Abstract
Background
The reconstruction of large defects after abdominoperineal resections and pelvic exenterations has traditionally been accomplished with vertical rectus myocutaneous flaps (VRAMs). For patients requiring two ostomies, robot-assisted abdominoperineal resections (APRs), and to avoid the morbidity of a VRAM harvest, the authors have used the gracilis muscle flap to reconstruct the large dead space in these patients.
Methods
A retrospective analysis of 16 consecutive APRs (10 with concomitant pelvic exenterations) reconstructed with gracilis flaps during a 2-year period was performed. Gracilis muscle flaps were used to obliterate the dead space after primary skin closure was ensured with adduction of the legs.
Results
All 16 patients had locally advanced cancers and had received neoadjuvant chemotherapy and radiation. Of these 16 patients, 10 had pelvic exenterations. All the patients had reconstruction with gracilis flaps (6 bilateral flaps). One major wound complication in the perineum occurred as a result of an anastomotic leak in the pelvis, but this was managed with conservative dressing changes. Three patients had skin separation in the perineum greater than 5 mm with intact subcutaneous closure. No patients required operative debridement or revision of their perineal reconstruction. No perineal hernias or gross dehiscence of the skin closure occurred.
Conclusions
Large pelvic and perineal reconstructions can be safely accomplished with gracilis muscle flaps and should be considered as an alternative to abdominal-based flaps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The reconstruction of perineal defects for abdominoperineal resections (APR) has undergone a shift as advances in neoadjuvant chemoradiation and surgical technique have increased the likelihood of sphincter preservation in advanced colorectal cancers.1,2 Consequently, at many institutions, reconstructions have been used largely for aggressive tumors that have been less responsive to neoadjuvant treatment, sarcomas, or recurrent disease. Many of the patients are left with a large skin defect in addition to a dead space in the unyielding floor of the pelvis. The combination of the wide cavity, pelvic fluid accumulations, and a wound bed damaged by radiation contribute to the increase in local complications in this group of patients.3–5 In fact, the complication rate for APR can be as high as 60 % for patients who have received preoperative radiation.
Traditionally, reconstructive options have included the vertical rectus abdominis (VRAM) myocutaneous flaps and thigh-based flaps. These flaps incorporate a skin paddle for the cutaneous perineal defect and a muscle component to obliterate the dead space in the pelvis.6–8 The workhorse for pelvic and perineal reconstructions has been the VRAM. However, the use of the VRAM is complicated by the need for two ostomy sites in many of the reconstructed patients, and ipsilateral ostomies are difficult to site and are associated with parastomal complications. Of the thigh-based flaps, the anterior lateral thigh flap provides the most reliable skin paddle and muscle bulk, but the sacrifice of part or all of the vastus lateralis muscle may result in significant disability. The gracilis flap’s utility in large pelvic and perineal defects is considered limited due to the smaller mass of the muscle, technical difficulty designing the skin island, and the high rate of associated complications.9
At our institution, we have used gracilis muscle flaps for perineal reconstruction whenever the skin can be reapproximated in the operating room. The muscle flap dissection is straightforward with a reliable vascular pedicle. Moreover, primary closure of the perineal skin obviates the need for a skin paddle from the gracilis, which can be unreliable. Although the gracilis flap has traditionally been described for reconstructing small abdominoperineal defects, we have found that this reconstruction is suitable for reconstructing large pelvic exenterations as well. This study aimed to review our experience using the gracilis flap for large APR and pelvic extenteration defects.
Patients and Methods
Between January of 2010 and December of 2012, 16 consecutive abdominoperineal reconstructions (10 with concomitant pelvic exenterations) were performed by a single oncologic team (G.B, V.M, and S.K.) and two reconstructive plastic surgeons (T.C. and M.SC) at the University of Texas Southwestern Medical Center. All the patients had primary closure of the perineum, and the size of the cutaneous defects ranged from 3 to 6 cm. The dead space was obliterated with pedicled gracilis muscle flaps (6 bilateral cases). The 16 patients (5 women and 11 men) had a mean age of 62 ± 8 years (range 53–78 years). The patients included one active smoker and nine former smokers. The comorbid conditions included diabetes mellitus (4 patients), kidney disease (2 patients), hypertension (8 patients), cardiovascular disease (2 patients), chronic obstructive pulmonary disease (1 patient), and obesity (7 patients). The mean body mass index (BMI) was 29 ± 7 kg/m2 (range 18–39 kg/m2).
