Abstract
This study was designed to synthesize quarternized chitosans (Q-CS) and explore their potential application in aqueous solubility enhancement of indomethacin (IND), a BCS class-II drug. Three different Q-CS; N,N,N-trimethyl chitosan chloride (TMC), N-(4-N‘-methylpyridinylmethyl) chitosan chloride (mPyCS), and N-(4-N’,N’,N’-trimethylaminobenzyl) chitosan chloride (TmBzCS) were synthesized and characterized through various spectroscopic analysis. Q-CS-based solid-dispersion (SD) composites of IND (Q-CS-IND) were prepared using the spray-drying method and characterized through Fourier transform infrared (FTIR), scanning electron microscopy (SEM), differential-scanning calorimetry (DSC), and powder X-ray diffraction (P-XRD). The solubility and dissolution profiles of SD-composites of IND were evaluated and compared with physical mixtures (PM). The IND contents were quantified and validated in the composites using UV-Vis spectrophotometer. FTIR and NMR analysis showed the successful preparation of Q-CS. TMC was found with the highest yield (55.13%) and mPyCS with the highest degree of quaternization (DQ) (63.37%). FT-IR analysis of IND-Q-CS composites demonstrated chemical interaction between carbonyl moieties of IND with functional groups of Q-CS. DSC and PXRD analyses demonstrated the transformation of IND in SD composites from crystalline to an amorphous form. All the IND-Q-CS composites were observed with a significant increase in the solubility and dissolution rate of the drug (1996.0 µg/min) compared to PM (1306.8 µg/min), which is higher than pure IND (791.6 µg/min). The contents of IND in TMC, mPyCS, and TmBzCS composites were 97.69–99.92%, 97.66-100.25%, and 97.18-100.11% respectively. Overall, the findings encourage the applications of Q-CS derivatives for increasing IND water solubility and warrant further in vivo biological profiling of IND composites.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poorly water-soluble drugs are associated with slow absorption resulting in low bioavailability that in turn limits their clinical applications [1]. Such drugs can be used only by parenteral routes or at high doses through the oral route, the convenient route of administration. One such example is indomethacin (IND), which is used as a non-steroidal anti-inflammatory drug for the treatment of pain and rheumatoid arthritis. The poor water solubility (BCS class-II) of IND results in its low absorption and bioavailability [2,3,4]. Therefore, to achieve effective pharmacokinetic and pharmacodynamic responses of the drug, it is necessary to formulate it by using alternative strategies. Various formulation strategies have been developed to improve the solubility or rate of dissolution and hence the oral bioavailability of water-insoluble drugs [5, 6]. Different techniques such as preparation of solid dispersion (SD) composites of IND based on poly-ethylene-glycol, complexation of IND with casein hydrolysates, preparation of polyamidoamine-based dendrimers of IND, liquisolid and compaction granulation, and polymeric nano-particulate formulations were attempted with the aim of enhancement of solubility and other physicochemical characteristics [7,8,9]. While many of these techniques showed promising results, enabling significant enhancement in the solubility of IND, each of these techniques presented different limitations such as low amorphization of the drug, structural complexities, low drug loading, poor chemical and physical stability, and premature drug leakage, low yield, drug precipitation upon dilution and scale-up problems [10,11,12]. All these limitations necessitate the continued search for more effective strategies for the solubility enhancement of IND.
A possible alternative strategy to cite the above-mentioned drawbacks could be the preparation of SD composites of IND using chemically modified chitosans. SD has been widely used to enhance the solubility, dissolution rate, and bioavailability of poorly soluble drugs [13]. These preparations involve dispersing the drug in an inert carrier matrix, which improves the drug’s dissolution rate and bioavailability. By converting the drug into an amorphous form or reducing its particle size, SD enhances the drug’s wettability and surface area, leading to improved solubility. This technique is particularly significant in pharmaceutical development as it allows for more effective drug delivery, improved therapeutic efficacy, and expanded clinical applications of drugs that would otherwise be limited by poor water solubility [8]. Concerning the carriers for SD, several water-soluble polymer carriers such as polyethylene glycol (PEG), polyvinyl pyrrolidone (PVP) [14], hydroxypropylmethylcellulose (HPMC) [15] and quarternized chitosans (Q-CS) [16] have been reported to improve the solubility and bioavailability of poorly water-soluble drugs. Among the polymers, chitosan, the N-deacetylated product of chitin, and its quarternized derivatives have attracted great interest due to their versatile properties such as biodegradability, biocompatibility, low toxicity, and permeation enhancement. Although the native chitosan has a wide range of applications, its intermolecular and intramolecular hydrogen bonds are highly crystalline, making it almost insoluble in water, only soluble in organic acidic solution with an apparent pKa of 5.5, thus limiting its use to some extent [17]. Therefore, improving the water solubility of chitosan is essential. Through chemical alterations, the native chitosan can be modified into derivatives with expanded solubility over a wide range of pH hence enhancing the diffusion of drug moiety across biological membranes in neutral/alkaline physiological conditions. The additional positive charge on the chitosan derivatives facilitates pronounced mucoadhesiveness, biocompatibility, and biodegradability, widening their biomedical applications [18,19,20,21]. Chitosan offers several reaction sites for substitution which make it easier to be modified chemically for the seek of improvement of the inherent properties. The various chemical modifications, such as acetylation, alkylation, carboxylation, esterification, etherification and quaternization, are performed at the C2, C3, and C6 positions of the CS backbone to enhance its physicochemical properties (such as increased aqueous solubility, improved absorption, and high bioavailability) [22,23,24,25,26]. Among the modifications, quaternization is regarded as the most impactful chemical modification in terms of enhancing the aqueous solubility of poorly water-soluble drugs as well as other physicochemical properties. Q-CS possesses permanent positive charges due to the introduction of quaternary ammonium groups. This increases their hydrophilicity and water solubility compared to native chitosan. Enhanced solubility facilitates better dispersion and dissolution of drugs in aqueous media [26]. There are several reports that have focused on the preparation of quaternization chitosan. N, N, N-trimethyl chitosan chloride (TMC) [27], N-(4-N’-methyl pyridinyl methyl) chitosan chloride (mPyCS) [28] and N-(4-N’,N’,N’-trimethylaminobenzyl) chitosan chloride (TmBzCS) [29] that are extensively studied for their potential biomedical role particularly in the improvement of physicochemical properties [30, 31]. There are numerous reports on the successful applications of quarternized chitosan derivatives in the development of various drug delivery vehicles for the purpose of enhancement of physicochemical properties of the encapsulated drug(s). For instance, curcumin which has low water solubility, is extensively investigated for solubility improvement by incorporating in various drug delivery vehicles using Q-CS as the carrier [32, 33]. Similarly, a wide range of chemotherapeutic agents belonging to various BCS classes are encapsulated in Q-CS-based vehicles with the aim of enhancing solubility and other physicochemical properties [26, 32, 34, 35].
The potential applications of Q-CS in the solubility enhancement of poorly water-soluble drugs fascinated our interest in designing the current study and extending the application of Q-CS to the solubility enhancement of IND. As, to the best of our knowledge and literature search the Q-CS are yet to explore for their promising impact on the aqueous solubility of IND. The aim of the current study was to evaluate the influence of Q-CS as drug carriers on the dissolution property of SD composites of IND prepared by spray drying technique and compared with physical mixtures of the carrier and IND. Three types of Q-CS derivatives; N,N,N-trimethyl chitosan chloride (TMC), N-(4-N’-methyl pyridinyl methyl) chitosan chloride (mPyCS), and N-(4-N’,N’,N’-trimethylaminobenzyl) chitosan chloride (TmBzCS) were synthesized and utilized as carriers.
