Abstract
Background
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy with a poor prognosis and few therapeutic options. Stathmin1 (STMN1) is a cytosolic protein involved in microtubule dynamics through inhibition of tubulin polymerization and promotion of microtubule depolymerization, which has been implicated in carcinogenesis and aggressive behavior in multiple epithelial malignancies. We aimed to evaluate expression of STMN1 in ACC and to elucidate how this may contribute to its malignant phenotype.
Methods
STMN1 was identified by RNA sequencing as a highly differentially expressed gene in human ACC samples compared with benign adrenal tumors. Expression was confirmed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR), Western blot, and immunohistochemical (IHC) staining of a tissue microarray (TMA) from two independent cohorts. The biologic relevance of STMN1 was investigated in NCI-H295R cells by lentivirus-mediated silencing.
Results
Differential gene expression demonstrated an eightfold increase in STMN1 messenger RNA (mRNA) in malignant compared with benign adrenal tissue. IHC showed significantly higher expression of STMN1 protein in ACC compared with normal and benign tissues. STMN1 knockdown in an ACC cell line resulted in decreased cell viability, cell-cycle arrest at G0/G1, and increased apoptosis in serum-starved conditions compared with scramble short hairpin RNA (shRNA) controls. STMN1 knockdown also decreased migration, invasion, and anchorage-independent growth compared with controls.
Conclusions
STMN1 is overexpressed in human ACC samples, and knockdown of this target in vitro resulted in a less aggressive phenotype of ACC, particularly under serum-starved conditions. Further study is needed to investigate the feasibility of interfering with STMN1 as a potential therapeutic target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adrenocortical carcinoma (ACC) is a rare but deadly malignancy accounting for 0.7–2.0 cases per million per years, with a 5-year overall survival of 37–47%.1,2,3 Although some patients present with symptoms from hormonal hypersecretion or mass effect, detection of ACC is often incidental and at an advanced stage. Surgical resection remains the only potential curative option for patients with ACC.4,5 For those unable to undergo resection, adrenolytic and conventional chemotherapeutic regimens yield modest results, further limited by toxicity.1,6,7,8 Despite significant efforts towards understanding ACC pathogenesis, novel agents have failed to improve clinical outcomes. Targeted therapies against vascular endothelial growth factor (VEGF) and insulin growth factor (IGF)-1 receptors have failed to demonstrate a survival benefit.9,10,11,12 Consequently, it is imperative to discover novel, effective therapeutic targets for ACC.
Given the recent unsuccessful clinical trials against transmembrane small-molecule inhibitors,9,10,11,12 we focused our attention on targetable cytosolic proteins that may be combined with conventional therapies for treatment of ACC patients. To explore the differential expression of these genes in malignant compared with benign adrenal cortical tissues, we performed RNA sequencing on 38 human adrenal samples comprised of normal adrenal cortex (NML), adrenal adenomas (AAs), and ACC.
Stathmin1 (STMN1, also known as p17, p18, p19, LAP18, metablastin, and oncoprotein18) is a 19kD cytosolic phosphoprotein involved in the regulation of microtubule dynamics by inhibition of tubulin polymerization and promotion of microtubule depolymerization.13 Its role in carcinogenesis has been investigated in a variety of tumor types and its expression has been associated with tumor progression and poor prognosis in breast, gastric, esophageal, endometrial, hepatocellular, oral squamous cell, and colorectal cancers.14,15,16,17,18,19,20 Furthermore, the role of STMN1 in oncogenesis is being explored, along with the therapeutic potential of suppressing STMN1. In colorectal cancer, silencing STMN1 inhibited colorectal cancer metastasis while enhancing response to 5-fluorouracil,21 and, in neuroblastoma, STMN1 suppression reduced lung metastases in vivo.22 Wang et al. showed that STMN1 knockdown improved sensitivity to the tubulin-targeting drugs paclitaxel and vinblastine in esophageal squamous cell carcinoma.23 Despite the observed role of STMN1 tumorigenesis across multiple tumor types, its role in ACC has not been elucidated.
