Abstract
Background
In the era of effective modern systemic chemotherapy (CT), the role of hepatic arterial infusion of fluoxuridine (HAI-FUDR) in the treatment of isolated unresectable colorectal liver metastasis (IU-CRCLM) remains controversial. This study aimed to compare the overall survival (OS) of HAI-FUDR in combination with modern systemic CT versus modern systemic CT alone in patients with IU-CRCLM.
Methods
This was a case–control study of IU-CRCLM patients who underwent HAI + modern systemic CT or modern systemic CT alone. Modern systemic CT was defined as the use of multidrug regimens containing oxaliplatin and/or irinotecan ± biologics.
Results
Overall, 86 patients met the inclusion criteria (n = 40 for the HAI + CT group, and n = 46 for the CT-alone group). Both groups were similar in demographics, primary and stage IV tumor characteristics, and treatment-related variables (carcinoembryonic antigen, use of biologic agents, total number of lines of systemic CT administered) (all p > 0.05). Additionally, both groups were comparable with respect to liver tumor burden [median number of lesions (13.5 vs. 15), percentage of liver tumor replacement (37.5 vs. 40 %), and size of largest lesion] (all p > 0.05). Median OS in the HAI + CT group was 32.8 months compared with 15.3 months in the CT-alone group (p < 0.0001). Multivariate analysis revealed HAI + CT (hazard ratio 0.4, 95 % confidence interval 0.21–0.72; p = 0.003), Eastern Cooperative Oncology Group status, and receipt of increasing number of lines of systemic CT to be independent predictors of survival.
Conclusions
In this case–control study of patients with IU-CRCLM, HAI in combination with CT was associated with improved OS when compared with modern systemic CT alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer (CRC) is the third most common cancer worldwide and the third leading cause of mortality among men and women. In 2015, an estimated 132,700 new cases led to nearly 49,700 deaths.1 Approximately 60 % of patients with CRC will develop liver metastases (LM), of which 10–25 % will have liver-only disease.2,3 In the era of effective modern-day multidrug chemotherapy (CT), patients with isolated unresectable CRC LM (IU-CRCLM) can have response rates of up to 60 % and a median overall survival (OS) of 2 years. Additionally, a subset of these patients (approximately 15–40 %) may convert to resectable, achieving 5-year survival rates of 30–40 % after resection.4–10 However, a significant number of IU-CRCLM fail to become resectable and are relegated to second-line CT, the response of which is generally poor (20–30 %), with a median survival of approximately 1 year.11–15
Regional hepatic therapy may have a role in patients with IU-CRCLM. Hepatic arterial infusion (HAI) therapy exploits the principle that CRCLMs derive their blood supply predominately from the hepatic artery.16 This unique characteristic, when coupled with the high first-pass extraction and short half-life of certain chemotherapeutics such as floxuridine (FUDR), allows for greater tumor–drug exposure with minimal systemic toxicity. Recent studies have reported impressive response rates for HAI-FUDR as first- and second-line therapy in IU-CRCLM,17,18 including a recent phase II study from Memorial Sloan Kettering Cancer Center (MSKCC) that combined modern systemic CT with HAI-FUDR and reported response rates of up to 80 % and a conversion rate of 47 %.19 Despite these encouraging data, the role of HAI therapy in the treatment of IU-CRCLM remains controversial, particularly in the era of effective contemporary CT.
Ideally, a randomized controlled trial of HAI + modern CT versus modern CT alone is needed to delineate the impact of HAI therapy on the survival of this patient subset. Since HAI therapy remains a modality employed only at specialized centers, and given the preference of the medical oncology community to exhaust effective systemic therapeutic options, accrual to such trials may prove to be difficult. Short of such level I evidence, a case–control study may be a practical alternative. Therefore, the aim of this study was to compare the OS of HAI + modern systemic CT versus modern systemic CT alone in patients with IU-CRLM.
Patients and Methods
Study Design, Definitions, and Patient Selection
This was a case–control study of IU-CRLM patients who underwent either HAI-FUDR + modern systemic CT (HAI + CT group) or modern systemic CT alone (CT group) at the University of Pittsburgh Medical Center (UPMC) from 2004 to 2014. This tertiary care center consists of a large network of hospitals, only one of which (UPMC’s flagship oncologic facility: UPMC Shadyside) offers HAI therapy. Thus, the decision to pursue HAI + CT (vs. CT alone) was dependent on whether the patient was referred to this core oncologic facility.
