esophageal SCC with stage cT1N + M0 or cT2-4aN0-3M0 according to the 7th TNM staging system of the American Joint Committee on Cancer (AJCC).17 Patients with macroscopically incomplete resection, those who died before hospital discharge, and those with incomplete records were excluded from the study.
For external validation, an independent cohort of patients with esophageal SCC who satisfied the inclusion criteria at the University of Texas MD Anderson Cancer Center (MDACC) between January 2001 and February 2015 were analyzed. This study was approved by the institutional review boards of both institutions, and informed consent was waived due to its retrospective nature.
All the patients had pretreatment evaluations including complete history, physical examination, standard laboratory tests, esophagogastroduodenoscopy (EGD) with endoscopic ultrasound (EUS) and biopsies, chest/abdominal computed tomography (CT), and/or positron emission tomography (PET). Each patient was evaluated by a multidisciplinary team before initiation of treatment according to institutional practice guidelines.
Treatment
All the patients in both the training and validation cohorts received concurrent platinum- or taxane-based chemotherapy during radiotherapy, and a fraction of the patients at MDACC received two to four cycles of induction chemotherapy before neoadjuvant CRT. Radiation was delivered using three-dimensional conformal radiotherapy (3DCRT), intensity-modulated radiotherapy, or proton beam therapy. Gross tumor volume (GTV) was defined as the primary tumor and positive lymph nodes on CT, EUS, and/or PET/CT. Clinical target volume was defined as the GTV plus 3-cm proximal and distal margins and a radial margin of 0.5–1.0 cm. The typical prescribed dose was 40 Gy in 20 fractions with 2 Gy per fraction once daily at SYSUCC, whereas the standard prescribed dose was 50.4 Gy in 28 fractions at MDACC.
Surgery was performed approximately 6–8 weeks after the completion of CRT. The surgical methods consisted of Ivor-Lewis esophagectomy, transhiatal three-field technique, and minimally invasive esophagectomy, as determined by the operating team. The pathologic stage was evaluated by two experienced pathologists. Pathologic complete response (pCR) was defined as complete absence of residual cancer cells in all layers of the esophagus and in the lymph nodes resected.
Follow-Up Assessment and Recurrences
After completion of treatment, patients were routinely followed up every 3 months during the first year, then every 6 months for the next 2 years, and yearly thereafter until 5 years in both the training and validation cohorts. The follow-up examinations included blood tests, periodic EGDs, chest/abdominal CT, and/or PET/CT. The pattern of first recurrence was used to classify locoregional or distant recurrence, which was established on histologic, cytologic, or explicit radiologic proof. Locoregional recurrences (LRRs) included recurrences within the esophagus or regional lymph nodes, whereas distant recurrences included non-regional lymph node metastases (supraclavicular and para-aortic nodes), distant organ metastases, and peritoneal carcinomatosis.
Statistical Analysis
Follow-up and survival times were defined from the date of surgery. The study calculated OS from the date of surgery until all-cause death or last follow-up visit, and RFS was defined as the interval between the date of surgery and the first event of disease recurrence. Patients who experienced recurrences before death also were counted as events.
The Kaplan–Meier method was used to analyze OS and RFS, and the Wilcoxon test was used to analyze the differences in survival distributions between cohorts. Statistical analyses were performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). All P values lower than 0.05 were considered statistically significant.
Results
Patient Characteristics
Of the 207 patients who underwent neoadjuvant CRT followed by esophagectomy at SYSUCC between 2002 and 2015, 34 patients were ineligible (8 due to macroscopically incomplete resection, 4 due to in-hospital death, and 21 due to incomplete records). The patient and treatment characteristics of 174 patients eligible for analysis in the training cohort are summarized in Table 1. The median age of this cohort was 55 years (range 42–73 years), and most tumors were located in the upper/middle third of the esophagus (79.3%). At baseline staging, 132 patients (75.9%) had stage 3 disease. The majority of the patients (81.6%) were treated with 3DCRT, and the median radiation dose was 40.0 Gy (range 40.0–50.4 Gy).