Herein, we report on the gene and protein expression of STMN1 in ACC compared with benign adrenal specimens. We further explored the role of STMN1 in ACC tumor cell survival and migration in vitro. Our data suggest that targeting STMN1 may have therapeutic benefit in this aggressive malignancy.
Materials and Methods
Tissue Specimens
With approval from the Weill Cornell Medical College Institutional Review Board, frozen adrenalectomy specimens from consented patients undergoing surgery at Weill Cornell Medical College/New York Presbyterian Hospital from June 2000 to September 2011 were identified. In addition, five tumor samples were obtained from the Cooperative Human Tissue Network, funded by the National Cancer Institute (NCI). Normal specimens were taken by an experienced attending endocrine pathologist (TS) from cortical adrenal tissue adjacent to and separate from an adenoma or from normal adrenal glands that were taken out as part of radical nephrectomies for other reasons. In total, 13 ACCs (9 non-functional, 2 cortisol-secreting, 2 testosterone-secreting), 14 AAs (6 aldosterone-secreting, 3 cortisol-secreting, 5 non-functional), and 11 NML samples reviewed by TS were chosen for further analysis.
RNA Extraction and Next-Generation RNA Sequencing
RNA extracted from 38 frozen tissue samples was submitted for next-generation sequencing at our institution’s core facility. Differentially expressed genes were identified by LIMMA analysis comparing ACC (malignant) and NML + AAs (benign). Data were restricted to cytosolic proteins using the Gene Ontology GO:005829 annotation.
For additional information on sample preparation, quantitative reverse transcription-polymerase chain reaction (qRT-PCR), Western blot, and Oncomine data analysis, refer to the electronic supplementary Methods.
Immunohistochemistry
The Cornell tissue microarray (TMA) was constructed from formalin-fixed paraffin-embedded (FFPE) tissue blocks from the 38 patients submitted for sequencing, with an additional 14 ACC samples only available as FFPE blocks. For each case, three cores of 0.6 mm were taken from areas of high tumor cell density, as identified by TS on corresponding hematoxylin and eosin-stained slides.
Blinded grading of STMN1 staining (refer to the electronic supplementary Methods for detailed staining protocol) was performed by TS using a semi-quantitative scoring system. An STMN1 score of 0, 1+, 2+, or 3+ represented no staining, weak staining, moderate staining, or strong staining, respectively. Appropriate positive and negative (incubation with secondary antibody only) controls were stained in parallel.
A validation TMA from the Translational Genomics Research Institute (TGEN) was used for confirmation of protein expression findings, consisting of 39 ACCs, 19 AAs, and 19 NML samples. STMN1 staining was performed and analyzed independently by a pathologist (SG).
Cell Culture
The ACC cell line NCI-H295R (ATCC) is a steroid-producing line isolated from a 48-year-old female with ACC.24 Cells were grown and maintained in DMEM/F12 supplemented with 1% ITS+Premix (BD Biosciences), 2.5% Nu-Serum (BD Biosciences), and 1% penicillin/streptomycin/amphotericin (P/S/A), in a standard humidified incubator at 37 °C in 5% CO2 atmosphere. For particular experiments, serum-free media was utilized for specific durations consisting of DMEM/F12 supplemented with 1% P/S/A only.
Lentivirus-Mediated Short Hairpin RNA Knockdown
OP18 short hairpin RNA (shRNA) transduction-ready lentiviral particles containing three STMN1-specific constructs added in equal ratios, and control shRNA encoding a non-targeting scramble sequence, were purchased (SantaCruz Biotechnology, Dallas, TX, USA). Transduction was carried out according to the manufacturer’s instructions, using puromycin (SantaCruz Biotechnology) selection. Successful knockdown of STMN1 expression was assayed by qPCR and Western blot.