Materials and Methods
Chemicals and Drugs
Chitosan was obtained from Bannawach Bio-Line Co., Ltd, Thailand, and Seafresh Chitosan Co., Ltd., Thailand. Their degree of deacetylation (% DD) and approximate average molecular weight were 80% DD and 30,000 g/mol. Glacial acetic acid, methanol, sodium hydroxide, and hydrochloric acid were analytical grade and purchased from Labscan®Asia, Thailand. Sodium cyanoborohydride was analytical grade and purchased from Fluka, Germany. Iodomethane and N-methyl-2-pyrrolidone (NMP) were analytical grade and obtained from Sigma-Aldrich, Germany. IND with 99.09% purity was purchased from Merck, Germany. A dialysis bag (cellulose tubular membrane MW cut off 12,000–14,000) was purchased from Membrane Filtration Products, USA.
Preparation of Q-CS Derivatives
Three types of Q-CS derivatives were prepared in this study namely, N, N, N-trimethyl chitosan chloride (TMC), N-(4-N’-methylpyridinylmethyl) chitosan chloride (mPyCS) and N-(4-N',N',N'-trimethylaminobenzyl) chitosan chloride (TmBzCS).
Preparation of N,N,N-trimethyl Chitosan Chloride (TMC) (3)
N,N,N-trimethyl chitosan chloride (TMC) (3) was prepared according to the method described by [29] with slight modifications. There are two steps in the synthesis of TMC (Fig. 1). In the first step, N,N,N-trimethyl chitosan iodide (2) was synthesized from chitosan (1) with iodomethane in basic condition, followed by the chloride ion exchange in the second step to give TMC (3).
Synthesis of N,N,N-trimethyl Chitosan Iodide (2)
Chitosan (1) (MW 30,000 g/mol) 3 g and 0.02 mol of glucosamine were dissolved in 150 mL of N-methyl-2-pyrrolidone (NMP) at room temperature. Then 5% w/v NaOH (3.0 mL) was added and continued stirring at 50 °C for 15 min. Subsequently, 3 mL (0.05 mol) of iodomethane (CH3I) was added in three portions at intervals of 4 h and continued stirring at 50 °C for 12 h. The reaction mixture becomes a yellow and clear solution. N,N,N-trimethyl chitosan iodide (2) was purified by precipitation using 300 mL of acetone. The N,N,N-trimethyl chitosan iodide (2) was used in the next step without further purification.
Synthesis of TMC (3)
N,N,N-trimethyl chitosan chloride (TMC) (3) was obtained by dissolving the precipitate of 2 in 15% (w/v) NaCl solution (150 mL), then an excess of chloride ion was eliminated by dialysis against water for 3 days (the water outside the dialysis bag was occasionally changed during dialysis process). TMC (3) was achieved by lyophilization using a freeze dryer (Dura-Dry™ Microprocessor Control, USA) [29].
Preparation of N-(4-N’-methyl pyridinyl methyl) Chitosan Chloride (mPyCS), (8)
N-(4-N’-methylpyridinylmethyl) chitosan chloride or mPyC (8), was synthesized in four steps (Fig. 2) using the method described [28]. The first step, N-(4-pyridinyl methyl) chitosan (5) was obtained by the reaction of chitosan (1) with 4-pyridine carboxaldehyde (4) in a mixture of 1% acetic acid and ethanol solution. The resulting product was further reduced using sodium cyanoborohydride (NaCNBH3) in a basic condition to give N-(4-pyridinyl methyl) chitosan (6) that was subsequently methylated using methyliodide in NMP in a basic condition to give N-(4-N’-methylpyridinylmethyl) chitosan iodide (7). The final product (8) was obtained by chloride ion exchange using a 15% NaCl solution.
Synthesis of N-(4-pyridinyl methyl) Chitosan (5)
Chitosan (1) 5 g (0.03 mol of glucosamine) was dissolved in 1% acetic acid 250 mL at room temperature. A solution of 4-pyridine carboxaldehyde (4) 0.03 g/mL in ethanol (70 mL) was added. The reaction mixture was stirred at room temperature for 24 h. The reaction mixture becomes a yellow suspension. The resulting product (5) was used in the next step without further purification.
Synthesis of N-(4-methylpyridinyl methyl) Chitosan (6)
The resulting mixture from the previous step was adjusted pH to 5 using 5% NaOH. Then, sodium cyanoborohydride (1.89 g, 0.03 mol) was added to the solution. The mixture was stirred at room temperature for 24 h. The solution was dialyzed against distilled water for 3 days (the water outside the dialysis bag was occasionally changed during the dialysis process) and lyophilization using a freeze dryer (Flexi-Dry™ Microprocessor Control, USA) to produce N-(4-methyl pyridinyl methyl) chitosan (6).
Synthesis of N-(4-N’-methyl pyridinyl methyl) Chitosan Iodide (7)
N-(4-pyridinyl methyl) chitosan (6) 3 g was dispersed in NMP 150 mL at room temperature. Then 5% w/v NaOH (3.0 mL) was added and continued stirring at 50°C for 15 min. Subsequently, 3 mL (0.05 mole) of iodomethane (CH3I) was added in three portions intervals during 4 h and continued stirring for 12 h at 50°C. The reaction mixture becomes a yellow and clear solution. N-(4-N’-methylpyridinylmethyl) chitosan iodide (7) was purified by precipitation using 300 mL of acetone and continued using in the next step.
Synthesis of N-(4-N’-methylpyridinylmethyl) Chitosan Chloride (8)
N-(4-N’-methylpyridinylmethyl) chitosan chloride (mPyCS) (8) was obtained by dissolving the precipitate of (7) in 15% (w/v) NaCl solution and stirred at room temperature for 2 h. Excess chloride ion was eliminated by dialysis against water for 3 days (the water outside the dialysis bag was occasionally changed during the dialysis process). mPyCS (8) was achieved by lyophilization and characterized by FT-IR and 1H-NMR spectroscopy and compared with the data from the previous report [28].
Preparation of 4-N‘, N‘, N‘-trimethylamine benzyl) Chitosan Chloride (TmBzCS, 13)
N-(4-N‘, N‘, N‘-trimethylamino benzyl) chitosan chloride, or TmBzCS (13), was synthesized in four steps (Fig. 3) using the method described [29]. N-(4-N‘, N‘-dimethylbenzyl) chitosan (10) was first obtained by the Schiff base formation reaction of chitosan (1) with 4-N, N-dimethylaminobenzaldehyde (9) in a mixture of 1% acetic acid and ethanol solution. The product was subsequently reduced by sodium cyanoborohydride (NaCNBH3) in a basic condition to give N-(4-N‘, N‘-dimethylaminobenzyl) chitosan (11). Then, (11) was subsequently methylated using methyliodide in NMP in a basic condition to give N-(4-N‘, N‘-dimethylbenzyl) chitosan iodide (12). The final product (13) was obtained by chloride ion exchange process using a 15% NaCl solution.
Synthesis of N-(4-N‘, N‘-dimethylbenzyl) Chitosan (10)
Chitosan (1) 5 g (0.03 mol of glucosamine) was dissolved in 1% acetic acid at room temperature. A solution of 4-N, N-dimethylaminobenzaldehyde (9) (0.03 g/mL) in ethanol 70 mL was added. The reaction mixture was stirred at room temperature for 24 h. The reaction mixture becomes a yellow suspension. The resulting product was utilized in the next reaction without further purification.
