Abstract
Purpose
Published data have shown heterogeneous outcomes for high-risk prostate cancer. Thus, we tried to identify more precise risk stratification system for contemporary high-risk prostate cancer.
Methods
Classifying patients according to National Comprehensive Cancer Network risk groups, we reviewed data of 1,905 men who underwent radical prostatectomy (RP) at our institution from 2006 to 2013. For our analyses, high-risk prostate cancers meeting at least one of two following factors were categorized as unfavorable high-risk prostate cancer: biopsy primary Gleason pattern 5 and/or multiple (≥2) high-risk criteria present. All other men with high-risk prostate cancer were designated as having favorable high-risk disease. Postoperative outcomes, including biochemical recurrence-free survivals were assessed and compared via log-rank test and Cox proportional hazards model.
Results
In multivariable analysis, primary Gleason 5 pattern on biopsy (p = 0.008) and multiple (≥2) high-risk criteria (p < 0.001) were observed to be independent predictors of the risk of biochemical recurrence amongst high-risk group undergoing RP. Favorable high-risk prostate cancer group showed a significantly higher 5-year biochemical recurrence-free survival than unfavorable high-risk group (56.35 vs. 18.75 %; log-rank test: p < 0.001). Favorable high-risk group demonstrated significantly lower 5-year biochemical recurrence-free survival than intermediate-risk group (56.07 vs. 82.05 %; log-rank test: p < 0.001).
Conclusions
A significant heterogeneity existed in biochemical outcomes of contemporary patients with high-risk prostate cancer who underwent definitive RP. According to primary Gleason pattern and number of high-risk criteria present, high-risk group should be stratified further into favorable and unfavorable disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With high risk of disease recurrence and progression, the management of high-risk prostate cancer (PCa) has remained a challenging task.1 Due to the lack of high-level evidence from randomized trial, optimal treatment for high-risk PCa is yet to be defined.2–6 Through the years, outcomes following radical prostatectomy (RP) in men with high-risk PCa have not improved significantly. Although RP is known to offer good prognosis in a proportion of patients with high-risk disease, many others still require secondary treatment after undergoing RP. Currently no reliable method is available to accurately differentiate between the two groups.
Published data demonstrate that outcomes of high-risk PCa vary considerably. Such phenomenon may be largely due to two factors. First, there is currently no definitive consensus on the definition of high-risk PCa.7–9 Depending on the definition applied, 5-year biochemical recurrence-free survival after RP in high-risk group has been reported to range from 49 to 80 %.10 Second, as heterogeneity in outcomes among high-risk patients defined by the same criteria also has been observed, subgroups within high-risk PCa may well exist. It can be hypothesized that currently defined high-risk group can be stratified further to identify subsets of men with relatively better and worse prognoses. Identification of such subgroups among patients with high-risk PCa using a tool that can easily be applied in actual clinical setting would contribute to the selection of candidates for adjuvant, multimodal treatment, or clinical trials.
Previous reports showed on the potential predictors of outcomes after RP among high-risk group.11–13 Meanwhile, considerable proportions of such RP series encompassed men who underwent RP before this millennium. With increase of prostate-specific antigen (PSA) screening, stage migration, and improvement in surgical technique since the introduction of D’Amico risk classification system in 1998, the characteristics of high-risk PCa may well have evolved with time as well.14 Contemporary high-risk group should be re-evaluated since the current risk-stratification system is derived from historical information. In addition, it should be reminded that the modifications of Gleason grading system were introduced by International Society of Urological Pathology (ISUP) in 2005.15
Thus, with goal of developing more precise risk stratification system for contemporary high-risk PCa that can be applied easily in clinical setting, we investigated the postoperative outcomes of patients who underwent RP for high-risk PCa during relatively recent period.
Materials and Methods
Patient Population
With approval from our institutional review board, we reviewed the records of 1,908 patients who underwent RP from 2006 to 2013 at our institution. Three patients were excluded due to lack of pathologic records. The National comprehensive cancer network (NCCN) risk classification of the 1,905 total patients was as the following: 655 (34.38 %) were low-risk, 704 (36.96 %) intermediate-risk, and 546 (28.66 %) high-risk.
