Abstract
Purpose
A meta-analysis of 22 randomized trials accrued from 1964 to 1986 demonstrated significantly higher rates of locoregional failure (LRF) and breast-cancer mortality in women with 1–3 positive nodes without postmastectomy radiotherapy (PMRT) after mastectomy (mast.). Recent data demonstrate that PMRT reduces distant metastases (DM) in women with pN1 disease. The challenge today is whether all patients with pathologic T1-2pN1 disease have similar substantial LRF/DM risk that routinely warrants PMRT.
Methods
We reviewed patients with pT1-2N1 breast cancer treated with mast. ± adjuvant systemic therapy without PMRT from 2000 to 2013. The endpoints were LRF and DM rates, estimated by cumulative incidence method.
Results
We identified 468 patients with median follow-up of 6.3 years. Most (71%) were estrogen receptor/progesterone receptor + human epidermal growth factor receptor 2 (HER2). There were 269 patients with 1+ node, 140 patients with 2+ nodes, and 59 patients with 3+ nodes. The 6-year LRF/DM rates were 4.1%/8.4%. Patients with 1+, 2+, and 3+ nodes had 6-year LRF of 2.3, 5.1 and 8.9%, respectively (p = 0.13). The 6-year DM rate was higher in patients with 3+ nodes versus 1–2+ nodes: 15.7% versus 7.4% (p = 0.02). Several subgroups had low 6-year LRF and DM rates, including T1/1+ node (0.8%/4.1% LRF/DM) and micrometastases (0%/5.8% LRF/DM).
Conclusions
Patients with pT1-2pN1 represent a heterogeneous group with a wide range of LRF/DM rates. In particular, patients with pT1 tumors and 1 + LN, and patients with micrometastases, had low event rates. These groups would derive small absolute reductions in LRF and DM with addition of PMRT, underscoring the importance of patient selection for PMRT in pT1-2pN1 breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The radiation management of T1–2 breast cancer with 1–3 positive lymph nodes (pT1-2pN1) treated with mastectomy remains controversial. Data from metaanalyses and randomized trials support that regional nodal irradiation (RNI) benefits those with pN1 disease by reducing locoregional failure (LRF) and distant metastasis (DM) and improving disease-free survival (DFS). The 2014 analysis of postmastectomy radiation therapy (PMRT) by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) reported results on > 8000 women enrolled on randomized trials from 1964 to 1986.1 In the subgroup of women with pN1 disease, PMRT significantly decreased the 10-year risk of LRF by 16.5%, resulting in a reduction in breast-cancer mortality (BCM) at 20 years of 7.9% (42.3 vs 50.2%, p = 0.01). In the subgroup of women with 1+ lymph node, PMRT also significantly reduced LRF risk (2.3 vs 17.8%) but did not significantly impact BCM.1 The chief criticism of the EBCTCG metaanalysis is that it represents an outdated treatment era, calling into question its applicability to current practice.
Cancer outcomes have improved for breast cancer with pN1 disease treated with mastectomy, reflecting smaller tumor burdens from cancer screening, changes in surgical approaches, and more effective systemic therapy.2,3,–4 However, two recent randomized trials reflecting a more modern treatment era also support use of PMRT and/or regional nodal irradiation (RNI) for breast cancer with pN1 disease. The European Organisation for Research and Treatment of Cancer (EORTC) 22922/10952 trial randomized 4004 patients after surgery to RNI/PMRT or no RNI/PMRT, with the majority having pN1 disease.5 The National Cancer Institute of Canada (NCIC) MA.20 trial similarly randomized 1832 women (85% pN1) treated with lumpectomy and breast radiation to RNI versus none.6 The EORTC trial resulted in a significant 3% absolute improvement in DFS and distant-DFS at 10 years [hazard ratio (HR) = 0.86] with a trend towards improvement in overall survival (OS) with use of RNI. The MA.20 trial similarly reported a significant 4% improvement in DFS and distant-DFS (HR = 0.76) from RNI. Reflecting the modern treatment era, more modest 10-year LRF rates of 9.5 and 7.8%, respectively, are noted in the control groups for the EORTC 22922 and NCIC MA.20 trials. Based on these findings, the American Society of Clinical Oncology (ASCO), American Society of Radiation Oncology (ASTRO), and Society of Surgical Oncology (SSO) published a guideline update on PMRT in 2016.7 The panel unanimously agreed that PMRT reduces LRF, any recurrence, and BCM in women with pT1-2pN1 disease.