All the patients had locally advanced cancers with primary diagnoses of colorectal adenocarcinoma (9 patients), pelvic sarcoma (2 patients), anal squamous cell carcinoma (2 patients), recurrent colorectal carcinoma (2), and recurrent prostate carcinoma (1 patient). All the patients underwent neoadjuvant chemotherapy and radiation treatment. The pelvic exenterations performed for 10 patients involved 7 ileal conduits, 4 prostatectomies, 3 vaginectomies/hysterectomies, and 2 extended pelvic sidewall resections. No sacrectomies were performed as part of the extirpation. All the patients were closed primarily, and the deep space was reconstructed with gracilis muscle flaps.
Surgical Technique and Postoperative Management
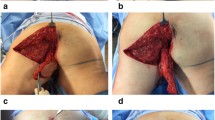
Reconstruction occurred after pelvic exenteration, ostomy maturation, and abdominal wall closure. When possible, the surgical oncologists placed an omental flap in the pelvis (in 5 of the 16 patients). However, the omentum did not provide enough bulk to obviate the need for a muscle flap in these patients. The patients were placed in lithotomy position during the operation, and the legs were adducted temporarily for assessment of the tension with primary skin closure using a temporary suture. Thereafter, the patient’s legs were abducted, and the gracilis flap was elevated with the legs in abduction (Fig. 1a). An incision was made posterior to the adductor longus to expose the gracilis muscle belly overlying the major pedicle (Fig. 1b). The dissection proceeded distally using lighted retractors toward the insertion at the medial tibial condyle. Next, an incision was made distally, and the flap was divided distally, then passed proximally. The flap was elevated from distal to proximal. The pedicle was identified and mobilized sufficiently to facilitate transposition of the gracilis muscle into the defect without compromising blood flow (Fig. 1c). Next, the flap was tunneled in a subcutaneous plane to the perineum (Fig. 1d). At this point, the adequacy of the flap for obliteration of the soft tissue defect was evaluated. If any residual dead space remained after the preliminary inset, a contralateral flap was elevated and used due to volume deficit (6 cases).
a Perineal defect after a robot-assisted abdominoperineal resection. Lone star retractor placed to facilitate retraction of the soft tissue. b Right sided gracilis harvested through a curvilinear incision just posterior to the adductor longus. Legs are still abducted during the flap elevation. c Gracilis muscle with pedicle exposed. The pedicle is not completely dissected. d Gracilis muscle tunneled in a subcutaneous plane into the pelvic defect. Adduction of the thigh permits further transposition and greater muscle volume in the wound
For the inset, the legs were brought out of abduction, and the flap or flaps were sutured circumferentially into the pelvic floor to obliterate the deep soft tissue defect. In bilateral cases, the flaps were sutured together with a PDS suture and sutured to the pelvic floor. Thereafter, the soft tissue was closed in multiple layers reconstructing the anatomic planes using PDS suture in an interrupted fashion. A closed suction drain then was placed into the perineum, and the skin was reapproximated with interrupted chromic sutures. The donor sites were closed after reapproximation of the superifical fascial layer followed by skin using absorbable sutures. A closed suction drain was placed into the donor sites as well.
Patients were on bed rest for at least 48 h but were placed in a sitting-up position by postoperative day 1. All the patients received deep venous thrombosis (DVT) chemoprophylaxis 12 h after the operation. Nursing care consisted of scheduled dressing changes at least three times daily to keep a dry dressing in the perineum. Patients were ambulating by postoperative day 3 with the assistance of a physical therapist.