This Institutional Review Board (IRB)-approved study was restricted to CRC patients with radiologically verifiable IU-CRCLM. All cross-sectional imaging (CT ± MRI ± PET of the chest, abdomen, and pelvis) was reviewed to document the absence of extrahepatic disease (EHD). Importantly, patients with equivocal imaging findings such as ‘indeterminate’, ‘small’, or ‘suspicious’ EHD were excluded. Similarly, ‘unresectability’ was confirmed by review of cross-sectional imaging and verification that the remnant liver volume was too small in relation to the extent of a resection ± ablation needed to extirpate all metastasis. Modern CT was defined as the use of multidrug regimens containing oxaliplatin and/or irinotecan ± biologics. Patients who underwent Y90 radioembolization at any point after the diagnosis of IU-CRCLM were not excluded.
Hepatic Arterial Infusion (HAI) + Chemotherapy (CT) Group
HAI therapy at our institution is usually undertaken in a pretreated cohort, since, based on inherent referral patterns, most CRCLM patients are typically first evaluated by medical oncology and systemic CT is administered as first-line therapy. Some of these patients are then referred for regional therapy to our division of surgical oncology, were all potential candidates for regional HAI-FUDR therapy undergo multidisciplinary assessment. Following HAI placement, these patients then undergo further systemic CT concurrently with HAI-FUDR.
The technique for HAI pump placement has previously been outlined.16 Our approach is to remove the primary tumor, if present, at the time of HAI pump insertion. HAI-FUDR is administered at 0.12 mg/(kg/day) × kg × pump volume/flow rate in addition to 30 mg of dexamethasone, 30,000 U heparin, and saline (all mixed to a total volume of 30 ml). Therapy is administered in a 4-week cycle; HAI therapy is started on day 1 of each cycle, and the pump is emptied and filled with heparin (30,000 units) and normal saline on day 15. Systemic CT treatment is started at least 2 weeks after HAI pump placement.
In order to fully evaluate the impact of HAI therapy in this patient subset, HAI patients were excluded if they had (i) concurrent isolated liver perfusion; (ii) resection of LM ± ablation at the time of HAI pump placement; (iii) resection of limited abdominal EHD; or (iv) presence of definite, suspected, or indeterminate EHD. Figure 1a details the search strategy for the HAI + CT group.
CT-Alone Group
The unique setup of the University of Pittsburgh Cancer Centers Network connects the main institution with >21 network community sites. The Medical Archival Software (MARS) system provides a centralized means of unifying the majority of electronic data in UPMC’s Hospital Information Systems, and allows analysis of all patients treated within this large network. Using an initial search for ‘CRCLM’, patients with unresectable CRCLM were identified between 2004 and 2014. Through a manual search of each patient’s EMR and cross-sectional imaging, the search was further refined to identify patients with IU-CRLM treated with modern systemic CT (Fig. 1b).
Statistical Analyses
Continuous data were summarized as medians and interquartile ranges, and categorical data were summarized as frequencies and percentages. To examine the baseline differences between the two groups, Fisher’s exact test (categorical variables) and Wilcoxon rank sum tests (continuous variables) were used. To avoid lead-in bias, OS (the primary endpoint of this study) was measured as the time from the date of diagnosis of IU-CRCLM to the date of death or last censored follow-up. Follow-up was complete for all patients. The Kaplan–Meier method was used to estimate the probability of OS and the log-rank test was used to compare survival functions. Univariate and multivariate analysis was based on Cox proportional hazards regression modeling. All analyses were performed using STATA 14.1 (StataCorp LP, College Station, TX, USA).