After neoadjuvant CRT, all the patients underwent esophagectomy within a median interval of 6.4 weeks (range, 3.9–13.3 weeks). After histopathologic examination, 78 patients (44.8%) achieved a pCR, and the remaining patients had the following characteristics: stage 1 (6.9%, n = 12), stage 2 (35.6%, n = 62), and stage 3 (12.6%, n = 22). The comparison between clinical and pathologic stage is shown in Table S1. Patients with clinical stage 1 or 2 disease had a higher pCR rate than those with stage 3 disease, but the difference was not statistically significant (52.4% vs. 42.4%; P = 0.258).
Follow-Up and Recurrences
The median follow-up period was 53.9 months for survivors in the training cohort, and 61 patients (35.1%) had died at the time of analysis. During the follow-up period, recurrences developed in 59 patients (33.9%). Among these patients, 66.1% (39 patients) had the recurrences proven pathologically. Among the 59 patients, 17 (9.8%) experienced LRR only, 32 (18.4%) experienced distant failure only, and 10 (5.7%) experienced concurrent LRRs and distant recurrences. For the patients who experienced recurrences, the median time to the first recurrence was 7.7 months (interquartile range 5.0–19.7 months) after surgery. The majority of the relapses (84.7%) for these patients occurred within 3 years after surgery. For the entire cohort, the 5-year OS rate was 63.7%, and the 5-year RFS rate was 65.0%.
Pathologic Stage and Recurrence Stratification
Because the total recurrence rates between pathologic stages 0 and 1 were comparable (P = 0.454), these two groups were combined for comparison with the other pathologic categories. As shown in Table 2, the total recurrence rates in the different pathologic categories were 22.2% for stages 0 and 1, 38.7% for stage 2, and 68.2% for stage 3 disease (P < 0.001), suggesting that patients with a higher pathologic stage had a significantly higher risk of recurrences (stages 0 and 1 vs. stage 2 [P = 0.028], stages 0 and 1 vs. stage 3 [P < 0.001], and stage 2 vs. stage 3 [P = 0.017]).
With regard to LRR, the patients with stage 3 disease had higher recurrence rates than those with stage 0 or 1 (36.4% vs. 8.9%, P = 0.001) or stage 2 disease (36.4% vs. 17.7%; P = 0.073). No statistical difference was noted in LRR rates between stage 0 or 1 and stage 2 disease (P = 0.105). However, the risk of distant recurrences was significantly higher for the patients with stage 2 than for those with stage 0 or 1 disease (32.3% vs. 16.7%; P = 0.025). The distant failure rates were comparable between stages 2 and 3 disease (32.3% vs. 31.8%; P = 0.97).
Recurrence timing and frequency according to pathologic categories are summarized in Table 2. More than 20% of the patients with stage 0 or 1 or stage 2 disease experienced late relapses after 3 years of follow-up evaluation, whereas all the patients with pathologic stage 3 disease had recurrences within 2 years. In particular, 62.5% of LRRs occurred within 2 years of follow-up evaluation for the patients with stage 0 or 1 versus 81.8% for stage 2 versus 100% for stage 3 disease, suggesting that LRRs occurred earlier for the patients with higher pathologic stage. Regarding distant recurrences, 73.4% of the recurrences occurred within 2 years after surgery for the patients with stage 0 or 1 versus 70% for those with stage 2 disease (P = 1.0). No patients with pathologic stage 3 disease experienced distant failure after 2 years of follow-up evaluation.