All biopsy and RP specimens were pathologically analyzed by at least two uro-pathologists. For our study, adverse pathologic features were defined as extracapsular extension (ECE) of tumor, seminal vesicle invasion (SVI), and lymph node invasion (LNI). We performed an extended PLND according to NCCN guidelines.1
Analysis of postoperative biochemical recurrence (BCR) was limited to patients who underwent RP from 2006 to 2012 (n = 197). BCR was defined as a PSA value ≥0.2 ng/ml on two consecutive measurements following RP.16
Favorable Versus Unfavorable High-Risk Disease
Within the high-risk cohort, high-risk PCa that met at least one of the following two factors were considered to be unfavorable high-risk group: biopsy Gleason score primary pattern 5 and/or the presence of two or three high-risk criteria. All other high-risk patients were considered to be favorable high-risk group.
Statistical Analysis
Characteristics of patients were analyzed by using ANOVA test and Chi-test. χ 2 analysis also was used to compare rates of adverse pathologic outcomes between patient groups. Cox univariable and multivariable regression models were used to identify the association between clinical risk factors and BCR. Postoperative BCR-free survivals of patient groups were calculated and compared using the Kaplan–Meier (K–M) with a log-rank test. SPSS v. 19.0 was used for all statistical analyses. p value <0.05 was considered statistically significant.
Results
Table 1 shows the baseline characteristics of patients according to the number of high-risk criteria. Of the 546 total high-risk PCa patients, 366 (67.03 %), 144 (26.37 %), and 36 (6.59 %) met one, two, and three risk criteria for high-risk PCa, respectively.
As for the rates of adverse pathologic features observed according to the type of high-risk criteria met by the patients, there were no significant differences among the three high-risk criteria (clinical stage ≥T3a, biopsy Gleason score ≥8, and PSA >20 ng/ml): ECE (59 vs. 48.4 vs. 56 %, p = 0.285), SVI (28.2 vs. 17.4 vs. 16.9 %, p = 0.237), and LNI (10.7 vs. 2.4 vs. 6.0 %, p = 0.128), respectively. Meanwhile, there were significant differences in the rates of adverse pathologic features between patients meeting only one high-risk criterion and those with two or three criteria: ECE (53 vs. 82.2 %, p < 0.001), SVI (18.3 vs. 47.8 %, p < 0.001), and LNI (4.9 vs. 12.1 %, p = 0.004).
Mean duration of postoperative follow-up was 45.1 months. Among high-risk patients who met only one high-risk criterion, no significant differences in 5-year postoperative BCR-free survivals were shown: clinical stage ≥T3a (n = 82, 22.4 %) versus biopsy Gleason score ≥8 (n = 139, 37.98 %; log-rank test: p = 0.410), clinical stage ≥T3a (n = 82, 22.4 %) versus PSA >20 ng/ml (n = 145, 39.62 %; log-rank test: p = 0.655), and biopsy Gleason score ≥8 (n = 139, 37.98 %) versus PSA >20 ng/ml (n = 145, 39.62 %; log-rank test: p = 0.689). However, patients with two or three high-risk criteria were observed to have significantly worse 5-year BCR-free survival than those with only one criterion (14.05 vs. 55.52 %; log-rank test: p < 0.001). When high-risk patients were stratified according to biopsy Gleason score, BCR rates for patients with Gleason 5 + 4, 5 + 3, 4 + 4, and 4 + 3 PCa were observed to be significantly different (82.6 vs. 50 vs. 42.1 vs. 39.3 %; log-rank test: p = 0.002).
In a multivariable Cox proportional hazards model, including age, prostate volume, clinical stage, PSA, primary Gleason pattern 5, and number of high-risk criteria, only the two variables of primary Gleason pattern 5 and number of high-risk criteria were observed to be independent predictors of BCR risk among the high-risk group (Table 2). As aforementioned, patients in the high-risk group with at least one of these two factors were designated as having unfavorable high-risk disease (196 patients, 35.9 %), whereas the others were considered to have favorable high-risk disease (350 patients, 64.1 %) for this study.
Comparison of adverse pathologic features between unfavorable and favorable high-risk groups showed significant differences. The unfavorable high-risk demonstrated significantly higher rates of adverse pathologic features as follows: ECE (52.3 vs. 81.1 %, p < 0.001), SVI (16.0 vs. 48.0 %, p < 0.001), and LNI (4.5 vs. 12.2 %, p = 0.002; Table 3).