Synthesis of N-(4-N‘, N‘-dimethylaminobenzyl) Chitosan (11)
The reaction mixture which was obtained from 3.3.1.3.1 was adjusted pH to 5 using 5% NaOH. Then, sodium cyanoborohydride (1.89 g, 0.03 mol) was added to the solution. The mixture was stirred at room temperature for 24 h. The solution was dialyzed against distilled water for 3 days (the water outside the dialysis bag was occasionally changed during the dialysis process) and lypophillized to produce N-(4-N‘, N‘-dimethylaminobenzyl) chitosan (11).
Synthesis of N-(4-N‘, N‘-dimethylaminobenzyl) Chitosan Iodide (12)
N-(4-N‘, N‘-dimethylaminobenzyl) chitosan (11) 3 g was dispersed in NMP 150 mL at room temperature. Then 5% w/v NaOH (3.0 mL) was added and continued stirring at 50 °C for 15 min. Subsequently, 3 mL (0.05 mol) of iodomethane (CH3I) was added in three portion intervals during 4 h and continued stirring for 12 h at 50 °C. The reaction mixture becomes a yellow and clear solution. N-(4-N‘, N‘, N‘-trimethylaminobenzyl) chitosan iodide (12) was purified by precipitation using 300 mL of acetone and used in the next step without further purification.
Synthesis of N-(4-N‘ ,N‘, N‘-trimethylaminobenzyl) Chitosan Chloride (13)
N-(4-N‘, N‘, N‘-trimethylaminobenzyl) chitosan chloride (TmBzCS, 13) was obtained by dissolving the precipitate from 3.3.1.3.3 in 15% (w/v) NaCl solution and continued 1 h. Excess sodium chloride was eliminated by dialysis against water for 3 days (the water outside the dialysis bag was occasionally changed during the dialysis process). TmBzCS (13) was achieved by lyophilization and characterized by FT-IR and 1H-NMR spectroscopy and compared with the previous report [29].
Characterization of Q-CS
Proton Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
1H NMR spectra of TMC (3), mPyCS (8), and TmBzCS (13) were obtained by FT-NMR spectrometer (500 MHz, Unity Inova, Varian, Germany) using D2O as a solvent for all Q-CS. The percent degree of quaternization (%DQ) was determined from 1H-NMR spectra and calculated using the equations given in Table I [36].
FTIR, DSC and XRD Analysis
FT-IR spectra of TMC (3), mPyCS (8), and TmBzCS (13) were determined by FT-IR spectrometer (Spectrum One, Perkin Elmer Ltd., UK). About 5 mg of the sample was mixed with 50 mg of dried potassium bromide (KBr) in a mortar. The KBr disk was obtained by compressing the powder under force in a hydraulic press. The transmission of the sample was recorded in a range of 450 to 4000 cm−1.
Thermal characteristics of TMC (3), mPyCS (8), and TmBzCS (13) were determined by Differential Scanning Calorimeter (DSC) (Perkin Elmer DSC7, USA). Accurately weighed each sample (5–8 mg) was placed in a sealed aluminum pan. An empty aluminum pan was served as a reference. Samples were heated on a Pyris 7 DSC (Perkin-Elmer, USA). The heating rate was 10 °C/min under and nitrogen purge at 50 mL/min.
The physical state of TMC (3), mPyCS (8), and TmBzCS (13) were assessed by the XRD technique. Powder X-ray diffraction spectra of the samples were obtained at room temperature using a powder X-ray diffractometer (Philips X’Pert MPD, Netherlands) with Cu as an anode material and graphite monochromator operating at a voltage of 40 kV. The samples were analyzed in a 2θ angle range from 20 o to 40 o. The process parameters were set as a step size of 0.05 o(θ) and a scan step time of 1.0 s.
Scanning Electron Microscopy
The morphologies characteristics of TMC (3), mPyCS (8), and TmBzCS (13) were observed by scanning electron microscope (SEM, Quanta 400, FEI, Czech Republic). The sample was mounted on a brass stub and then coated with gold by sputtering technique. The samples were then observed under vacuum at magnification ranging from 1,000 to 5,000.
Water Solubility test
The water solubility of the synthesized Q-CS derivatives was carried out by weighing 100 mg of Q-CS derivatives and transferring them to the respective test tube as described [37]. Then 200 µL of distilled water into test tubes using a micropipette at room temperature. The mixture was shaken and checked for solubility. If the compound was not dissolved, another 200 µL of distilled water was added until a clear solution was obtained. The accurate solubility value was calculated.
Preparation of Q-CS-IND Composites
Preparation of Q-CS-IND Composites by Physical Mixing
Physical mixtures of IND and Q-CS were obtained by mixing with a spatula until getting homogeneity. The various weight ratios of IND: Q-CS (1:1, 1:2, 1:3, and 1:5) were chosen in this preparation.
Preparation of Q-CS-IND Composites by Spray Drying Method
SD in Q-CS: drug composites in various weight ratios (IND: Q-CS = 1:1, 1:2, 1:3, and 1:5) were obtained by spray drying technique as follows. Q-CS solutions (4% w/v) were prepared by dissolving 2 g of Q-CS in distilled water (50 mL) at room temperature. IND (2 g, 1 g, 0.67 g, and 0.4 g according to the ratio) was dissolved in 95% ethanol and then added to the Q-CS aqueous solution while stirring. The mixture was stirred at room temperature for 24 h and the resulting solution was subjected to a spray dryer (Mini Spray Dryer B-290, BÜCHI, Switzerland) (inlet temp = 100 °C, outlet temp = 100 °C) to obtain the composites as the dried powder.
Determination of Drug Content in the Composites
The drug content capacity of Q-CS-IND composites prepared by physical mixture and SD was determined by accurately weighing the amount of the sample about 10 mg and transferring it to a 100 mL volumetric flask. A solution of 50% methanol in water was added and adjusted to volume. A 5 mL of this solution was transferred to a 10 mL volumetric flask and adjusted to 10 mL with distilled water. The IND content was analyzed by measuring absorbance using a UV-Vis spectrophotometer (Hewlett Packard 8452 A, Diode Array Spectrophotometer, USA) at 318 nm. The experiment was performed in triplication. The concentration of IND in sample solutions was calculated using the regression equation of the standard curve of IND standard solutions and expressed as %drug content.
Physicochemical Characterization
The physicochemical properties of IND-Q-CS composites both prepared by physical mixing and spray drying methods were studied and compared with pure drug.
Fourier Transform Infrared (FT-IR) Spectroscopy
FT-IR spectra of IND, Q-CS-IND physical mixtures, and Q-CS-IND SD composites were determined by FT-IR spectrometer (Spectrum One, Perkin Elmer Ltd., UK). The potassium bromide pellet method was used for determination. About 5 mg of the sample was mixed with dried potassium bromide 50 mg in a mortar, the KBr disk was obtained by compressing the powder under force in a hydraulic press. The transmittance of the sample was recorded in a frequency range of 450 to 4000 cm−1.
Characterization by Using Differential Scanning Calorimetry (DSC)
Thermal characteristics of pure IND, Q-CS-IND physical mixtures, and Q-CS-IND SD composites containing various proportions of IND in Q-CS were determined by Differential scanning calorimeter (Perkin Elmer DSC7, USA). Accurately weighed samples (5–8 mg) were placed in sealed aluminum pans. An empty aluminum pan was served as a reference. Samples were heated on a Pyris7 DSC (Perkin-Elmer, USA). The heating rate was 10 °C/min under the nitrogen stream with a flow rate of 50 mL/min.