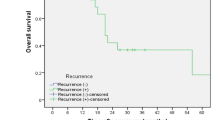
The 5-year RFS rates were 74.7% for those with stage 0 or 1, 61.4% for stage 2, and 20.9% for stage 3 disease (stages 0 and 1 vs. stage 2 [P = 0.039], stages 0 or 1 vs. stage 3 [P < 0.001], and stage 2 vs. stage 3 [P < 0.001]; Fig. 1a). Likewise, the patients with pathologic stage 0 or 1 disease demonstrated a significantly more favorable OS than those with stage 2 or 3 disease (stages 0 and 1 vs. stage 2 [P = 0.035], stage 0 and 1 vs. stage 3 [P < 0.001], and stage 2 vs. stage 3 [P = 0.007]; Fig. 1b).
External Validation of Risk Stratification
Table 3 lists the clinical characteristics of the 51 eligible patients in the validation cohort. The median age was 63 years (range 43–78 years), and 64.7% of the patients had stage 3 disease. After neoadjuvant CRT and esophagectomy, patients in the following pathologic categories were identified: stage 0 (49.0%, n = 25), stage 1 (5.9%, n = 3), stage 2 (35.3%, n = 18), and stage 3 (9.8%, n = 5) disease. As shown in Table S2, the patients with clinical stage 1 or 2 disease had a significantly higher pCR rate than those with stage 3 disease (82.2% vs. 36.4%; P = 0.014).
The median follow-up time was 42.1 months for the survivors in this cohort, and 30 patients (58.8%) had died at the time of the analysis. During the follow-up period, 16 patients (31.4%) experienced recurrences. Of the 16 patients, 6 (11.8%) had LRR only, 7 (13.7%) had distant failure only, and 3 (5.9%) had concurrent LRRs and distant recurrences. The total recurrence rates according to pathologic categories were 14.3% for stages 0 and 1, 44.4% for stage 2, and 80.0% for stage 3 disease (P = 0.005). Regarding recurrence frequency, 75% of the recurrences occurred within 2 years after surgery for the patients with stage 0 or 1 or stage 2 disease. Similar to the training cohort, all the patients with stage 3 disease experienced recurrences within 2 years of follow-up evaluation in the validation cohort. The 3-year RFS rates were 78.6% for pathologic stages 0 and 1, 45.4% for stage 2, and 20.0% for stage 3 disease (P = 0.008; Fig. 2). No significant difference in survival distributions was noted for any of the pathologic categories between the training cohort and the validation cohort (stages 0 and 1 [P = 0.199], stage 2 [P = 0.057], stage 3 [P = 0.554]).
Discussion
The current study evaluating the correlation between pathologic stage and recurrence risk stratification in esophageal SCC after neoadjuvant CRT demonstrated that patients with a higher pathologic stage were associated with a significantly higher risk of recurrences and worse survival. Additionally, these findings appear generalizable because this was reproducible in a validation cohort from a large institution in the United States with vastly different ethnic backgrounds. Therefore, risk-based surveillance strategies and clinical decisions could be developed for different pathologic categories.
The association between pathologic stage and survival as well as recurrence risk for patients with esophageal adenocarcinoma has been previously documented.12,13,14 Davies et al.13 indicated that OS was strongly determined by pathologic stage after neoadjuvant chemotherapy for esophageal adenocarcinoma rather than by clinical stage at baseline. Furthermore, Taketa et al.12 studied 518 patients with esophageal adenocarcinoma and found an excellent association between pathologic stage and frequency/type/timing of relapses after trimodality therapy. Regarding esophageal SCC, several studies have shown that pCR, tumor regression grade, perineural invasion, lymphovascular invasion, and pathologic lymph node status after neoadjuvant CRT are important prognostic factors for OS.18,19,20 However, whether these factors can customize the recurrence risk stratification in SCC is unclear. On this issue, our study demonstrated that pathologic stage was associated with both recurrence risk and survival in esophageal SCC after trimodality therapy, which is similar to adenocarcinoma. Wang et al.20 reported that patients with pathologic stage 1 disease had survival outcomes comparable with those for patients demonstrating pCR, which is consistent with our findings. Thus, according to recurrence risk stratification, we combined pathologic stages 0 and 1 disease and classified patients into three risk categories: stages 0 and 1, stage 2, and stage 3 disease.