RESULTS
Overall Cohort Characteristics
Between 2004 and 2014, a total of 163 HAI and 369 CT-alone patients with metastatic unresectable CRC were identified using our initial search strategy. Based on the exclusion criteria described above (Figs. 1a, b), 40 HAI + CT and 46 CT-alone patients met the inclusion criteria of the study and hence formed the two patient cohorts in this case–control study. The median age for the entire group (N = 86) was 59 years (63 % males), and 90 % of patients had synchronous LM at presentation (Table 1). Liver tumor burden was extensive, with a median of 15 lesions and 40 % replacement of liver parenchyma. Primary tumors were removed in 76 % of patients, and over two-thirds of these had node-positive disease. Expectantly, two-thirds of the tumors were moderately differentiated and 40 % had KRAS mutations. Median duration of first-line systemic CT was 6 months.
HAI-Related Treatment Characteristics
The median duration between the diagnosis of IU-CRCLM and HAI pump placement was 8.5 months. All HAI pumps were placed after first-line CT had previously been administered for IU-CRCLM, with a median duration of pre-pump CT of 6 months. The median number of HAI-FUDR cycles administered was 4 (interquartile range 2–7).
Comparison of HAI + CT Versus CT
Table 2 compares the demographic and treatment characteristics of both groups. Median time from the first diagnosis of CRC to the diagnosis of unresectable disease was less than 1 month in both groups, consistent with the fact that most patients had synchronous disease at the time of presentation. Demographics, including age, sex, body mass index (BMI), race, and Eastern Cooperative Oncology Group (ECOG) status, were similar between both groups. Primary CRC tumor characteristics, including location, lymph node status, tumor grade, lymphovascular invasion (LVI), perineural invasion, Kras, and microsatellite instability, were also similar (all p > 0.05). As expected, the primary tumor was removed in all HAI + CT patients, but only in 54 % of the CT-alone group (p < 0.001). Metastatic liver tumor burden was comparable between both groups, with a similar median number of liver lesions (13.5 vs. 15), percentage of liver tumor replacement (37.5 vs. 40 %), size of largest liver lesion (5.6 vs. 6 cm), and carcinoembryonic antigen (CEA) at diagnosis of IU-CRCLM (210 vs. 167) [all p > 0.05]. Additionally, both groups were comparable with regard to the total number of lines of CT, duration of first- and second-line CT, and the use of biologic agents.
Survival Analysis
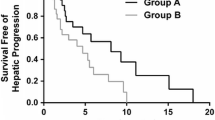
Median survival for the HAI + CT group was 32.8 months versus 15.3 months for the CT-only group (p < 0.0001) (Fig. 2). On univariate analysis, age, ECOG status, resection status of the primary, lymph node status, grade, LVI, duration of second-line CT, use of biologic agent, number of lines of systemic CT, and the use of HAI pump therapy displayed a significant association with OS (Table 3). On multivariate analysis, HAI + CT treatment (hazard ratio 0.39, 95 % confidence interval 0.21–0.72; p = 0.003), ECOG status, and number of lines of systemic CT remained as independent predictors of OS.
Discussion
This case–control study demonstrates that HAI-FUDR in combination with modern systemic CT is associated with improved OS when compared with modern systemic CT alone for patients with IU-CRCLM.
CT remains the mainstay of treatment for patients with unresectable CRCLM. Recent trials of modern CT report response rates of up to 60 % and a median survival of up to 24 months in the first-line setting using contemporary regimens, including FOLFOX/FOLFIRI in combination with anti-vascular endothelial growth factor inhibitors or anti-epidermal growth factor receptor inhibitors.20–23 Response and survival in the second-line setting is significantly worse. Since HAI therapy in this study can be considered to have been administered in the second-line setting (a median of 6 months pretreatment with systemic CT prior to HAI insertion), the median OS of 33 months is encouraging, particularly in view of the heavy liver tumor burden. In addition to HAI, low ECOG status and receipt of multiple lines of systemic therapy were also associated with improved survival. Not surprisingly, patients with good performance status can expect better longevity by virtue of tolerating successive lines of CT. Interestingly, resection of the primary tumor exhibited a strong association with survival on UVA, narrowly failing to reach significance in the MV model (p = 0.065). Two recent studies have questioned the contemporary assumption that resection of the asymptomatic primary tumor harbors no survival benefit,24,25 and this issue is now being investigated in two phase III trials.26–28 Although both groups were similar with respect to all other treatment characteristics, more patients underwent radioembolization (Y90) in the CT arm, however this was not an independent predictor of survival.