Characterization by Powder X-ray Diffractometry (P-XRD)
The physical state of pure IND, Q-CS-IND physical mixtures, and Q-CS-IND SD composites were assessed by the XRD technique. Powder X-ray diffraction spectra of the samples were obtained at room temperature using a powder X-ray diffractometer (Philips X’Pert MPD, Netherlands) with Cu as an anode material and graphite monochromator operating at a voltage of 40 kV. The samples were analyzed in a 2θ angle range from 20o- 40 o. The process parameters were set as a step size of 0.05 o(θ) and a scan step time of 1.0 s.
Morphologies Observation
The morphologies characteristics of pure IND, Q-CS-IND physical mixture, and Q-CS-IND SD composites were observed by Scanning Electron Microscope (SEM, Quanta 400, FEI, Czech Republic). These samples were mounted on a brass stub and then coated with gold by sputtering technique. The samples were then observed under vacuum at magnification ranging from 500 to 15,000.
Solubility Studies
The solubility of pure IND and Q-CS-IND physical mixtures and SD composites of Q-CS-IND were studied using the modified method described by [8]. The sample equivalent to about 10 mg of IND was added to test tubes containing 10 mL of distilled water (pH = 7.10). The tube was shaken in a water bath shaker (WB-14, Memmert, Germany) at 37 ± 0.5 °C until equilibrium for 24 h. Then, the supernatant was filtered through a 0.22 μm membrane filter. The filtrate was suitably diluted with distilled water and determined concentration of dissolved IND was by measuring absorbance with a UV-Vis spectrophotometer (Hewlett Packard 8452 A, Diode Array Spectrophotometer, USA) at 318 nm. The experiment was performed in triplicate.
Dissolution Studies
The dissolution of pure IND, from the physical mixture, and the SD were determined. The dissolution studies were modified from USP32 (USP32 & NF27) using USP dissolution apparatus type II (the paddle method). The dissolution mediums (750 mL) were phosphate buffer pH 6.8 maintained at 37 ± 0.5 °C. The paddles rotations were set at 100 rpm. A sample containing about 20 mg of IND was added to the surface of the dissolution medium. At suitable time intervals (5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 90, 120, 150, and 180 min), a 2.0 mL aliquot sample was withdrawn from the dissolution medium and the same volume of preheated dissolution medium was infused into the dissolution flask after each sample was taken to maintain a constant volume of the dissolution medium throughout the test. The withdrawn sample solution was filtered through a 0.22 μm membrane filter before measurement. The amount of IND was determined by UV-Vis spectrophotometer (Hewlett Packard 8452 A, Diode Array Spectrophotometer, USA) at 318 nm. The concentration of IND was calculated using the regression equation of the standard curve of IND standard solutions and expressed as a percentage of drug dissolved from the mean of three determinations.
UV-Visible Spectrophotometric Method Validation
The drug content capacity of Q-CS-IND composites, drug solubility, and dissoluble drugs were analyzed by UV-Vis spectrophotometric method. This technique was utilized in this study due to its simplicity, rapidity, and suitability for the quantitation of IND in the samples. The spectroscopic method is also economical in terms of cost-effectiveness and time consumption.
Instrumental and Analytical Conditions for UV-Visible Spectrophotometric Method
UV-visible spectrophotometric analyses were carried out on a Hewlett-Packard 8452 A Diode Array Spectrophotometer. The UV-visible spectrum of IND solutions was recorded in a range of 200 to 800 nm. In the spectral analysis, the wavelength 318 nm was defined for the quantitation of IND.
Preparation of Standard Solutions for Spectrophotometric Analysis
IND stock standard solutions were prepared by approximately weighing about 100 mg of IND and transferred to a 100 mL volumetric flask. Methanol was added and adjusted to 100 mL and mixed well to obtain a solution having a concentration of 1000 µg/mL. This solution was used to study for the validation of the spectrophotometric method.
Validation of the Assay
The method validation was performed according to the International Conference on Harmonization (ICH) guidelines for validation of analytical procedures (ICH, 1996).
Linearity and Calibration Curve
IND standard solutions of 10, 15, 20, 25, and 30 µg/mL were prepared by transferring 100, 150, 200, 250, and 300 µL of the IND stock standard solution into a 10 mL volumetric flask. Methanol was added and adjusted to volume and mix well. Three replicate analyses of each solution were performed in a day. Three determinations were carried out for each solution (n = 3). Linearity was obtained by plotting the absorbance values at 318 nm against the concentrations of the standard solutions and analyzed using the linear least squares regression equation. Linearity was expressed as a coefficient of determination (r2) which should be more than 0.999.
Precision
The measurements of intra-day and inter-day variability were utilized to determine the precision of the method. For intra-day precision, five concentrations of IND standard solutions at 10, 15, 20, 25, and 30 µg/mL in the calibration range were prepared with methanol in triplicate (n = 3). For inter-day precision of IND standard solutions were assessed by repeating the intra-day precision on three different days. Concentrations of IND standard solutions from the experiments were calculated according to the linear equation of the standard curve. Precision was calculated and expressed as a percentage relative standard deviation (%RSD).
Accuracy
The accuracy of the method was performed by recovery study using the method of standard additions. The recovery was determined by recovery of known amounts of IND reference standard used at three concentration levels of 16, 20, and 24 µg/mL (80%, 100%, and 120%). The Q-CS (100 mg) was placed in 100 mL of distilled water in a 100 mL Erlenmeyer flask. The IND reference standard was added in a different amount to each flask to obtain the final solution having concentrations of 16, 20, and 24 µg/mL. The samples were sonicated for 30 min. All solutions were prepared in triplicate. The clear solution was taken to determine the amount of IND by using a UV-Vis spectrophotometer at 318 nm. The percentage recovery of IND was calculated using a linear equation of the standard curve.
Specificity
Specificity is a method of producing a response for only an analyte accurately and specifically in the presence of other components in the sample matrix. The specificity of the analytical method was confirmed by analysis of 20 µg/mL of IND standard solution in methanol. The resulting solutions were used to assess the UV-Vis absorption spectrum in a range of 200–400 nm. The specificity was performed by assessing the presence of only the IND spectrum at 318 nm without the inference absorption from the absorption spectra of Q-CS (TMC, mPyCS, and TmBzCS).
The solution of quarternized chitosans was prepared by dissolving each Q-CS of about 100 mg in distilled water (100 mL) and stirring at room temperature with a magnetic stirrer for 1 h. A 2 mL of this solution was transferred to a 10 mL volumetric flask and adjusted to 10 mL with distilled water to obtain the Q-CS solutions having a concentration of 20 µg/mL.
Limit of Detection (LOD) and Limit of Quantitation (LOQ)
The limit of detection is the lowest concentration of analyte that is detectable at the most sensitive instrument settings but not necessarily quantitated, under the stated experimental conditions. The limit of quantification is the lowest concentration of analyte that can be determined with acceptable precision and accuracy, under the stated experimental conditions. Standard solutions of IND were analyzed in the concentrations of 10, 15, 20, 25, and 30 µg/mL. LOD and LOQ were determined based on the response and slope of the regression equation from the calibration curve using the following equations (ICH, 19,946).
- σ:
-
The standard deviation of the response
- S:
-
The slope of the calibration curve
Statistical Analysis
The results from the study were compared with determined by using one-way ANOVA. The differences were considered statistically significant when p < 0.05. The statistical analysis was performed by using the Statistical Package for the Social Sciences (SPSS) Version 17.0 software package.