Taketa et al.12 reported that the LRR rates were similar across all pathologic stages, whereas distant relapses increased with higher stage for patients who had adenocarcinoma treated with neoadjuvant CRT. The detailed recurrence pattern of SCC differed from that of adenocarcinoma. Our results indicated that SCC patients with stage 3 disease had significantly higher LRR rates than those with stage 0 or 1 or stage 2 disease, indicating that strategies to reduce the risk of LRRs should be studied for this high-risk cohort. On the other hand, we found that the risks of distant failure were comparable between stage 2 and stage 3 disease, suggesting that no specific strategy might be needed to detect distant relapses for a specific category in SCC.
A rational surveillance regimen should be defined on the basis of tumor recurrence risk, timing, and frequency. In the study reported by Taketa et al.,12 more than 99% of all recurrences occurred within 3 years of follow-up evaluation for adenocarcinoma patients with pathologic stage 0 or 1 disease. Therefore, the authors suggested that the surveillance should be terminated after 3 years for this cohort. Unlike the findings for adenocarcinoma, our study showed that 20% of late recurrences occurred 3 years after surgery for SCC patients with stage 0 or 1 disease. Similarly, 21.4% of SCC patients with stage 2 disease experienced late relapses after 3 years. In line with our results, Steffen et al.21 also observed that more patients with SCC experienced late relapses after 4 years than patients with adenocarcinoma. Based on these data, follow-up assessment every 6–12 months from years 3 to 5 may be warranted for SCC patients with pathologic stage 0, 1, or 2 disease. Compared with patients who have earlier-stage disease, SCC patients with pathologic stage 3 disease tended to experience recurrences not only significantly sooner but also more frequently. Given that all relapses occurred within 2 years after surgery, surveillance should be more frequent during the first 2 years, and intensive follow-up evaluation after 2 years may be unnecessary for SCC patients with pathologic stage 3 disease.
To date, no genetic susceptibility for esophageal SCC in the Western population has been identified. On the contrary, a large genome-wide association study has identified two susceptibility genes (PLCE1 and C20orf54), which were highly correlated with esophageal SCC in the Chinese population.22 In addition, Zhang et al.23 reported that patients with esophageal SCC from Eastern and Western countries had several different clinical features, such as age and female proportion, which also was confirmed by our study. However, despite the differences in gene mutational frequencies and clinical characteristics, Eastern SCC patients showed an OS comparable with that of Western patients in this population-based study. Consistently, our study found that the recurrence patterns and survival distributions were similar between the training cohort from China and the validation cohort from the United States.
Our study had several drawbacks that should be noted. First, the results may have been inevitably influenced by selection bias due to its retrospective nature. Second, the baseline characteristics, proportion of patients receiving induction chemotherapy, chemotherapy regimens, radiation dose, and radiation methods were not balanced between the training cohort and the validation cohort, which may have affected the outcomes. However, despite some discrepancies between the two cohorts, the major findings were successfully reproduced in the external validation cohort. Finally, due to the rarity of esophageal SCC in the United States, the number of patients was limited in the validation cohort.
Conclusions
After neoadjuvant CRT and esophagectomy, esophageal SCC patients with a higher pathologic stage were associated with a significantly higher risk of recurrences and worse survival outcomes. This finding was successfully validated in a small Western cohort from a single institution. Therefore, multicenter and prospective validation is warranted to confirm our findings.
References
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92.
Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8.
Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. 2014;32:385–91.
Sudo K, Taketa T, Correa AM, et al. Locoregional failure rate after preoperative chemoradiation of esophageal adenocarcinoma and the outcomes of salvage strategies. J Clin Oncol. 2013;31:4306–10.