Soft Agar Anchorage-Independent Growth
Two-layered soft agar assays were performed in six-well plates. The bottom layer of agar (1 mL/well) contained 0.5% agar (Sigma-Aldrich, St Louis, MO, USA) in maintenance medium. 50,000 cells in maintenance medium were mixed with agarose for a 0.35% solution, and allowed to solidify atop the agar layer. This was overlaid with complete media and allowed to grow at 37 °C. After 3 weeks, cell colonies were stained with 0.005% crystal violet solution and examined by microscopy. Colonies were counted in four separate fields per well and summed for each of six replicates. The experiment was performed twice.
Cell Proliferation
The Vybrant MTT Cell-Proliferation Assay Kit (LifeTechnologies) was used to assess cell proliferation. 30,000 viable cells were seeded per well in 96-well plates in phenol-free complete media and phenol-free serum-free media. Every 24 h, media was replaced and a plate was subjected to the assay by adding 10 uL of 12 mM MTT stock solution. Absorbance was read using a microplate reader (iMARK, BioRad) at 490 nm. Experiments were duplicated.
Cell Cycle and Apoptosis
For cell-cycle analysis, 5 × 105 cells were seeded in six-well plates. Cells were harvested and fixed after 48 h, stained with propidium iodide, and analyzed by flow cytometry (Beckman-Coulter, Brea, CA, USA). Cell-cycle stages were determined using the Kaluza software (Beckman-Coulter). For apoptosis, a PE-Annexin V Apoptosis Detection Kit was used (BD Biosciences). Similarly, after 48 h, supernatant was harvested, cells stained with Annexin V and 7-aminoactinomycin D (7-AAD), and analyzed by flow cytometry as above. The experiments were duplicated.
Cell Migration
A scratch assay was used to determine cell migration. Cells seeded in six-well plates were grown to 80% confluence. A 200 µL pipette tip was used to create a horizontal scratch at three locations. Cells were photographed daily and percentage migration was measured in six fields using ImageJ software. The experiment was duplicated.
Cell Invasion
Using the Cytoselect 24-Well Cell Invasion Assay (Cell-Biolabs, Inc., San Diego, CA, USA), 2 × 105 cells in serum-free media were seeded onto precoated extracellular basement membrane inserts. Inserts were placed in a 24-well plate containing complete media. After 48 h of incubation, cells that invaded the matrix to the lower surface of the membrane were stained and counted under a light microscope. Four fields in four separate quadrants of each membrane were counted and averaged. The experiment was duplicated.
Paclitaxel Sensitivity
Forty-thousand cells were plated in triplicate in 96-well plates in regular growth media. After overnight incubation, cells were subjected to various concentrations of paclitaxel (0–2000 nM). After 72 h of drug treatment, cell proliferation was measured by MTT assay, as described previously. Experiments were duplicated.
Statistical Analysis
Calculations to determine significance in experiments were carried out using Student’s t test or one-way analysis of variance, as appropriate. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using STATA version 13.0 (StataCorp LLC, College Station, TX, USA).
Results
Stathmin1 (STMN1) Expression is Markedly Increased in Adrenocortical Carcinoma Compared with Adrenal Adenomas and Normal Adrenal Cortex
RNA-sequencing analyses identified more than 300 genes that met the inclusion criteria for differentially expressed transcripts of cytosolic proteins. Figure 1a represents the top 20 most differentially expressed genes between benign (AA+NML) and malignant samples (ACC). STMN1 was among the top differentially expressed genes in ACC, with a more than eightfold increase in STMN1 expression in malignant over benign adrenal tissue (p < 2.4E–07) (Fig. 1b). As represented in Fig. 1c, we found similar messenger RNA (mRNA) expression data in an independent, publically available dataset available at Oncomine.org (p = 7.5 × 10−9).