Given the consistent impact on DFS demonstrated, it is likely that the proportion of women who receive PMRT for pT1-2pN1 disease will increase over time as a result of the 2014 EBCTCG metaanalysis, the 2015 EORTC 22922/NCIC MA.20 randomized trials, and the 2016 ASCO/ASTRO/SSO PMRT guidelines. However, routine PMRT use in these patients may lead to increased toxicity such as cardiac morbidity, pneumonitis/lung fibrosis, and lymphedema.
As a group, pT1-2pN1 breast cancer represents a broad spectrum of disease that includes an equally broad range of risks for disease recurrence. Prior EBCTCG metaanalyses have demonstrated that the benefit from radiation is proportionally linked to the amount of disease risk without radiation.8 For this population treated with mastectomy, it is anticipated that the benefit of PMRT similarly will depend on baseline risk without radiation. Therefore, identification of subgroups with low risk of recurrence within this population will allow selection of patients in whom the benefits of PMRT are outweighed by the associated toxicity risk.
In this study, we aim to evaluate the LRF and DM rates in breast cancer patients with pT1-2pN1 disease treated with mastectomy. We specifically set out to establish the recurrence risk for 1+ versus 2+ versus 3+ nodes at our institution. We hypothesize that there are subgroups of patients within the pT1-2pN1 spectrum who have sufficiently low recurrence risk that routine use of PMRT is not indicated.
Patients and Methods
We retrospectively reviewed patients in our institutional cancer registry with pT1-2pN1 breast cancer treated with initial mastectomy and adjuvant systemic therapy from 2000 to 2013. Patients who received neoadjuvant chemotherapy were excluded. This treatment timeline was chosen to have a representative patient population treated with modern systemic therapy (ST). This study was approved by our Institutional Review Board.
We collected the following data: age, tumor laterality, tumor size, tumor grade, lymphovascular space invasion (LVSI), extracapsular extension (ECE), subtype [hormone-sensitive, HS (ER+ or PR+, HER2−), HER2+ (ER−/PR−/HER2+), triple negative (TN)], nodal evaluation (axillary lymph node dissection or sentinel lymph node biopsy only), absolute number of axillary lymph nodes (ALN) removed, size of lymph node metastases (macrometastasis or micrometastasis), number of lymph nodes involved (1, 2, or 3), and type of systemic therapy delivered (chemotherapy and/or antiendocrine).
The primary endpoint was cumulative incidence of LRF, defined as a first recurrence in either the ipsilateral chest wall, axilla, supraclavicular fossa, and/or internal mammary nodes with or without simultaneous distant recurrence. Secondary endpoints included cumulative incidence of DM, DFS (any recurrence or death), and OS. We used the cumulative incidence method to estimate the LRF and DM rates. Gray’s p value was used to compare outcomes for these endpoints. DFS and OS analysis were performed by Kaplan–Meier method (log-rank test was used to compare survival between groups). Cox regression analysis was used to perform univariate and multivariate models of LRF and DM. Variables with p < 0.20 on univariate analysis were entered in the multivariate analysis to calculate adjusted hazard ratios. p value < 0.05 was considered statistically significant. Data were analyzed using SAS version 9.4.
Results
Patient Characteristics
We identified 468 eligible patients with baseline characteristics summarized in Table 1. Median age was 53 years, 61% had pathologic T2 disease, 48% had lymphovascular space invasion, and ~ 21% had extracapsular extension (ECE). The distribution of patients by approximate subtype was 71% HS, 11% triple negative (TN), and 17% HER2+. The median number of lymph nodes dissected was 18, and 10% had sentinel lymph node biopsy only. No patients had positive surgical margins. ST included chemotherapy (CT) in 78% of patients and endocrine therapy (ET) in 85% of HS patients, and 31 patients did not receive ST. Type of ST is detailed in Table S1.
There were 269 patients (58%) with 1+ node, 140 patients (30%) with 2+ nodes, and 59 patients (12%) with 3+ nodes. There were 134 patients (28.6%) with T1 tumors and 1+ node. There was high correlation between the number of involved nodes and ECE, with increasing number of involved nodes associated with higher rate of ECE: 10.8% (29/269) for 1+ node versus 28.6% (40/140) for 2+ nodes versus 49.2% (29/59) for 3+ nodes (p < 0.0001). Presence of ECE was associated with higher odds of a patient having 3+ nodes [odds ratio (OR) = 4.76, p < 0.0001].