Outcomes
Demographic data and patient outcomes were retrospectively analyzed from a prospective database maintained by the University of Texas Southwestern Cancer Center and the Department of Plastic Surgery. Major perineal complications were defined as any perineal wound requiring debridement and open packing. Minor perineal wounds were defined as any wound separations greater than 5 mm that required dressing changes. Perineal hernias were defined as any herniation of abdominal viscera through the perineal floor with resultant bulge. Abdominal wound- and donor-site complications were defined as any wound infection, dehiscence (>5 mm), or persistent seroma (requiring aspiration or prolonged drainage).
All patients were followed weekly until their wounds were healed and then every 3 months thereafter. The minimum follow-up period was 5 months, and the longest follow-up period was 40 months.
Statistical Analysis
No statistical analysis was performed. Values are expressed as means ± standard deviations.
Results
Our series had no intraoperative deaths and only one 30-day mortality. The patient who died was a 73-year-old man with multiple comorbidities and a history of bilateral renal cell carcinoma, metastatic prostate adenocarcinoma, and pleiomorphic pelvic sarcoma. He died of pulmonary and renal failure on postoperative day 20 after his palliative resection and reconstruction. The average age for our cohort was 62 ± 8 years. The majority of our patients (87 %) had neoadjuvant chemotherapy and radiation treatment (Table 1).
Due to the complexity of the procedures and the multiple surgical teams involved, the mean operative time was 10:34 ± 2:32 h. The reconstructive portion of the operation accounted for 2:39 ± 1:03 h of the total operating room time. Seven patients had robot-assisted pelvic dissections. Six patients required bilateral gracilis flaps to obliterate the deep pelvic defect (all the bilateral gracilis patients had pelvic exenterations). Female patients were more likely to require bilateral gracilis flaps (3 of 5) but not more likely to have pelvic exenteratations (3 of 5 females vs. 7 of 11 males). The mean width of the defect was 4.28 ± 1.13 cm, and all skin defects were closed primarily (Table 2).
The patients ambulated 4 ± 2 days after the operation on the average, and the average hospital stay was 13 ± 6 days. Three patients experienced DVT (Table 3).All the patients had manual compression stockings in the operating room and received DVT chemoprophylaxis within 12 h after surgery. No postoperative bleeding complications occurred that required operative management (data not shown).
One patient had a major wound complication in the perineum. This patient had an anastomotic leak that was well managed conservatively with drainage catheters but manifested as an abscess in the perineum. This was treated with local wound care and antibiotics, and the patient recovered without further complications. Additionally, one patient experienced a bladder leak, which drained via the perineum, but had no compromise of the perineal closure. This wound healed without any local dehiscence or wound care once the bladder was decompressed.
Overall, the incidence of major perineal wound complication was 6 %, and the incidence of minor perineal complication was 19 %. Three patients (19 %) had a surgical-site infection of the gracilis donor site, all of which responded to drainage and antibiotics. Four patients (25 %) had abdominal surgical-site infections, which were managed as well with drainage, local wound care, and antibiotics (Table 4). There were no abdominal, parastomal, or perineal hernias or bulges and no flap losses.
Other major complications included respiratory complications in three patients and tachyarrhythmias in three patients. These patients who experienced arrhythmias were monitored in the telemetry unit and remained asymptomatic after rate control before discharge.
Discussion
This study demonstrated that perineal reconstructions, even for large pelvic extirpations, can be successfully accomplished using a gracilis muscle flap to obliterate the dead space. For patients who require an ileal conduit and an end colostomy, the extirpative surgeons prefer to place the ostomies on contralateral sides due to the difficulties managing ipsilateral stomas. Consequently, we prefer to avoid the vertical rectus abdominis myocutaneous flap in patients who will require two ostomies. This eliminates the abdominal morbidity associated with harvesting a VRAM, the concomitant difficulties with abdominal closure, and the possible need for a component separation.10 Moreover, a significant number of our perineal reconstructions (n = 7) are performed after robot-assisted APR without access to the peritoneal cavity from a transabdominal approach.