a Heatmap of unsupervised clustering results from RNA sequencing of 11 NMLs, 14 AAs, and 13 ACCs depicting the top 20 most differentially expressed genes. b Quantitative expression of STMN1 in ACC compared with AAs and NML. c Affymetrix microarray-based expression of STMN1 mRNA in 10 NML, 22 AA, and 33 ACC samples from the Giordano Adrenal 2 dataset (oncomine.org), where Student’s t-test was used for comparison between groups. NML normal adrenal, AA adrenal adenoma, ACC adrenocortical carcinoma, STMN1 Stathmin1, mRNA messenger RNA
To investigate the potential contribution of hormone secretion to STMN1 expression, we compared expression of STMN1 among non-functional adenomas compared with non-functional carcinomas. We found a greater than tenfold higher expression of STMN1 in non-functional ACCs compared with non-functional AAs. Expression of more ubiquitously expressed genes related to hormone production, such as 17-α-hydroxylase, showed equal RNA expression between the NML, AA and ACC groups.
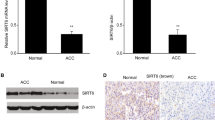
Differential expression of STMN1 between the malignant and benign samples was validated by qPCR and immunoblot (Fig. 2a, b), which demonstrated higher expression of STMN1 in primary ACC samples and NCI-H295R, compared with NML and AAs.
a Relative STMN1 expression in adrenocortical tissue and cell lines. mRNA expression of STMN1 was normalized to GAPDH using the (2−ΔΔCT) method. Values are relative to NML. b Representative expression of STMN1 protein by Western blot (n = 3). c–e Representative expression of the STMN1 protein by immunohistochemistry in NML c AA d and ACC e. f Immunohistochemical staining of Cornell TMA for STMN1 (NML: n = 10; AA: n = 14; ACC: n = 27). g Immunohistochemical staining of TGEN TMA for STMN1 (NML: n = 19; AA: n = 19, ACC: n = 39). The ANOVA statistical test was used for comparisons between groups. STMN1 Stathmin1, mRNA messenger RNA, NML normal adrenal, AA adrenal adenoma, ACC adrenocortical carcinoma, GAPDH glyceraldehyde-3-phosphate dehydrogenase, TMA tissue microarray, TGEN Translational Genomics Research Institute, ANOVA analysis of variance
To analyze STMN1 protein expression in a larger sample size, we further investigated protein expression using immunohistochemistry (IHC) on a TMA of 51 samples, and found higher STMN1 expression compared with NML and AAs (p < 0.01) (Fig. 2c–f). We confirmed these findings using an independent TMA (TGEN TMA), and found a stronger staining pattern overall and similarly high expression of STMN1 in ACC samples relative to both NML (p < 0.05) and AAs (p < 0.01) (Fig. 2g). Available clinical characteristics of patients represented in the two TMAs, including age, sex, biochemical profile, histopathologic features (e.g., Ki-67 index), and length of follow-up, are shown in electronic supplementary Table 1.
Effect of STMN1 Knockdown on Malignancy Potential in NCI-H295R
Stable knockdown of STMN1 (ΔSTMN1) was achieved via lentiviral shRNA infection using puromycin selection in the NCI-H295R cell line. We achieved 70% knockdown of SMTN1 expression at the mRNA level and 78% knockdown of protein expression compared with control cells transfected with a scrambled sequence (electronic supplementary Fig. 1).
To evaluate tumorigenicity, we performed a soft-agar, anchorage-independent growth assay. ΔSTMN1 cells formed significantly fewer colonies (637 colonies) in soft agar after 3 weeks of incubation compared with the control (837 colonies; p < 0.01) (Fig. 3a, b).