Outcomes
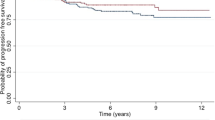
At median follow-up of 6.3 years, there were 26 LRF events, 52 DM events, 102 DFS events, and 73 deaths. Figure 1a demonstrates that the 6-year LRF rate was 4.1%. The 6-year LRF rate by number of involved nodes was 2.3% (1+ node), 5.2% (2+ nodes), and 8.9% (3+ nodes) (p = 0.13, Fig. 1b). The 6-year DM rate was 8.4% (Fig. 2a). Figure 2b demonstrates that patients with 1+ or 2+ nodes had similarly low rates of DM, with the rate significantly increasing for patients with 3+ nodes: 7.5% (1+ node) versus 7.2% (2+ nodes) versus 15.7% (3+ nodes) (p = 0.054). Grouped together, patients with 1+ or 2+ nodes had significantly lower 6-year DM rate: 7.4 versus 15.7% (p = 0.016).
Findings for all primary and secondary endpoints are summarized in Table 2. DFS and OS rates were numerically lower in patients with 3+ nodes, although this did not reach statistical significance (Table 2). Certain subgroups of patients had very low 6-year LRF and DM rates (Table 2). This included the 134 patients with T1 tumors and 1 + LN (6-year LRF = 0.8%, 6-year DM = 4.1%), 90 patients with N1mic disease only (6-year LRF = 0%, 6-year DM = 5.8%), and 199 patients with HS subtype and 1 + LN (6-year LRF = 2.7%, 6-year DM = 7.0%).
Outcomes by Receipt of Systemic Therapy
When examining the rates of LRF and DM by receipt of ST, no significant differences were seen. Twenty-three of the 26 LRF events and 47 of the 52 DM events occurred in the 437 patients who received ST. The 6-year LRF rate for the ST versus no ST groups was 3.5 versus 9.4% (p = 0.319), and the 6-year DM rates was 8.6 versus 7.0% (p = 0.310) (Fig. S1).
We performed a more detailed analysis of recurrence rates by receipt of ST in the HS subtype. The 6-year LRF rates were 2.2% (CT + ET), 6.0% (ET only), 10.6% (CT only), and 14.4% (no ST) (p = 0.124, Fig. S2). Similarly, the DM rates were 6.1% (CT + ET), 10.7% (ET only), 10.5% (CT only), and 5.1% (no ST) (p = 0.469, Fig. S2).
Univariate and Multivariate Analyses for LRF/DM
On univariate analysis, CT was significantly associated with a decreased hazards ratio for LRF [HR = 0.41, 95% confidence interval (CI) 0.18–0.93, p = 0.03] and ECE was significantly associated with increased risk of LRF, while patients with 3+ versus 1–2+ nodes (HR = 2.16, p = 0.08) and macrometastases (HR = 2.91, p = 0.15) had nonsignificantly increased hazards for LRF (Table S2). On multivariate analysis, ECE and CT remained independently prognostic for LRF (Table S3).
Tables S4 and S5 summarize the results for DM. On univariate analysis, T2 tumors, 3+ nodes, ECE, and grade 3 were associated with increased risk of DM. Given the strong association between the number of positive nodes and ECE, the multivariate analysis that includes both variables shows a strong trend towards increased risk of DM for each variable, although neither variable reached statistical significance (Table S5). When excluding ECE, patients with 3+ nodes (versus 1–2+ nodes) had significantly increased hazard for developing DM after controlling for other key variables. The multivariate results were similar for ECE when excluding 3+ nodes. Tables S6 and S7 demonstrate the regression analyses for LRF and DM in patients who received ST, with overall similar results.
Discussion
We found that, while the 6-year LRF and DM rates are modest (4.1 and 8.4%, respectively) in a modern cohort of patients with pT1-2pN1 disease treated with mastectomy ± adjuvant ST without PMRT, this ranged from 0 to 25% for LRF and 4 to 37.5% for DM, depending on the subset studied (Table 2). For example, we found that 6-year LRF and DM rates are numerically higher in patients with 3+ nodes (8.9 and 15.7%, respectively) as compared with 1–2+ nodes (2.3–5.2 and 7.2–7.5% respectively). This underlies the broad range of disease risk encompassed in the pT1-2pN1 group. In addition, various subgroups have extremely low rates of 6-year LRF and DM.