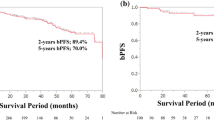
As for postoperative BCR-free survival, we found a significant difference between the favorable and the unfavorable high-risk group (5-year BCR-free survival rate: 56.35 vs. 18.75 %; log-rank test: p < 0.001; Fig. 1). Still, compared with intermediate-risk group, favorable high-risk group was shown to have significantly worse biochemical outcome (5-year BCR-free survival rate: 56.07 vs. 82.05 %; log-rank test: p < 0.001).
When biochemical outcomes of unfavorable high-risk patients having both of the two unfavorable risk factors (n = 22) and those having only one unfavorable risk factor (n = 140) were compared, a significant difference was observed in BCR rates (60 vs. 86.4 %; log-rank test: p = 0.013).
Discussion
In this study, we observed heterogeneous pathologic and biochemical outcome among contemporary patients who underwent RP for high-risk PCa. Specifically, we found that high-risk PCa patients undergoing RP could be stratified further into the two subsets of favorable and unfavorable high-risk groups according to the presence of primary Gleason pattern 5 on biopsy and/or the number of high-risk criteria. Having significantly shorter postoperative BCR-free survival than intermediate-risk group, patients in the favorable high-risk group were shown to have significantly longer BCR-free survival than the unfavorable high-risk group. Overall, our findings support more precise characterization of high-risk PCa than currently offered by NCCN risk group.
Assessing the outcome of RP in patients with pretreatment PSA >20 ng/ml, Spahn et al. observed that PSA >20 ng/ml associated with biopsy Gleason score ≤7 resulted in 10-year PCa-specific mortality rate of 5 %, whereas 10-year PCa-specific mortality rate was 35 % when associated with biopsy Gleason score ≥8.17 From their findings, they suggested that biopsy Gleason score was the strongest predictor of progression and mortality in men with PSA >20 ng/ml undergoing RP. In a multi-institutional study of patients undergoing RP for high-risk PCa, Walz et al. found that men who met only one high-risk criterion had most favorable 5-year BCR-free survival (50.3 %) relative to patients meeting two or more high-risk criteria (27.5 %).18 Also, they observed that men with Gleason score 8–10 had significantly worse BCR-free survival than those who had cT3 tumor, demonstrating the heterogeneity of high-risk PCa according to the type of risk criteria. From assessing the outcome of 798 men who underwent RP for high-risk PCa, Reese et al. found that the 5-year BCR-free survival was 51.2 % for men with only 1 high-risk criterion, significantly higher than 40.0 % for men with multiple criteria present.12 Meanwhile, Reese et al. also observed no significant difference in BCR-free survival rates according to the type of criterion met among the patients who met only one high-risk criterion. Similar to our finding, only a trend of high (≥8) Gleason score being a stronger prognostic factor than two other high-risk criteria was observed in Reese et al.’s series. As the clinical significances of Gleason score and/or number of high-risk criteria among high-risk group have been confirmed in these relevant studies, it can be suggested that findings from these published studies corroborates our results.
Gleason pattern 5 is defined as adenocarcinoma with essentially no glandular differentiation, composed of solid sheets, cords, or single cells (comedocarcinoma with central necrosis surrounded by papillary cribriform or solid masses).15 One of the notable finding from our study would be that the primary Gleason pattern 5 on biopsy, instead of the Gleason sum, was shown to be an independent predictor of BCR-free survival among the high-risk group. When stratifying our subjects by biopsy Gleason patterns, men with Gleason 5 + 3 tumors were shown to have significantly worse biochemical outcome than those with Gleason 4 + 4 tumor. Admittedly, Gleason sum may well have proven to be a significant prognostic factor in this study with a larger cohort or different endpoint, such as metastasis-free or PCa-specific survival. Although patients with high Gleason score was not observed to have statistically worse BCR-free survival than others among men with a single high-risk criterion in our study, we believe that our findings on primary Gleason pattern 5 provide further evidence that Gleason score is a strong predictor of outcome even among high-risk group. Sundi et al. tested 28 different permutations of adverse grade, stage, and cancer volume by comparing their hazards ratio for metastasis and cancer-specific mortality in a cohort of 753 men who underwent RP for high-risk localized PCa.11 Similar to our findings, they observed that a subgroup of high-risk group who have particularly poor oncologic outcome, which they designated as very high-risk group, was best defined by primary Gleason pattern 5, ≥5 cores with Gleason score 8–10, or multiple high-risk criteria. Meanwhile, in addition to reports of the presence of Gleason pattern 5 being associated with outcome after primary treatments of PCa, others have observed that the proportion of Gleason pattern 4 or 5 may be a strong predictor of long-term outcome following definitive treatment as well.19–25 D’Ambrosio et al. reported from evaluating patients who underwent primary radiotherapy that the percentage of biopsy tissue containing Gleason pattern 4 or 5 was shown to be an independent predictor of BCR and furthermore, only significant predictor of distant metastasis following radiotherapy.26 Even though we could not assess the percentage of biopsy tissue containing Gleason pattern 4 or 5 in this study, such parameter should be evaluated as a potential tool to upgrade the current risk-based classification system in the future.