Several clinical trials have evaluated HAI therapy in the setting of unresectable CRCLM;22–26 however, most of these have compared HAI therapy alone with CT or best supportive care. A meta-analysis of those trials reported no survival advantage for HAI,27 the reasons for which are multifactorial but include the fact that some of these trials used 5-fluorouracil rather than FUDR as the chemoperfusate; the former agent does not possess the high hepatic tumor uptake seen with FUDR. Additionally, although HAI therapy may reduce the risk of disease progression in the liver, a significant proportion of patients with liver-only disease will progress in extrahepatic sites due to occult EHD; such patients will benefit from the addition of systemic CT.28,29 Importantly, HAI pump placement and maintenance requires technical expertise that may not be readily available outside a few specialized high-volume centers, invariably leading to higher dropout rates in some of the HAI arms of those trials. A recent phase II clinical trial by D’Angelica et al. more accurately depicts the outcomes of unresectable CRCLM when HAI is combined with contemporary CT at a high-volume specialized center, with an overall RR of 76 % (72 % in previously treated patients) and a median OS of 38 months.19
The ability of modern CT to convert unresectable disease to resectable status is an important endpoint. Adam et al. previously showed that 12.5 % of initially unresectable patients can undergo surgery after CT and achieve survival similar to resectable patients.5,30 In the recent PEAK trial, 15 % of patients converted to resection.21 Others have shown conversion rates as high as 24–46 % but many of these reports are limited by less stringent definitions of unresectability, and the exclusion of pretreated patients.6,31–33 HAI therapy in combination with modern-day CT seems to be associated with favorable conversion rates, as shown by three trials from the MSKCC.17–19 In the recent trial by D’Angelica et al. mentioned above, the conversion rate was 47 %, which is significantly higher than the conversion rate in this series.19 Although cross comparison between both reports is not feasible, it is noteworthy that 35 % of the MSKCC cohort were chemo-naive, and that the ‘converted’ group had a median number of nine liver tumors. In contrast, all the HAI patients in this series were heavily pretreated (median time on CT prior to HAI pump placement = 6 months), and had a relatively larger hepatic tumor burden (median number of tumors = 15; hepatic replacement by tumor = 40 %). Interestingly, 73 % of the ‘non-converted’ cohort in the MSKCC trial were treated with HAI in the second-line setting, and those patients had a median of 17 tumors—characteristics more in keeping with our current report. Taken together, these data suggest that HAI in combination with contemporary CT is associated with prolonged survival in the salvage setting, even when conversion is not achieved (33 months in our report and 32 months in the MSKCC trial). Even more provocative, based on the D’Angelica report, is that HAI + CT may need to be considered in the first-line setting since this strategy is associated with impressively high conversion rates and even longer survival (3-year survival of 91 %; median OS not reached.19)
This case–control study has several limitations. First, despite the well-balanced groups, this retrospective analysis suffers from an inherent selection and referral bias in the HAI group. The decision to administer HAI + CT therapy was performed in multidisciplinary fashion at UPMC’s flagship oncologic facility, whereas the CT-alone group originated from any of the UPMC’s 21 satellite community centers. Such differences in expertise and lack of valuable multidisciplinary assessment could have negatively influenced patient survival in the CT-alone group. Second, and due to the strict inclusion criteria, the sample size in both groups was small. This was necessary however to minimize the potential for including confounding patients with EHD. Third, although all patients received contemporary multidrug regimens, there was inherent heterogeneity in the type of regimen used. Finally, the median survival of patients treated with systemic CT alone (approximately 15 months) is inferior to recently reported historic controls (up to 2 years). This can be explained by the large tumor burden in our cohort but may also be a consequence of less stringent and objective definitions of ‘unresectability’ in other trials.
Conclusions
This case–control study of patients with IU-CRCLM suggests that the addition of HAI-FUDR therapy to modern systemic CT is associated with improved OS when compared with modern systemic CT alone.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–9.
Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–17.
Masi G, Loupakis F, Pollina L, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009;249(3):420–5.
Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27(11):1829–35.
Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11(1):38–47.
Wong R, Cunningham D, Barbachano Y, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22(9):2042–8.