Results and Discussion
Q-CS Derivatives
Three types of Q-CS were successfully synthesized by the previously reported procedures [28, 29]. TMC (3) is an N-alkyl substituted derivative, whereas, mPyCS (8) and TmBzCS (13) are N-aryl substituted derivatives. TMC (3) was obtained as a yellow solid (Fig. 4) with a 60.94% yield after the lyophilization process with a 55.13% (DQ). TMC was well dissolved with a solubility value of 12.25 mg/mL in water. Similarly, mPyCS (8) was obtained as a yellow solid in 63.37% yield. It was found that the %(DQ) of the primary amine of chitosan (%DQ-CS) was 15.51% and the (DQ) at N-atom of pyridine ring (%DQpy) was 34.56% and therefore gave the total (DQ) equal to 50.07%. mPyCS was well dissolved with a solubility value of 16.25 mg/mL. TmBzCS (13) was obtained as a yellow solid in a 62.42% yield. The (DQ) of 13 was determined by 1H-NMR spectroscopy and found that the %(DQ) of primary amines of chitosan (%DQ-CS) was 20.29% and %(DQ) at aminobenzyl groups was 47.03%. Hence, the total (DQ) of 13 was 67.32%. The water solubility value of 13 was 17.88 mg/mL. The water solubility of Q-CS was increased when increasing the %DQ due to more quaternary ammonium group. However, mPyCS with a DQ of 50.07% was found with higher solubility in water than TMC with a DQ of 55.13%. That may be due to mPyCS containing more hydrophilic groups than TMC. The water solubility of chitosan (MW 3000) was < 0.025 mg/mL and from the definition of USP and it is in the range of description of practically insoluble [38]. The synthesized Q-CS could enhance water solubility. The water solubility of TMC, mPyCS, and TmBzCS were 12.52, 16.25, and 17.88 mg/mL in the range description form sparingly soluble.
Characterization of Q-CS
The synthesized chitosans were characterized by 1H-NMR and FT-IR. The results of 1H-NMR and IR spectrum of TMC are displayed in Fig. 4. While the FTIR and Proton NNR spectra of mPyCS and TmBzCS are given in Figs. 2S and 3S. The FT-IR spectra of the obtained quarternized derivatives were similar to the previous report [18, 27, 39] which shows the characteristic of quaternary ammonium substituents. The absorption band at 1476.4 cm−1 of TMC, at 1473.7 cm−1 of mPyCS, and 1474.3 cm−1 of TmBzCS are due to the C-H symmetric bending of methyl group on quaternary ammonium substituents [40]. All the spectra exhibited the characteristic 1H-NMR pattern of chitosan i.e. the multiplet at 4.4–3.5 ppm due to protons on chitosan rings and at 2.2–2.4 ppm due to the N-acetyl protons of GlcNAc, respectively. It exhibits characteristic resonances at δ 3.2 which is attributed to the N, N, N trimethyl protons [41]. Resonance signals at δ 3.42 are assigned to the N - methyl pyridinyl protons, and two doublet of a doublet at 8.3–9.1 ppm due to the protons of the pyridine ring [42]. Because the peaks of pyridine ring protons did not overlap the proton resonances of the GlcN, therefore, the integral area of pyridine ring protons signals in 1H-NMR was used to determine the degree of substitution (Table I). The signals of aromatic protons appear as a doublet in the region of δ 7.95–8.2 ppm. The broad signal at δ 4.4 ppm belongs to the benzylic protons of the N-benzyl group, and at δ 3.9, 3.35, and 3.1 ppm are assigned to N-methyl, N, N, N-trimethyl, and N, N-dimethyl protons, respectively [28, 29, 43]. The degree of substitution was calculated based on the integral area of aromatic protons since these proton signals appear in the downfield region compared to the GlcN protons.
Preparation and Characterization of Q-CS-Drug Composites
Product Yield and %drug Content
Q-CS (TMC, mPyCS, and TmBzCS) based composites of IND in different weight ratios (IND: Q-CS, 1:1, 1:2, 1:3, and 1:5) were prepared by spray drying method, and their product yields and % drug content in each composite are summarized in Table II. All TMC: IND composites were obtained as a light yellow dried powder (Figs. 14S and 15S). SD composites of TMC: IND in weight ratios 1:1, 1:2, 1:3, and 1:5 were obtained with yields of 71.27, 78.59, 79.54, and 76.33% respectively. The composites of mPyCS: IND prepared through the physical mixing process were achieved as dark yellow dried powder (Fig. 16S) while the mPyCS-based SD composites prepared through the spray drying method were achieved in blackish-yellow color (Fig. 17S). Slightly lower product yields were obtained when compared to that of TMC: IND composites. The product yields of mPyCS: IND composites in weight ratios of 1:1, 1:2, 1:3, and 1:5 were 69.13, 67.18, 71.21 and 70.03%, respectively. The TmBzCS-IND composites were obtained as light yellow dried powder (Figs. 18S and 19S). TmBzCS-IND composites in weight ratios 1:1, 1:2, 1:3, and 1:5 gave the product 61.11, 62.37, 60.62 and 60.47%, respectively. High percent drug contents (from 97.66 to 100.25%) were observed in all SD composites (Table II).
Composites of quarternized chitosans (TMC, mPyCS, and TmBzCS) with IND in different weight ratios (IND: Q-CS, 1:1, 1:2, 1:3, and 1:5) were also prepared by the physical mixing method and their product yields and % drug contents in each mixture are summarized in Table II. Mixtures of TMC: IND in weight ratios 1:1, 1:2, 1:3, and 1:5 were obtained at 99.67, 99.24, 99.17, and 99.79%. The product yields of mPyCS: IND mixtures in weight ratios of 1:1, 1:2, 1:3, and 1:5 were 99.54, 100.01, 99.92, and 99.68%, respectively. TmBzCS - IND physical mixtures in weight ratios 1:1, 1:2, 1:3, and 1:5 gave the product 99.32, 99.53, 99.24 and 100.02%, respectively. High percent drug contents similar to that observed for SD were observed in all composites, in the range of 99.48 to 100.17%.
FT-IR Spectroscopy
FT-IR Spectroscopy is a useful method to detect the interaction of functional groups of IND Q-CS in Q-CS-IND composites. FT-IR spectrum of pure IND (Fig. 4S) shows absorption bands of the carboxylic group at 1717 cm−1 and of the amide group at 1691 cm−1. The findings of the current study were consistent with previous reports [44]. This absorption remained in the FT-IR spectrum obtained from the composites prepared by the physical mixing process using TMC (Fig. 5a), mPyCS, or TmBzCS (Figs. 5Sa-6Sa) at different weight ratios. The results indicated that no interaction between both carbonyl moieties of IND, with any functional groups of Q-CS in physical mixtures.
In contrast, the results obtained from the FT-IR spectrum of the SD composites prepared by spray drying method. The carbonyl peaks of both carboxylic and amide groups of IND were shifted to the lower frequencies in all types of composites prepared by using TMC (Fig. 5b), mPyCS (Fig. 5Sb) and TmBzCS (Fig. 6Sb). Two carbonyl peaks were observed in the FT-IR spectrum of Q-CS-IND composites prepared by using different weight ratios (1:1, 1:2, 1:3, and 1:5) at about 1592 and 1550 cm−1. The possible causes of the peak shifting could be due to some interactions between functional groups of IND with functional groups of Q-CS. The major interactions could be hydrogen bonding between carbonyl of amide and carbonyl of carboxylic groups with protons on the quaternary ammonium groups or with protons on the chitosan chain [45]. Another possible interaction that may occur in the composites is the primary ionic interaction due to the deprotonation of the carboxylic group to give carboxylate moieties and interact with the positively charged amino group of the chitosan network (Fig. 7S). It is also evident from the FT-IR spectra which show a shift in the absorption band to 1590–1595 cm−1 (Fig. 5).