Table 3 summarizes modern series reporting clinical–pathologic characteristics and recurrence risks for pT2pN1 breast cancer treated with mastectomy without PMRT. Every report includes LRF rates. However, our study further reports DM rates, which may be the more compelling endpoint given that the EORTC 22922 and NCIC MA.20 trials demonstrated an approximate 20% relative reduction in DM with RNI/PMRT.
In terms of LRF, our results are consistent with those reported by McBride et al.2 from The University of Texas MD Anderson Cancer Center, Moo et al.4 from Memorial Sloan Kettering Cancer Center, and Miyashita et al.3 from Tohoku University. The 5-year (or 8-year reported by Miyashita et al.) LRF rates in pT1-2pN1 disease after mastectomy with or without PMRT are < 5% in all of these series. Since the women included in these analyses were treated from 1996 to 2013, it is likely that these lower LRF rates reflect treatment in a more modern era that includes screening detection, improved ST, and meticulous attention to surgical margins. Zeidan et al.9 reported results of a secondary analysis of the Breast International Group 02-98 trial that specifically looked at outcomes in the subset of women with pT1-2pN1 disease treated with and without PMRT. In the patients treated without PMRT, the 10-year LRF rate was 6.5%.
In contrast, other groups have found higher LRF rates. He et al.10 reported a 5-year LRF of 11.1% in 618 patients with pT1-2pN1 disease treated with surgery and adjuvant ST without PMRT from 1998 to 2007. Over two-thirds of the patients had pT2 disease, which could have influenced the LRF rate. In addition, Tendulkar et al.11 reported a 5-year LRF of 8.9% in 271 women with pT1-2pN1 disease treated with surgery and adjuvant ST without PMRT from 2000 to 2007.
Identifying individual patients with pT1-2pN1 disease who may benefit from PMRT remains challenging and is based mostly on clinical and pathologic features. In the current study, the HS subtype had low LRF and DM rates at 6 years. However, longer follow-up for HS patients is necessary. In a large metaanalysis of women who were disease free after 5 years of endocrine therapy, Pan et al.12 found that, after discontinuation of endocrine therapy, DM events continued to occur at a steady rate of ~ 2% in the subsequent 5–15 years in women with ER+ disease.
In women with HS breast cancer, evidence is growing that the 21-gene recurrence score (RS) assay can be used to identify subgroups of luminal A breast cancers with pN1 disease that do not derive a benefit from adjuvant systemic therapy.13,14,15,–16 In addition, the RS is proving to be prognostic for LRR as well. Woodward et al.17 analyzed 251 women with ER+, node-positive disease treated with mastectomy without radiation therapy on Southwest Oncology Group (SWOG) 8814. Patients with low RS had significantly lower 10-year LRR compared with patients with intermediate/high RS (7.8 vs 16.8%, p = 0.018). Those authors conclude that those results argue for a prospective trial to investigate omission of PMRT in women with pT1-2pN1 disease and low RS.17 Mamounas et al. examined the role of the RS assay in node-positive patients treated on the National Surgical Adjuvant Breast and Bowel Project (NSABP) B28 clinical trial.18 In that trial, women were randomized to receive four cycles of adriamycin and cyclophosphamide (AC) or four cycles of AC followed by four cycles of paclitaxel; women aged ≥ 50 years and women < 50 years with ER- or PR-positive tumors received 5 years of tamoxifen. The RS assay was performed on a subset of 1065 patients, and the 10-year cumulative incidence of locoregional recurrence was significantly associated with RS: 3.3% (low RS) versus 7.2% (intermediate RS) versus 12.3% (high RS). In the subgroup of 386 patients treated with mastectomy with 1–3+ nodes (PMRT was not allowed), the 10-year LRR rates by RS were 2.4% (low RS) versus 4.1% (intermediate RS) versus 6.0% (high RS).18 Therefore, the role of RS in identifying women treated with mastectomy with 1–3+ nodes who may not benefit from PMRT warrants further study. Prospective, randomized data are needed to further clarify the role of PMRT in women with pT1-2pN1 breast cancer. Incorporation of a biologic assay in women with HS breast cancer is well suited for a phase III trial of PMRT/RNI. The upcoming Tailor RT study (Canadian Cancer Trials Group MA39) will use the 21-gene RS to randomize women with RS < 18 and 1–3+ nodes after lumpectomy or mastectomy to RNI/PMRT versus no RNI/no PMRT.18,19
Our study has several limitations. First, this is a retrospective, single-institution analysis, and the results can be viewed as hypothesis-generating. Only a small proportion of patients had HER2+ or TN disease, particularly in the subgroup that did not receive PMRT. Therefore, the subgroup analysis of outcomes by subtype and number of involved nodes presented in Table 2 is most reliable for the HS patients. In addition, given that the vast majority of patients had HS disease, longer follow-up (≥ 10 years) is needed to ensure that the patterns seen in this study persist.