Uetake H, Yasuno M, Ishiguro M, et al. A multicenter phase II trial of mFOLFOX6 plus bevacizumab to treat liver-only metastases of colorectal cancer that are unsuitable for upfront resection (TRICC0808). Ann Surg Oncol. 2015;22(3):908–15.
Folprecht G, Gruenberger T, Bechstein W, et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann Oncol. 2014;25(5):1018–25.
Alberts SR, Horvath WL, Sternfeld WC, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23(36):9243–9.
Van Cutsem E, Bajetta E, Valle J, et al. Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29(15):2004–2010.
Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–506.
Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12.
Bendell JC, Nemunaitis J, Vukelja SJ, et al. Randomized placebo-controlled phase II trial of perifosine plus capecitabine as second- or third-line therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2011;29(33):4394–400.
Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28(31):4706–13.
Leal JN, Kingham TP. Hepatic artery infusion chemotherapy for liver malignancy. Surg Oncol Clin N Am. 2015;24(1):121–48.
Kemeny N, Jarnagin W, Paty P, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol. 2005;23(22):4888–96.
Kemeny NE, Melendez FDH, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27(21):3465–71.
D’Angelica MI, Correa-Gallego C, Paty PB, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015;261(2):353–60.
Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75.
Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32(21):2240–7.
Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–9.
Venook AP, Niedzwiecki D, Lenz H-J, et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). ASCO Meeting Abstracts 2014;32(18 Suppl):LBA3.
Tarantino I, Warschkow R, Worni M, et al. Prognostic relevance of palliative primary tumor removal in 37,793 metastatic colorectal cancer patients: a population-based, propensity score-adjusted trend analysis. Ann Surg. 2015;262(1):112–20.
Gresham G, Renouf DJ, Chan M, et al. Association between palliative resection of the primary tumor and overall survival in a population-based cohort of metastatic colorectal cancer patients. Ann Surg Oncol. 2014;21(12):3917–23.
Rahbari NN, Lordick F, Fink C, et al. Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS - a randomised controlled multicentre trial (ISRCTN30964555). BMC Cancer. 2012;12(1):1–11.
Lam-Boer J, Mol L, Verhoef C, et al. The CAIRO4 study: the role of surgery of the primary tumour with few or absent symptoms in patients with synchronous unresectable metastases of colorectal cancer: a randomized phase III study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer. 2014;14:741.
Anwar S, Peter MB, Dent J, et al. Palliative excisional surgery for primary colorectal cancer in patients with incurable metastatic disease. Is there a survival benefit? A systematic review. Colorectal Dis. 2012;14(8):920-30.
D’Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18(4):1096-103.
Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–57; discussion 657-8.
Ardito F, Vellone M, Cassano A, et al. Chance of cure following liver resection for initially unresectable colorectal metastases: analysis of actual 5-year survival. J Gastrointest Surg. 2013;17(2):352–9.
Barone C, Nuzzo G, Cassano A, et al. Final analysis of colorectal cancer patients treated with irinotecan and 5-fluorouracil plus folinic acid neoadjuvant chemotherapy for unresectable liver metastases. Br J Cancer. 2007;97(8):1035–1039.
Skof E, Rebersek M, Hlebanja Z, et al. Capecitabine plus irinotecan (XELIRI regimen) compared to 5-FU/LV plus irinotecan (FOLFIRI regimen) as neoadjuvant treatment for patients with unresectable liver-only metastases of metastatic colorectal cancer: a randomised prospective phase II trial. BMC Cancer. 2009;9:120.
Conflict of Interest
Mashaal Dhir, Heather L. Jones, Yongli Shuai, Amber K. Clifford, Samantha Perkins, Jennifer Steve, Melissa E. Hogg, M. Haroon A. Choudry, James F. Pingpank, Matthew P. Holtzman, Herbert J. Zeh III, Nathan Bahary, David L. Bartlett, and Amer H. Zureikat have no conflicts of interest involving this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhir, M., Jones, H.L., Shuai, Y. et al. Hepatic Arterial Infusion in Combination with Modern Systemic Chemotherapy is Associated with Improved Survival Compared with Modern Systemic Chemotherapy Alone in Patients with Isolated Unresectable Colorectal Liver Metastases: A Case–Control Study. Ann Surg Oncol 24, 150–158 (2017). https://doi.org/10.1245/s10434-016-5418-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5418-6