Recently, a relatively simple pretreatment prognostic model for high-risk PCa was reported. By constructing multivariable regression models to predict PCa-specific survival as a function of high-risk criteria, Joniau et al. stratified 1,360 high-risk PCa patients who underwent RP at 8 European centers based on a model that includes 3 subgroups: good prognosis subgroup (1 single high-risk criterion); intermediate prognosis subgroup (PSA >20 ng/ml and stage cT3–4); and poor prognosis subgroup (GS 8–10 in combination with at least 1 other high-risk criterion).13 According to this classification system, the 5- and 10-year PCa-specific survival rates were 98.7 and 95.4, 96.5 and 88.3, and 88.8 and 79.7 %, for the good, intermediate, and poor prognosis subgroups, respectively, demonstrating that outcomes significantly worsened in a stepwise fashion from the good to the poor prognosis subgroups. Meanwhile, more than 30 % of men included in this study underwent RP during the period of 1987 to 1996, more than half undergoing RP before year 2000. Since RP has been considered as a first-line treatment option for high-risk PCa in more recent period, subjects included in their study may well be a highly selected group of patients. Stage migration and change in the profile of PCa diagnosed since 1990s also should be considered.27,28
In our study, we observed that unfavorable high-risk group with two unfavorable risk factors (Gleason pattern 5 and also having more than 2 high-risk criteria) had significantly worse biochemical outcome than those with only a single risk factor. Such finding would justify further stratification of the unfavorable high-risk group. However, because the number of patients with two unfavorable risk factors was relatively small in our study, further evaluation of such strategy of substratification would be needed.
We acknowledge that this study has some limitations. First, our study design is retrospective. Second, distribution of patients among various subgroups in our study was not even, mostly due to the modest size of our cohort. In addition, because only patients with high-risk PCa opting for RP were included in this study, our findings may not be applicable to high-risk patients undergoing radiotherapy or systemic therapy. Furthermore, follow-up duration of our subjects did not allow analyses of metastasis-free or cancer-specific survival.
Conclusions
A distinct heterogeneity exists in both pathologic and biochemical outcomes among the contemporary patients with high-risk PCa who underwent definitive RP. Due to the significantly different prognosis according to the presence of primary Gleason pattern 5 and the number of high-risk criteria, the high-risk group should be substratified into favorable and unfavorable subgroups. Such minimal modification of the NCCN risk stratification system would be easy to use and help to select optimal candidates for adjuvant or multimodal treatments and clinical trials.
References
NCCN. Clinical Practice Guidelines in Oncology (NCCN Guideline®). Prostate cancer v4.2013. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Bolla M, van Poppel H, Tombal B, et al; European Organisation for Research and Treatment of Cancer, Radiation Oncology and Genito-Urinary Groups. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380:2018–27.
Swanson GP, Hussey MA, Tangen CM, et al; SWOG 8794. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol. 2007;25:2225–9.
Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–30.
Messing EM, Manola J, Yao J, et al. Eastern Cooperative Oncology Group study EST 3886. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–9.
Dorff TB, Flaig TW, Tangen CM, et al. Adjuvant androgen deprivation for high-risk prostate cancer after radical prostatectomy: SWOG S9921 study. J Clin Oncol. 2011;29:2040–5.
van den Ouden D, Hop WC, Schröder FH. Progression in and survival of patients with locally advanced prostate cancer (T3) treated with radical prostatectomy as monotherapy. J Urol. 1998;160:1392–7.
Van Poppel H, Goethuys H, Callewaert P, Vanuytsel L, Van de Voorde W, Baert L. Radical prostatectomy can provide a cure for well-selected clinical stage T3 prostate cancer. Eur Urol. 2000;38:372–9.