Our results are consistent with previous reports [46]. They prepared SD composites of IND by solvent evaporation method and using three types of poly-(vinylpyrrolidone) (PVP 12PF, PVP K30, and PVP K90) as carriers. In FT-IR spectra of the SD showed the shift of all C = O peaks of IND in the co-evaporated system to 1614, 1590 and 1577 cm-1, respectively They explained that the hydrogen bonding formation between the amide carbonyl of PVP and the hydroxyl group of the IND would occur [46]. Patel and co-workers prepared SD composites of tacrolimus by a combination of melt and adsorption techniques and using Gelucire 44/14® and Gelucire 50/13® as carriers [47]. In FT-IR spectra tacrolimus shows C = O (ester and ketone) stretching vibrations at 1,733 and 1,690 cm-1. However, in SD composites, the absorption bands of 1733 and 1690 cm-1 were weakened and shifted to a lower frequency. They suggested that the C = O functional groups of tacrolimus interacted with the functional group of Gelcuire® at the molecular level in SD composites.
Differential Scanning Calorimetric (DSC) Analysis
Differential scanning calorimetry is a thermal analysis technique used to measure changes in heat flows associated with material transition. DSC is commonly utilized to determine the glass transition temperature and crystalline melting point of polymeric material [48]. In this study, all samples including pure IND and composites obtained from both physical mixing and spray drying methods were subjected to DSC analysis. DSC thermogram of pure IND shows a melting endothermic peak at about 162.16 °C (Fig. 6). Previous reports show the melting point range of IND in the range of 158–165 °C [9, 49]. TMC (Fig. 6), mPyCS (Figs. 8S and 9S), and TmBzCS displayed broad endothermic peaks at 123.54 °C, 132.82 °C, and 146.50 °C, respectively. Sharp melting endotherm peaks of IND were still observed in DSC thermograms of physical mixtures of TMC-IND with a slight shift in melting point. TMC-IND physical mixtures in weight ratios of 1:1, 1:2, 1:3 and 1:5 showed melting endothermic peaks at 164.48 °C, 161.95, °C, 158.37 °C and 147.65 °C, respectively. In contrast to the DSC thermograms of TMC-IND composites prepared by the spray drying method, no sharp endothermic peak was observed at any weight ratio. Broad peaks with back tailing were detected in DSC thermograms of mPyCS-IND physical mixtures. The melting endotherm of mPyCS: IND physical mixtures at weight ratios of 1:1, 1:2, 1:3, and 1:5 were found at 151.11 °C, 151.14, °C, 140.05 °C and 140.03 °C, respectively. No melting endothermic peaks were observed in all DSC thermograms of mPyCS-IND composites prepared by the spray drying method. Moreover, DSC thermograms of TmBzCS-IND physical mixtures in the weight ratios of 1:1, 1:2, 1:3, and 1:5 demonstrated the melting endothermic peaks of IND at 158.60 °C, 157.02, °C, 156.33 °C and 156.30 °C, respectively. No endothermic peak was noticed in the DSC thermograms of TmBzCS-IND composites prepared by the spray drying method at any weight ratio. Overall, these results demonstrated that IND in all physical mixtures using TMC, mPyCS, and TmBzCS as a drug carrier, remained in the crystalline state. Broaden with less intense peaks of IND in all DSC thermograms of physical mixtures indicated a slight decrease in its crystallinity due to the mixing process. The disappearance of the endothermic peaks of IND in the DSC thermograms of IND composites prepared by spray drying methods indicates the amorphization of the drug that caused the increase in the water solubility of IND. The findings of this study were in correlation with previous reports on the thermal properties of IND composites prepared by physical mixing and spray drying methods [9, 50].
Powder X-Ray Diffractometry (P-XRD)
Powder X-ray diffraction (PXRD) is a rapid analytical technique primarily used for phase identification and classification of materials. The advantage of this method includes rapid and non-destructive analysis of multi-component mixtures without the need for extensive sample preparation. This technique could also be able to use for the analysis of material interaction characteristics [51]. Samples that have crystallinity properties show a crystallinity pattern consisting of a series of sharp peaks, amorphous materials, however, produce broad background signals. In this study, powder X-ray diffractometry was used to determine the crystallinity properties of pure IND and IND in composites both prepared by physical mixing and spray drying methods at various weight ratios. The powder X-ray diffractometry pattern of pure IND shows several distinctive sharp peaks at 20.29, 21.81, 22.81, 24.03, 26.63, 29.35, 30.29, 33.49 (2θ) indicates crystalline structure (Fig. 7). These sharp peaks of IND were still observed in the PXRD patterns of all physical mixtures prepared by using TMC (Fig. 7), mPyCS (Fig. 10Sa), and TmBzCS (Fig. 11Sa) as diluents, but at lower intensity. Therefore, IND’s presence in physical mixtures remains in the crystalline state. The height of the peaks varied from the highest of 1:1 to the smallest of 1:5, depending on the diluting factor from the preparation procedure. In contrast to the PXRD patterns which were obtained from the SD, IND changed the solid state of the drug from crystalline to amorphous. SD composites obtained by using TMC, mPyCS, and TmBzCS gave similar results, demonstrating that IND in these SD was in amorphous form. Our findings are in correlation with previous reports by [52]. Similarly, Fujii and coworkers (2005) studied the SD composites of IND using crospovidone (CrosPVP) as a carrier [53]. Five formulations of SD without solvent were prepared by dissolving the drug in a molten carrier followed by cooling and subjected to PXRD determination. The result found that no IND peak was observed in all PXRD patterns of SD composites. The authors suggested that IND changed its crystallinity to an amorphous state due to the spray drying process could reduce drug particle size and polymer was observed to reduce crystal growth rates relative to the growth rate of the drug.
Scanning Electron Microscopy
The SEM micrographs of the synthesized TMC, mPyCS, and TmBzCS are illustrated in Fig. 8. All the obtained Q-CS have shown a bulky appearance. TMC existed as a loose flake, mPyCS existed as a compact bulky structure and TmBzCS existed as a loose bulky structure. IND consisted of a mixed size of crystalline particles with different shapes (Fig. 9a). In physical mixtures, IND was found distributed among the carrier particles. TMC-IND physical mixture (Fig. 9b) showed IND crystals mixed with TMC particles. In the mPyCS-IND physical mixture (Fig. 9C) smaller sizes of IND crystals were found distributed along with the mPyCS carrier. IND crystals were observed intercalated within the TmBzCS particles (Fig. 9d). Their changes affect the drug solubility property. Different morphologies were observed when using different carriers and the samples were prepared by the spray drying method. The surface appearances of SD composites of IND were different from the composites prepared through physical mixing. SEM of SD composites based on TMC-IND was displayed in Fig. 10 which were prepared by using 1:1 (a), 1:2 (b), 1:3 (c), and 1:5 (d) IND: Q-CS weight ratios, respectively. The crystal characteristic of IND and the original morphology of TMC disappeared and new morphologies with irregular sizes were presented. A compact rod-like structure aggregated to form a large particle was seen in TMC-IND (1:1 ratio), whereas irregular shape particles were detectable in TMC-IND 1:2, 1:3, and 1:5 ratios. It is noticeable that hollow spheres were observed in SD composites based on TMC-IND at 1:2, 1:3, and 1:5 ratios, to different extents and sizes. The mPyCS-based composites showed needle-like particles mixed with hollow sphere particles but to a different extent (Fig. 12S). This might be due to the limited surface recrystallization of IND when the carrier was used in a higher concentration. The thermal analysis did not show any peak for the respective crystal which indicates that the recrystallization of IND is low and has no effect on the dissolution rate of IND from the respective mPyCS-based SD composites [54, 55]. However, in the case of using TmBzCS as a drug carrier in SD preparation, only hollow spheres were observed. In a 1:1 ratio, the spheres were molten to form a matrix particle (Fig. 8a), whereas, obviously seen hollow spheres were found in other TmBzCS-IND composites prepared through spray drying in 1:2, 1:3 and 1:5 ratios (Fig. 13b and d). The size of those hollow spheres varied from less than 1 μm to 3 μm. The disappearance of IND crystals in SD composites could be due to the reduction in particle size and could be able to incorporated into the polymer particle [56]. The formation of hollow spheres with varying particle sizes could be because of using the spray drying technique. The bubbles which were the same during the spraying process, collapsed or burst during the drying process to give opened or hollow spheres [57].