Robb WB, Messager M, Dahan L, et al. Patterns of recurrence in early-stage oesophageal cancer after chemoradiotherapy and surgery compared with surgery alone. Br J Surg. 2016;103:117–25.
van Hagen P, Wijnhoven BP, Nafteux P, et al. Recurrence pattern in patients with a pathologically complete response after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br J Surg. 2013;100:267–73.
Abate E, DeMeester SR, Zehetner J, et al. Recurrence after esophagectomy for adenocarcinoma: defining optimal follow-up intervals and testing. J Am Coll Surg. 2010;210:428–35.
Xi M, Hallemeier CL, Merrell KW, et al. Recurrence risk stratification after preoperative chemoradiation of esophageal adenocarcinoma. Ann Surg. 2018;268(2):289–95.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers: V.2.2018. Fort Washington, PA: NCCN.
Taketa T, Sudo K, Correa AM, et al. Post-chemoradiation surgical pathology stage can customize the surveillance strategy in patients with esophageal adenocarcinoma. J Natl Compr Cancer Netw. 2014;12:1139–44.
Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014;32:2983–90.
McNamara MJ, Rybicki LA, Sohal D, et al. The relationship between pathologic nodal disease and residual tumor viability after induction chemotherapy in patients with locally advanced esophageal adenocarcinoma receiving a tri-modality regimen. J Gastrointest Oncol. 2016;7:196–205.
Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2007;17:38–44.
Deng J, Chen H, Zhou D, et al. Comparative genomic analysis of esophageal squamous cell carcinoma between Asian and Caucasian patient populations. Nat Commun. 2017;8:1533.
Edge SB, Byrd DR, Compton CC, et al (eds). AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010.
Robb WB, Dahan L, Mornex F, et al. Impact of neoadjuvant chemoradiation on lymph node status in esophageal cancer: post hoc analysis of a randomized controlled trial. Ann Surg. 2015;261:902–8.
Tu CC, Hsu PK, Chien LI, et al. Prognostic histological factors in patients with esophageal squamous cell carcinoma after preoperative chemoradiation followed by surgery. BMC Cancer. 2017;17:62.
Wang CC, Cheng JC, Tsai CL, et al. Pathological stage after neoadjuvant chemoradiation and esophagectomy superiorly predicts survival in patients with esophageal squamous cell carcinoma. Radiother Oncol. 2015;115:9–15.
Steffen T, Dietrich D, Schnider A, et al. Recurrence patterns and long-term results after induction chemotherapy, chemoradiotherapy, and curative surgery in patients with locally advanced esophageal cancer. Ann Surg. 2017. https://doi.org/10.1097/SLA.0000000000002435.
Wang LD, Zhou FY, Li XM, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet. 2010;42:759–63.
Zhang J, Jiang Y, Wu C, et al. Comparison of clinicopathologic features and survival between eastern and western population with esophageal squamous cell carcinoma. J Thorac Dis. 2015;7:1780–6.
Acknowledgment
This work was supported by Grants from the Natural Science Foundation of Guangdong Province (2015A030310044), the Science and Technology Planning Project of Guangdong Province (2013B021800156), and the Guangdong Esophageal Cancer Institute Science and Technology Program (M201715).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
Steven H. Lin has received research funding from STCube Pharmaceuticals, Peregrine Pharmaceuticals, Hitachi Chemical Diagnostics, and Roche/Genentech, is on the advisory board for AstraZeneca, and has received honoraria from US Oncology and ProCure. The other authors have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, S., Liu, S., Zhang, L. et al. Recurrence Risk Based on Pathologic Stage After Neoadjuvant Chemoradiotherapy in Esophageal Squamous Cell Carcinoma: Implications for Risk-Based Postoperative Surveillance Strategies. Ann Surg Oncol 25, 3639–3646 (2018). https://doi.org/10.1245/s10434-018-6736-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6736-7