In summary, women with pT1-2pN1 breast cancer treated with initial mastectomy have historically been grouped together. However, our study emphasizes that pT1-2pN1 breast cancer is a heterogeneous cohort, implying that considering this entity as one group is not sufficient to tailor treatment recommendations. Instead, we found that this population has a wide range of LRF and DM. Women with 3+ nodes and TN subtype had excessively high rates of LRF and/or DM. However, the HS subtype predominated in this study, and this group had overall low rates of LRF/DM. In particular, patients with pT1 tumors and 1 + LN, HS subtype with 1 + LN, and patients with micrometastases only had extremely low event rates. These groups would derive small absolute reductions in LRF and DM with addition of PMRT, underscoring the importance of patient selection for PMRT in pT1-2pN1 breast cancer.
References
McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135.
McBride A, Allen P, Woodward W, et al. Locoregional recurrence risk for patients with T1,2 breast cancer with 1–3 positive lymph nodes treated with mastectomy and systemic treatment. Int J Radiat Oncol Biol Phys. 2014;89(2):392–398.
Miyashita M, Tada H, Suzuki A, et al. Minimal impact of postmastectomy radiation therapy on locoregional recurrence for breast cancer patients with 1 to 3 positive lymph nodes in the modern treatment era. Surg Oncol. 2017;26(2):163–170.
Moo TA, McMillan R, Lee M, et al. Selection criteria for postmastectomy radiotherapy in T1–T2 tumors with 1 to 3 positive lymph nodes. Ann Surg Oncol. 2013;20(10):3169–3174.
Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317–327.
Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307–316.
Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. J Clin Oncol. 2016;34(36):4431–4442.
Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106.
Zeidan YH, Habib JG, Ameye L, et al. Postmastectomy radiation therapy in women with T1–T2 tumors and 1 to 3 positive lymph nodes: analysis of the Breast International Group 02-98 Trial. Int J Radiat Oncol Biol Phys. 2018;101(2):316–324.
He ZY, Wu SG, Zhou J, et al. Postmastectomy radiotherapy improves disease-free survival of high risk of locoregional recurrence breast cancer patients with T1–2 and 1 to 3 positive nodes. PLoS ONE. 2015;10(3):e0119105.
Tendulkar RD, Rehman S, Shukla ME, et al. Impact of postmastectomy radiation on locoregional recurrence in breast cancer patients with 1–3 positive lymph nodes treated with modern systemic therapy. Int J Radiat Oncol Biol Phys. 2012;83(5):e577–e581.
Pan H, Gray R, Braybrooke J, et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846.
Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65.
Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829–1834.
Gluz O, Nitz UA, Christgen M, et al. West German Study Group Phase III PlanB Trial: first prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol. 2016;34(20):2341–2349.
Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26(25):4063–4071.
Woodward WA, Barlow WE, Jagsi R, et al. The 21-gene recurrence score and locoregional recurrence rates in patients with node-positive breast cancer treated on SWOG S8814. Int J Radiat Oncol Biol Phys. 2016;96(2):146.
Mamounas EP, Liu Q, Paik S, et al. 21-Gene recurrence score and locoregional recurrence in node-positive/ER-positive breast cancer treated with chemo-endocrine therapy. J Natl Cancer Inst. 2017. https://doi.org/10.1093/jnci/djw259.
Canadian Cancer Trials Group. 2018. https://www.ctg.queensu.ca/public/breast/breast-disease-site. Accessed 6 March 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bazan, J.G., Majithia, L., Quick, A.M. et al. Heterogeneity in Outcomes of Pathologic T1-2N1 Breast Cancer After Mastectomy: Looking Beyond Locoregional Failure Rates. Ann Surg Oncol 25, 2288–2295 (2018). https://doi.org/10.1245/s10434-018-6565-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6565-8