Lentiviral-assisted stable knockdown of STMN1 in NCI-H295R cells. a Expression of STMN1 mRNA without transfection (H295R) and after transfection with scramble shRNA (control) and STMN1 shRNA (ΔSTMN1) normalized to GAPDH using the (2−ΔΔCT) method. b Representative Western blot demonstrating decreased protein expression of knockdown cells (ΔSTMN1) compared with untreated cells (H295R) and cells treated with scramble shRNA (control). STMN1 Stathmin1, mRNA messenger RNA, shRNA short hairpin RNA, GAPDH glyceraldehyde-3-phosphate dehydrogenase
STMN1 Knockdown on Cellular Proliferation, Cell Cycle and Apoptosis In Vitro
Using the MTT assay to assess cell viability, we found no significant difference between the control and STMN1 knockdown NCI-H295R cells (ΔSTMN1) (Fig. 4a). Next, we cultured cells in serum-free conditions. Cell viability was modestly reduced in ΔSTMN1 compared with the control when serum was removed (p < 0.01) (Fig. 4b). Cell-cycle analysis (Fig. 4c) demonstrated a slightly greater proportion of cells remaining in G0/G1 under serum-free media (76.9% vs. 73.5%; p < 0.01 in ΔSTMN1 vs. the control, respectively) compared with regular growth media (68.7% vs. 66.2%; p = 0.09 in ΔSTMN1 vs. the control, respectively). However, in assessing apoptosis, we found a significantly greater proportion of viable cells (41.5% vs. 13.4%; p < 0.01) and fewer apoptotic cells (39.6% vs. 73.3%; p < 0.01) in the control group under serum-free conditions (Fig. 4d). No significant difference was seen in cell cycle or apoptosis between the ΔSTMN1-infected cells and control cells in regular serum-containing media.
a, b Effect of STMN1 knockdown on cellular proliferation, representing mean data from two independent experiments performed in quintuplet. STMN1 knockdown and control NIH-H295R cells were grown in a normal media and b SF media. c Effect of STMN1 knockdown on cell cycle. Cells were grown in regular media and SF media for 48 h prior to analysis. Data represent two independent experiments. d Effect of STMN1 knockdown on apoptosis. Cells were grown in regular media and SF media for 48 h prior to analysis. Experiments were performed in duplicate. Student’s t-test was used for pairwise statistical comparisons. STMN1 Stathmin1, SF serum-free
Effects of STMN1 Knockdown on Cell Migration and Invasion
To investigate the role of STMN1 in cellular motility and invasive potential, we subjected infected cells to a scratch assay for 5 days. ΔSTMN1 cells were found to migrate 11% less than control cells transfected with scrambled shRNA (p < 0.05) (Fig. 5a, b). Using a Boyden Chamber assay after 48 h of incubation, cells transfected with STMN1 shRNA demonstrated less invasive ability than control cells (p < 0.05) (Fig. 5c).
a Representative images of cell migration at T = 0 and T = 5 days in the control and STMN1 knockdown cells. b Quantitative assessment of cell migration from two independent experiments. c Effect of STMN1 knockdown on cell invasion showing significantly decreased invasion ability of STMN1 knockdown cells. Data represent two independent experiments. Student’s t-test was used for pairwise statistical comparisons in b and c. T = time, STMN1 Stathmin1
Effect of STMN1 Knockdown on Sensitivity to Paclitaxel
Paclitaxel was tested at various concentrations and its effect on cellular proliferation was compared between ΔSTMN1 and control H295R cells (electronic supplementary Fig. 2). We found the half maximal inhibitory concentration (IC50) to be non-statistically different (IC50 of ΔSTMN1: 337.3 nM; IC50 of control cells: 374.1 nM), suggesting that knockdown of STMN1 does not change the sensitivity of paclitaxel on H295R cells.
Discussion
STMN1 is a cytosolic protein that plays an important regulatory role in microtubule dynamics. It was originally described as being overexpressed in leukemia and lymphoma,25 and STMN1 overexpression has since been documented in multiple solid organ human malignancies. STMN1 overexpression has been correlated with aggressive cellular behavior and poor clinicopathologic and prognostic features in other cancers.14,16,17,19,26 Furthermore, studies of STMN1 inhibition have suggested that STMN1 may hold promise as a potential therapeutic target.21,22,23 We sought to understand whether STMN1 contributes to the pathogenesis of ACC as a gateway for further investigation into its therapeutic potential.