For cases in which the perineal skin cannot be closed primarily (due to radiation or extensive resection) and the abdomen is unavailable, our algorithm is for thigh-based flaps. Unfortunately, the skin island of the gracilis flap is reliant on skin perforators on the proximal portion of the muscle, with unreliable perfusion of the distal skin island. It is this distal portion that would reconstruct the perineum. Excellent results have been obtained, however, with the use of pedicle anterior lateral thigh flaps for reconstruction of perineal wounds.11 However, a significant amount of tunneling is involved (deep to the rectus femoris), and the risk of inadequate length remains. In these situations, the flap would have to be converted to a free flap. The VRAM flap can be used with a subcutaneous tunnel if the patient does not have adequate thigh tissue to facilitate coverage of large skin defects in the perineum.
Nonetheless, in our patients, we find that the gracilis muscle flap or flaps provide sufficient vascularized bulk to reconstruct the pelvic defect in APRs and pelvic exenterations. In our series, six patients required bilateral gracilis flaps, all for pelvic exenterations. However, five other pelvic exenteration patients were reconstructed with just a unilateral gracilis. None of those patients had a major perineal complication, and only one patient had a minor perineal wound problem. We have noted an increased incidence of bilateral flaps in female patients, which may be attributable to their relatively wider pelvis and correspondingly larger volume deficit. However, none of these patients have experienced any wound-healing complications.
Our low complication rate compares favorably with VRAM and ALT flap-based reconstructions in the literature.1,11–16 Overall, our major perineal wound complication rate was 6 %, and our minor perineal complication rate was 19 %. We defined any wound separation greater than 5 mm that could be managed with conservative dressing changes as a minor complication. The major perineal wound was a result of an intrapelvic process after an anastomotic leak, and it could be argued that this was not a primary wound complication.
None of our patients required an additional trip to the operating room for management of their perineal wound, and most wounds were small superificial dehiscences with intact subcutaneous closures. Perineal complications have been consistently higher in published series when flap-based reconstructions are not used, especially in irradiated fields.6–8 These complication rates range from 7 to 60 % overall. With the interposition of vascularized tissue, these rates decrease to between 20 and 30 %. These studies used myocutaneous flaps with skin islands comprising a portion of the reconstruction.
Our current algorithm consists of approximating the perineal skin without tension after gracilis muscle flap interposition. The perineum is closed with the legs in adduction. This allows us to close defects as wide as 6 cm. These measurements were taken from pathology reports and not in situ and thus may under represent the width of some of these wounds in the operating room. Patients are not placed sitting up until postoperative day 1 and are not allowed to ambulate per protocol until postoperative day 2. However, a few patients in our series had their ambulation delayed or temporarily suspended due to medical complications related to respiratory distress, DVT, bladder leaks, and the like. Nonetheless, they ambulated on the average by postoperative day 4, and no frank dehiscences occurred after activity was initiated. Moreover, none of the patients reported long-term difficulty with sitting or ambulation after primary closure (data not shown). Additionally, they did not have any of the attendant weakness or abdominal morbidity associated with a VRAM flap.
Although the vastus lateralis is one of many extensors in the leg, it is one of the largest, and the sacrifice of this muscle may result in some weakness17. Nonetheless, it is in our algorithm for pelvic and perineal wounds requiring a large skin island that cannot be approximated primarily.
Based on this approach to complex perineal reconstructions, we find that the gracilis muscle flap is a safe and easily performed flap for reconstructing APR and even pelvic exenteration defects in the irradiated patient with low complication rates and minimal morbidity.
References
Lu JY, Xiao Y, Qiu HZ, Wu B, Lin GL, Xu L, Zhang GN, Hu K. Clinical outcome of neoadjuvant chemoradiation therapy with oxaliplatin and capecitabine or 5-fluorouracil for locally advanced rectal cancer. J Surg Oncol. 2013; 108:213–9.
Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13:1152–60.