Carver BS, Bianco FJ Jr, Scardino PT, Eastham JA. Long-term outcome following radical prostatectomy in men with clinical stage T3 prostate cancer. J Urol. 2006;176:564–8.
Yossepowitch O, Eggener SE, Bianco FJ Jr, Carver BS, Serio A, Scardino PT, Eastham JA. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J Urol. 2007;178:493–9.
Sundi D, Wang VM, Pierorazio PM, et al. Very-high-risk localized prostate cancer: definition and outcomes. Prostate Cancer Prostatic Dis. 2014;17:57–63.
Reese AC, Pierorazio PM, Han M, Partin AW. Contemporary evaluation of the National Comprehensive Cancer Network prostate cancer risk classification system. Urology. 2012;80:1075–9.
Joniau S, Briganti A, Gontero P, et al. European Multicenter Prostate Cancer Clinical and Translational Research Group (EMPaCT). Stratification of high-risk prostate cancer into prognostic categories: a European multi-institutional study. Eur Urol. 2014. doi:10.1016/j.eururo.2014.01.020.
D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74.
Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–42.
Abdollah F, Karnes RJ, Suardi N, et al. Predicting survival of patients with node-positive prostate cancer following multimodal treatment. Eur Urol. 2014;65:554–62.
Spahn M, Joniau S, Gontero P, et al. Outcome predictors of radical prostatectomy in patients with prostate-specific antigen greater than 20 ng/ml: a European multi-institutional study of 712 patients. Eur Urol. 2010;58:1–7.
Walz J, Joniau S, Chun FK, et al. Pathological results and rates of treatment failure in high-risk prostate cancer patients after radical prostatectomy. BJU Int. 2011;107:765–70.
Hashine K, Yuasa A, Shinomori K, Shirato A, Ninomiya I, Teramoto N. Tertiary Gleason pattern 5 and oncological outcomes after radical prostatectomy. Jpn J Clin Oncol. 2011;41:571–6.
Trock BJ, Guo CC, Gonzalgo ML, Magheli A, Loeb S, Epstein JI. Tertiary Gleason patterns and biochemical recurrence after prostatectomy: proposal for a modified Gleason scoring system. J Urol. 2009;182:1364–70.
Stock RG, Cesaretti JA, Hall SJ, Stone NN. Outcomes for patients with high-grade prostate cancer treated with a combination of brachytherapy, external beam radiotherapy and hormonal therapy. BJU Int. 2009;104:1631–6.
Sabolch A, Feng FY, Daignault-Newton S, et al. Gleason pattern 5 is the greatest risk factor for clinical failure and death from prostate cancer after dose-escalated radiation therapy and hormonal ablation. Int J Radiat Oncol Biol Phys. 2011;81:e351–60.
Vis AN, Roemeling S, Kranse R, Schröder FH, van der Kwast TH. Should we replace the Gleason score with the amount of high-grade prostate cancer? Eur Urol. 2007;51:931–9.
Nanda A, Chen MH, Renshaw AA, D’Amico AV. Gleason Pattern 5 prostate cancer: further stratification of patients with high-risk disease and implications for future randomized trials. Int J Radiat Oncol Biol Phys. 2009;74:1419–23.
Cheng L, Koch MO, Juliar BE, Daggy JK, Foster RS, Bihrle R, Gardner TA. The combined percentage of Gleason patterns 4 and 5 is the best predictor of cancer progression after radical prostatectomy. J Clin Oncol. 2005;23:2911–7.
D’Ambrosio DJ, Hanlon AL, Al-Saleem T, et al. The proportion of prostate biopsy tissue with Gleason pattern 4 or 5 predicts for biochemical and clinical outcome after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:1082–7.
Gallina A, Chun FK, Suardi N, et al. Comparison of stage migration patterns between Europe and the USA: an analysis of 11 350 men treated with radical prostatectomy for prostate cancer. BJU Int. 2008;101:1513–8.
Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101:1280–3.
Disclosure
All authors have no conflict of interest with any institution or product.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jo, J.K., Kook, H.R., Byun, SS. et al. Stratification of Contemporary Patients Undergoing Radical Prostatectomy for High-risk Prostate Cancer. Ann Surg Oncol 22, 2088–2093 (2015). https://doi.org/10.1245/s10434-014-4183-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4183-7