In this study, we have shown that STMN1 expression was eightfold higher in ACCs by RNA sequencing and IHC compared with benign adrenal samples. Additionally, lentiviral-mediated knockdown of STMN1 expression resulted in significant effects on cellular viability, cell-cycle progression, apoptosis, and invasion. Our data suggest that STMN1 overexpression contributes to the invasive nature in ACC cells and that silencing its expression or inhibiting its function may be an approach to mitigate the contribution of STMN1 to malignant potential.
Several groups have proposed that as a microtubule depolymerizing protein, STMN1 overexpression allows cells to transit rapidly through mitosis and thereby result in unrestricted proliferation. This behavior results in an aggressive clinical phenotype associated with poorer prognostic features, including progression-free survival and overall survival.27,28,29,30
Others have sought to understand how STMN1 overexpression may contribute to treatment response to microtubule-altering chemotherapeutics such as taxanes, which stabilize microtubules and prevent microtubule depolymerization, and vinca alkaloids, which destabilize microtubules by binding tubulin and detering microtubule assembly.31 Specifically, in breast cancer, Alli et al. showed that overexpression of STMN1 decreased sensitivity to paclitaxel and vinblastine, and suggested it did so by altering drug binding to microtubules. This was associated with growth arrest at the G2/M boundary where these drugs are most effective in altering microtubule dynamics.32 In a follow-up study, using RNA interference, Alli et al. demonstrated improved sensitivity to these drugs by targeting STMN1 expression, inducing microtubule polymerization, and promoting G2/M progression.33 In esophageal cancer, Wang et al. showed that silencing STMN1 similarly increased sensitivity to antimicrotubule drugs.23
While the exact mechanism behind the effect of STMN1 on cellular processes and microtubule-altering drugs is unclear, Wang et al. recently showed, in osteosarcoma cell lines, that knockdown of STMN1 enhanced chemosensitivity to paclitaxel through inhibition of autophagy,34 while in non-small cell lung cancer, downregulation of STMN1 resulted in a decrease of autophagy and sensitized cells to radiation.35 Although a small molecule inhibitor to better investigate the synergistic effect of STMN1 inhibition with taxane therapy is not yet commercially available, Wang et al. are investigating a liposome-based carrier to deliver an anti-STMN1 RNA-interfering plasmid in a phase I trial.36
Our results failed to show a synergistic effect between STMN1 knockdown and paclitaxel. We also did not see a significant difference in the cellular proliferation between control and knockdown cells in regular, serum-containing media. This may be because STMN1 in ACC has more apparent effects on migration and invasion than proliferation. Another potential explanation is incomplete STMN1 knockdown. In regard to paclitaxel, there may be an entirely different underlying mechanism of STMN1 interaction with microtubule-altering drugs in ACC compared with other cell types. Further study is necessary to determine the applicability of taxanes in ACC, as well as investigate other drugs that may act synergistically with STMN1 knockdown.
Conclusion
STMN1 is highly overexpressed in ACC. STMN1 knockdown alters ACC phenotype in vitro by making cells less aggressive and less tumorigenenic. A mechanism to selectively target STMN1 in cancer cells remains to be discovered, but work in other cancer types has shown promise in independent and combination therapy with conventional therapeutics. Our study offers a novel cytosolic target for investigation in the treatment of ACC, and future studies will focus on testing directed STMN1 inhibition in vitro and in vivo to determine its applicability in human ACC.
References
Fassnacht M, Libe R, Kroiss M, Allolio B. Adrenocortical Carcinoma: A Clinician’s Update. Nat Rev Endocrinol. 2011;7:323–35.
Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(12):4551–64.
Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, et al. Adrenocortical carcinomas: Surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons Study Group. World J Surg. 2001;25(7):891–7.
Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113(11):3130–6.
Datta J, Roses RE. Surgical Management of Adrenocortical Carcinoma: An Evidence-Based Approach. Surg Oncol Clin N Am. 2016;25(1):153–70.
Libé R. Adrenocortical carcinoma (ACC): diagnosis, prognosis, and treatment. Front Cell Dev Biol. 2015;3:45.
Creemers SG, Hofland L, Korpershoek E, Franssen GJ., van Kemenade FJ, de Herder WW, et al. Future directions in the diagnosis and medical treatment of adrenocortical carcinoma. Endocr Relat Cancer. 2016;23(1):R43-69.
Wortmann S, Quinkler M, Ritter C, Kroiss M, Johanssen S, Hahner S, et al. Bevacizumab plus capecitabine as a salvage therapy in advanced adrenocortical carcinoma. Eur J Endocrinol. 2010;162:349–56.
O’Sullivan C, Edgerly M, Velarde M, Wilkerson J, Venkatesan AM, Pittaluga S, et al. The VEGF inhibitor axitinib has limited effectiveness as a therapy for adrenocortical cancer. J Clin Endocrinol Metab. 2014;99(4):1291–7.
Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–35.
Costa R, Carneiro BA, Tavora F, Pai SG, Kaplan B, Chae YK, et al. The challenge of developmental therapeutics for adrenocortical carcinoma. Oncotarget. 2016;7(29):46734–49.
Berruti A, Sperone P, Ferrero A, Germano A, Ardito A, Priola M, et al. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur J Endocrinol. 2012;166:451–8.
Rubin CI, Atweh GF. The role of stathmin in the regulation of the cell cycle. J Cell Biochem. 2004;93(2):242–50.
Akhtar J, Wang Z, Yu C, Zhang ZP, Bi MM. STMN-1 Gene: A Predictor of Survival in Stage IIA Esophageal Squamous Cell Carcinoma After Ivor-Lewis Esophagectomy. Ann Surg Oncol. 2014;21(1):315–21.
He X, Liao Y, Lu W, Xu G, Tong H, Ke J, et al. Elevated STMN1 promotes tumor growth and invasion in endometrial carcinoma. Tumour Biol. 2016;37(7):9951–8.
Hsieh S, Huang S, Yu M, Yeh T, Chen T, Lin Y, et al. Stathmin1 overexpression associated with polyploidy, tumor-cell invasion, early recurrence, and poor prognosis in human hepatoma. Mol Carcinog. 2010;49(5):476–87.
Kang W, Tong JHM, Chan AWH, Lung RWM, Chau SL, Wong QWL, et al. Stathmin1 Plays Oncogenic Role and Is a Target of MicroRNA-223 in Gastric Cancer. PLOS One. 2012;7(3):33919.
Kouzu Y, Uzawa K, Koike H, Saito K, Nakashima D, Higo M, et al. Overexpression of stathmin in oral squamous-cell carcinoma: correlation with tumour progression and poor prognosis. Br J Cancer. 2006;94:717–23.
Kuang X, Chen L, Zhang Z, Liu Y, Zheng Y. Stathmin and phospho-stathmin protein signature is associated with survival outcomes of breast cancer patients. Oncotarget. 2015;6(26):22227–38.
Zhang H, Guo X, Guo S, Wang Q, Chen X. STMN1 in colon cancer: expression and prognosis in Chinese patients. Eur Rev Med Pharmacol Sci. 2016;20:2038–44.
Yu W, Tan XF, Tan HT, Lim TK, Chung MCM. Unbiased Proteomic and Transcript Analyses Reveal that Stathmin-1 Silencing Inhibits Colorectal Cancer Metastasis and Sensitizes to 5-Fluorouracil Treatment. Mol Cancer Res. 2014;12(12):1717–28.