Wiatrek RL, Thomas JS, Papaconstantinou HT. Perineal wound complications after abdominoperineal resection. Clin Colon Rectal Surg. 2008;21:76–85.
Luna-Pérez P, Rodríguez-Ramírez S, Vega J, Sandoval E, Labastida S. Morbidity and mortality following abdominoperineal resection for low rectal adenocarcinoma. Rev Invest Clin. 2001;53:388–95.
Farid H, O’Connell TX. Methods to decrease the morbidity of abdominoperineal resection. Am Surg. 1995;61:1061–4.
Butler CE, Gündeslioglu AO, Rodriguez-Bigas MA. Outcomes of immediate vertical rectus abdominis myocutaneous flap reconstruction for irradiated abdominoperineal resection defects. J Am Coll Surg. 2008;206:694–703.
Chessin DB, Hartley J, Cohen AM, Mazumdar M, Cordeiro P, Disa J, Mehrara B, Minsky BD, Paty P, Weiser M, Wong WD, Guillem JG. Rectus flap reconstruction decreases perineal wound complications after pelvic chemoradiation and surgery: a cohort study. Ann Surg Oncol. 2005;12:104–10.
Shibata D, Hyland W, Busse P, Kim HK, Sentovich SM, Steele G Jr, Bleday R. Immediate reconstruction of the perineal wound with gracilis muscle flaps following abdominoperineal resection and intraoperative radiation therapy for recurrent carcinoma of the rectum. Ann Surg Oncol. 1999;6:33–7.
Nelson RA, Butler CE. Surgical outcomes of VRAM versus thigh flaps for immediate reconstruction of pelvic and perineal cancer resection defects. Plast Reconstr Surg. 2009;123:175–83.
Baumann DP, Butler CE. Component separation improves outcomes in VRAM flap donor sites with excessive fascial tension. Plast Reconstr Surg. 2010;126:1573–80.
Wong S, Garvey P, Skibber J, Yu P. Reconstruction of pelvic exenteration defects with anterolateral thigh-vastus lateralis muscle flaps. Plast Reconstr Surg. 2009;124:1177–85.
Lefevre JH, Parc Y, Kernéis S, Shields C, Touboul E, Chaouat M, Tiret E. Abdominoperineal resection for anal cancer: impact of a vertical rectus abdominis myocutaneus flap on survival, recurrence, morbidity, and wound healing. Ann Surg. 2009;250:707–11.
Shibata D, Hyland W, Busse P, Kim HK, Sentovich SM, Steele G Jr, Bleday R. Immediate reconstruction of the perineal wound with gracilis muscle flaps following abdominoperineal resection and intraoperative radiation therapy for recurrent carcinoma of the rectum. Ann Surg Oncol. 1999;6:33–7.
Buchel EW, Finical S, Johnson C. Pelvic reconstruction using vertical rectus abdominis musculocutaneous flaps. Ann Plast Surg. 2004;52:22–6.
Arnold PG, Lovich SF, Pairolero PC. Muscle flaps in irradiated wounds: an account of 100 consecutive cases. Plast Reconstr Surg. 1994;93:324–7.
Hinojosa MW, Parikh DA, Menon R, Wirth GA, Stamos MJ, Mills S. Recent experience with abdominal perineal resection with vertical rectus abdominis myocutaneous flap reconstruction after preoperative pelvic radiation. Am Surg. 2009;75:995–9.
Hanasono MM, Skoracki RJ, Yu P. A prospective study of donor-site morbidity after anterolateral thigh fasciocutaneous and myocutaneous free flap harvest in 220 patients. Plast Reconstr Surg. 2010;125:209–14.
Disclosure
There is no financial interest or commercial association for any of the authors that might pose or create a conflict of interest with the information presented in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chong, T.W., Balch, G.C., Kehoe, S.M. et al. Reconstruction of Large Perineal and Pelvic Wounds Using Gracilis Muscle Flaps. Ann Surg Oncol 22, 3738–3744 (2015). https://doi.org/10.1245/s10434-015-4435-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4435-1