Solubility Studies
The aqueous solubility of a drug is a primary concern of its dissolution rate. The solubility of IND and all Q-CS-IND composites (10 mg) in 10 mL in distilled water (pH = 7.1) were performed at room temperature and the results are summarized in Figs. 11 and 12. The solubility of pure IND was found to be 0.227 ± 0.078 mg/mL. The intrinsic aqueous solubility of IND is reported to be 8.8 µg/mL [58]. Both physical mixtures and SD composites could enhance the water solubility of IND (Figs. 11 and 12). The solubility of physical mixture samples of TMC-IND composites was in the range of 0.313 ± 0.069 to 0.489 ± 0.067 mg/mL. The solubility of physical mixture samples of mPyCS-IND composites was slightly higher than TMC-IND composites in the mixture in the range of 0.424 ± 0.079 to 0.997 ± 0.057. The highest solubility of Q-CS-IND composites prepared by the physical mixture method was observed in TmBzCS-IND composites in the range of 0.453 ± 0.069 to 0.569 ± 0.066. The solubility of physical mixtures of TMC-IND composites was significantly (p < 0.05) lower than mPyCS-IND and TmBzCS-IND composites prepared by the physical mixture method. When increasing the amount of Q-CS was used in the preparation process, the solubility of IND was increased in all samples. The solubility of IND-Q-CS physical mixtures in weight ratios 1:3 were significantly (p < 0.05) higher than the physical mixtures in weight ratios 1:1 and 1:2 but, the solubility of IND in IND-Q-CS physical mixtures in weight ratios 1:3 was not significantly different when compared with 1:5 (p > 0.05). The physical mixtures of TMC-IND, mPyCS-IND, and TmBzCS-IND composites could improve the water solubility of IND by 2.15 folds, 2.48 folds, and 2.50 folds in weight ratios of 1:5, respectively.
The solubility of IND in SD composites was significantly (p < 0.05) higher than in physical mixture samples at the same drugs-to-polymer ratio. The solubility of IND-Q-CS-based SD composites in weight ratios 1:3 was significantly (p < 0.05) higher than in weight ratios 1:1 and 1:2. However, the solubility of IND in IND-Q-CS-based SD composites in weight ratios 1:3 was not significantly (p > 0.05) different when compared to that at the weight ratio 1:5. The SD composites based on TMC-IND, mPyCS-IND, and TmBzCS-IND composites could improve water solubility of IND by 4.25 folds, 4.39 folds and 4.40 folds respectively in weight ratio of 1:5. Several factors may be attributed to these results, firstly, the SD preparation technique could reduce the drug’s particle size to the molecular level. Secondly, Q-CS carriers can assist in the solubilizing of the drug by enhancing of wettability of the drug. Better solubility of IND was observed in SD composites than in physical mixtures could also be due to the better enhancement of the surface area and the higher dispersed state of the drug in the carriers. Moreover, the effect of the preparation procedure of drug: carriers’ solid dispersion could cause IND to become amorphous and increase the solubility of IND, whereas, IND in a physical mixture remained in crystalline form. These can be explained by the powder X-ray diffraction patterns and DSC thermograms. In addition, the ionic interaction between the molecule of the drug with the carriers may enhance the ionizable property of the drug. Since water solubility of the acidic drugs could be improved by additional basic reagents during solution preparation. The Q-CS contains basic functional groups (-NH2) that could be utilized as the basic reagent and hence deprotonate the protons from the carboxylic group of IND to give an ionized form of IND, which has better water solubility property. Our findings are in correlation with previous reports [59].
Dissolution Profile
The in vitro dissolution profiles of indomethacin (IND) from Q-CS-IND composites in various weight ratios (Q-CS: IND = 1:1, 1:2, 1:3, 1:5) were evaluated in phosphate buffer (pH 6.8) over time intervals of 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 90, 120, and 180 min (Fig. 13a and f). Pure IND dissolved slowly from 5 to 45 min, reaching a maximum dissolution of 44.32% (0.012 mg/mL) with no further increase until 180 min. IND has a pKa of 4.5 and water solubility of 0.937 mg/L at room temperature (Windholz, 1976). Given that the phosphate buffer pH was 6.8, a pH higher than its pKa, IND was able to dissolve due to its ionizable nature, although its solubility was limited to 0.012 mg/mL. Figure 13a shows the dissolution profiles of IND in phosphate buffer (pH 6.8) from TMC-based SD composites in different ratios (IND: Q-CS = 1:1 to 1:5). All SD composites produced significantly higher dissolution rates for IND compared to pure IND. The drug release from SD composites began within the first 5 min and continued, reaching a maximum at 45 min for 1:3 and 1:5 ratios, and at 60 min for 1:1 and 1:2 ratios. The maximum percentage of drug dissolved from IND: TMC-based SD in ratios of 1:1, 1:2, 1:3, and 1:5 were 95.65%, 98.63%, 100.12%, and 100.26%, respectively. SD composites of TMC: IND in all ratios showed significantly better in vitro release characteristics than TMC: IND physical mixtures (p < 0.05) (Fig. 13b). TMC-IND composites prepared by physical mixing in all ratios exhibited a significantly higher dissolution rate (p < 0.05) than pure IND. The physical mixture of IND: TMC in ratios of 1:3 and 1:5 showed similar release patterns, achieving a maximum drug release of 70% at 45 min, which was significantly higher than the physical mixture in ratios of 1:1 and 1:2.