Byme F, Yang L, Philliips P, Hansford L, Fletcher J, Ormandy C, et al. RNAi-mediated stathmin suppression reduces lung metastasis in an orthotopic neuroblastoma mouse model. Oncogene. 2014;33(7):882–90.
Wang S, Akhtar J, Wang Z. Anti-STMN1 therapy improves sensitivity to antimicrotubule drugs in esophageal squamous cell carcinoma. Tumour Biol. 2015;36(10):7797–806.
Gadzar A, Oie H, Shackleton C, Chen T, Triche T, Myers C, et al. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. 1990;50(17):5488–96.
Roos G, Brattsand G, Landberg G, Marklund U, Gullberg M. Expression of oncoprotein 18 in human leukemias and lymphomas. Leukemia. 1993;7(10):1538–46.
Rana S, Maples P, Senzer N, Nemunaitis J. Stathmin1: A novel therapeutic target for anticancer activity. Expert Rev Anticancer Ther. 2008;8(9):1461–70.
Akhtar J, Wang Z, Yu C, Li C-S, Shi Y-L, Liu H-J. STMN-1 is a potential marker of lymph node metastasis in distal esophageal adenocarcinomas and silencing its expression can reverse malignant phenotype of tumor cells. BMC Cancer. 2014;14:28.
Watanabe A, Suzuki H, Yokobori T, Tsukagoshi M, Altan B, Kubo N, et al. Stathmin1 regulates p27 expression, proliferation and drug resistance, resulting in poor clinical prognosis in cholangiocarcinoma. Cancer Sci. 2014;105(6):690–6.
Yuan R, Jeng Y, Chen H, Lai P, Pan H, Hsieh F, et al. Stathmin overexpression cooperates with p53 mutation and osteopontin overexpression, and is associated with tumour progression, early recurrence, and poor prognosis in hepatocellular carcinoma. J Pathol. 2006;309:549–58.
Reyes HD, Miecznikowski J, Gonzalez-Bosquet J, Devor EJ, Zhang Y, Thiel KW, et al. High stathmin expression is a marker for poor clinical outcome in endometrial cancer: an NRG oncology group/gynecologic oncology group study. Gynecol Oncol. 2017;146(2):247-253.
Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–65.
Alli E, Bash-Babula J, Yang J-M, Hait WN. Effect of Stathmin on the Sensitivity to Antimicrotubule Drugs in Human Breast Cancer. Cancer Res. 2002;62(23):6864–9.
Alli E, Yang J, Ford J, Hait W. Reversal of stathmin-mediated resistance to paclitaxel and vinblastine in human breastcarcinoma cells. Mol Pharmacol. 2007;71(5):1233–40.
Wang Z, He R, Xia H, Wei Y, Wu S. Knockdown of STMN1 enhances osteosarcoma cell chemosensitivity through inhibition of autophagy. Oncol Lett. 2017; 13(5) 3465–70.
Zhang X, Ji JF, Yang Y, Zhang J, Shen LF. Stathmin1 increases radioresistance by enhancing autophagy in non-small-cell lung cancer cells. Onco Targets Ther. 2016;9:2565–74.
Wang Z, Jay CM, Evans, C, Kumar P, Phalon C, Rao DD, et al. Preclinical Biodistribution and Safety Evaluation of a pbi-shRNA STMN1 Lipoplex after Subcutaneous Delivery. Toxicol Sci. 2017;155(2):400–8.
Funding
This study was supported by the Weill Cornell Clinical and Translational Science Center NIH/NCATS Grant TL1TR000459.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aronova, A., Min, I.M., Crowley, M.J.P. et al. STMN1 is Overexpressed in Adrenocortical Carcinoma and Promotes a More Aggressive Phenotype In Vitro. Ann Surg Oncol 25, 792–800 (2018). https://doi.org/10.1245/s10434-017-6296-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6296-2