Dissolution profiles of a IND and TMC-IND composites prepared by spray drying in weight ratios 1:1, 1:2, 1:3, and 1:5; b IND and TMC-IND composites prepared by physical mixing in weight ratios 1:1, 1:2, 1:3 and 1:5; c IND and mPyCS-IND composites prepared by spray drying in weight ratios 1:1, 1:2, 1:3, and 1:5; d IND and mPyCS-IND composites prepared by physical mixing in weight ratios 1:1, 1:2, 1:3 and 1:5; e IND and TmBzCS-IND composites prepared by spray drying in weight ratios 1:1, 1:2, 1:3, and 1:5; f IND and TmBzCS-IND composites prepared by physical mixing in weight ratios 1:1, 1:2, 1:3 and 1:5
Figure 13c and f show the dissolution profiles of IND in SD composites, physical mixture (PM) using TMC as a carrier at the same weight ratios and pure IND at the same results were observed in all weight ratios. SD composites consistently displayed significant enhancement in the IND solubility higher than that of physical mixtures. Moreover, physical mixtures could also significantly improve IND solubility better than pure IND. The drug dissolution rate (DR) in the first 20 min [60] was calculated and summarized in Table III. The rate could increase from 388.56 µg/min to about 486–822.4 and 1436.2–1611.2 µg/min from physical mixtures and SD composites, respectively. Similar results were observed when mPyCS was applied as the carrier. mPyCS: IND SD composites in all weight ratios (Fig. 13c) could enhance IND solubility much better than pure IND. All composites showed a release of IND up to a maximum (almost 100%) in 20 min. IND: mPyCS composites in weight ratios 1:3 and 1:5 gave the best release patterns, followed by 1:2 and 1:1, respectively. The highest drug solubility was observed at 35 min and the release was in the range of 63.15–68.80%. At the same weight ratio, SD based on mPyCS as a carrier demonstrates better drug solubility enhancement than physical mixtures (Fig. 13c and d). In the case of using mPyCS as a carrier, the 20 min drug dissolution rate could improve from 388.56 µg/min to about 998.9–1139.6 µg/min from physical mixtures and to about 1856–1984.8 µg/min from the SD composites. Almost the same effect was observed when using TmBzCS as a carrier compared to that of mPyCS. SD composites in all weight ratios showed better in-vitro release characteristics than those physical mixtures and pure IND. The drug could release up to almost 100% within 20 min (Fig. 13e). The highest drug release could be seen from the SD composites using TmBzCS: IND in weight ratios 1:3 and 1:5. SD composites in weight ratios of 1:1 and 1:2 was slightly lower than at the ratio of 1:3 and 1:5. IND could release in the range of 95.65–98.63% at 20 min. A lower extent of IND was released from the physical mixture when TmBzCS was used as carriers, compared to that of SD composites but higher than pure IND (Fig. 13F). The same release patterns were noticed from the physical mixtures in weight ratios 1:3 and 1:5, which are slightly better than at the weight ratios 1:1 and 1:2. The maximum solubility could be found at 40 min of the dissolution study. Once again, all SD composites markedly improved the IND dissolution characteristics than physical mixtures at the same weight ratio and pure IND (Fig. 13e and f). The 20-minute drug dissolution rates could improve from 388.56 µg/mL of IND to 1270.6–1384.4 µg/mL from physical mixtures and to 1849.6–1993.6 µg/mL from SD composites (Table III). mPyCS and TmBzCS-based composites (both SD and PM) of IND showed better dissolution of the drug compared to the TMC-based composites at the same weight ratio. mPyCS and TmBzCS in SD composites showed 100% drug release within 30 min, whereas, TMC needed a slightly longer time (about 60 min) which may be attributed to the higher water solubility of mPyCS and TmBzCS than TMC. Moreover, pyridine in mPyCS has chemical properties similar to tertiary amines. Therefore, it is easily attacked by alkylating agents in the methylation step to give N-alkylpyridinium salts [61, 62]. TmBzCS has an amino group in the aromatic ring in the methylation step to produce a quaternary ammonium group in the aromatic ring. mPyCS and TmBzCS contain more hydrophilic groups than TMC. Therefore, mPyCS and TmBzCS have higher water solubility than TMC and that is why the former two can enhance the solubility of IND in the composites more than the latter. Using TMC in SD composites in 1:3 and 1:5 weight ratios, drug solubility increased up to 100%, but, a slightly lower amount (about 91% and 94%) of the drug as released from SD composites based on TMC in 1:1 and 1:2 weight ratios. Similar results were observed when using Q-CS as drug carriers in physical mixtures. However, a lower amount of the drug (from 57 to 69%) could be released from these samples. mPyCS and TmBzCS enhanced the drug release rate better than TMC at all weight ratios. Using TMC in weight ratios of 1:3 and 1:5 was found to improve drug solubility characteristics, but not the drug release rate.
DR = The 20-minute drug dissolution rate; TD = Total drug content; D = Percent release of drug at 20 min.
Validation of the Visible Spectrophotometric Method for Quantitative Determination of IND in Samples
Method validation is a process of establishing the performance characteristics of the analytical method for the quantitative analysis of the analyte in the sample. The methods validation process for analytical procedures begins with the planned and systemic collection by the applicant of the validation data to support analytical procedures [63]. The methods were validated according to International Conference on Harmonization (ICH) guidelines for the validation of analytical procedures [64]. This study aimed to develop and validate UV-visible spectrophotometric methods for the determination of IND in Q-CS-IND composites. The UV-visible spectrum absorption was recorded using a UV-visible spectrophotometer (Hewlett Packard 8452 A, Diode Array Spectrophotometer, USA) at 318 nm. The wavelength of 318 nm was used in the study since it is the maximum absorption wavelength of IND. The results from the method validation are as follows:
Linearity and Calibration Curve
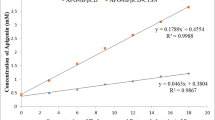
The calibration curve for standard IND was obtained by plotting the absorbance values versus concentrations of standard solutions of IND in methanol. Linearity was found to be in the range of 10 to 30 µg/mL (Fig. 14) with a significantly high value of correlation coefficient r2 = 0.999. The representative equation was y = 0.023x + 0.148. The quantitative parameters for the determination of IND are listed in Table IV. The low R.S.D value (< 2%) indicated the high precision of the calibration curve and was accepted for further analysis method. It should be noted that at the time of the determination, a new standard curve is constructed for each experiment interpretation.
Precision
The precision analyses result of intra-day (n = 3) and inter-day (n = 3) of various concentrations of IND were obtained and reported as %RSD values. The linear calibration curves of the standard solutions of IND having concentrations of 10, 15, 20, 25, and 30 µg/mL were obtained at three different determination times on the same day. The obtained calibration curves are displayed in Fig. 15. When the standard solutions concentration (10–30 µg/mL) were prepared on different days and used for absorbance determination at 318 nm, the straight line of standard curves was also achieved (Fig. 15). The %RSD values of intra-day and inter-day precision were 0.11 to 0.97% and 0.39 to 1.08% respectively (Table V). All relative standard deviation values were lower than 2% indicating that the developed method has high precision value. It is, therefore, the method that was suitable for use in determining the drug content of Q-CS-drug composites, drug solubility, and amount of soluble drug.
Accuracy
Accuracy was investigated employing a standard IND addition experiment, at three concentration levels (16, 20, and 24 µg/mL) in triplicate. The mean recoveries of each concentration are shown in Table VI High amount of IND was recovered (99%) by the extraction method that was used in the analytical procedure. This can be assumed that the true concentration of IND in all samples can be obtained by this analytical method.
Specificity
The analytical method used for qualitative determination of IND in samples was found to have specificity. Since no other absorption band was found interference at 318 nm in the UV-visible spectra of sample solutions prepared from blank Q-CS. Only the absorbance peak of IND was found in the UV-visible spectra of sample solutions (Fig. 16). Therefore, the determination of IND was performed at 318 nm to evaluate the concentration of IND in all samples.
Detection and Quantitation Limits
From the data obtained by the UV-visible spectrophotometric analysis of IND standard solutions, the detection limit and quantitation limit can be calculated. The limit of detection (LOD) calculated using the parameters of the calibration curve was 1.58 µg/mL. It was possible to identify the absorption band of IND at 318 nm. The limit of quantification (LOQ) determined based on the parameters of the calibration curve was 4.78 µg/mL and showed a good regression coefficient (r2 = 0.999).
Conclusions
This study highlighted the significant utility of Q-CS derivatives as carriers and solubility enhancers of poorly water soluble drugs. The Q-CS derivatives; N,N,N-trimethyl chitosan chloride (TMC), N-(4-N‘-methylpyridinylmethyl) chitosan chloride (mPyCS), and N-(4-N’,N’-trimethylaminobenzyl) chitosan (TmBzCS) - were synthesized with good yields (60.94%, 63.37%, and 62.42% respectively). The Q-CS were then used as carriers in the development of IND composites, prepared through spray drying method (SD composites) and physical mixing methods, with varying weight ratios of IND to Q-CS (1:1, 1:2, 1:3, and 1:5). High yields were achieved (60.11–79.54% for SD composites, 97.18–100.25% for physical mixtures). The composites were analyzed for drug content using UV-Vis spectroscopy, showing high drug content (97.18–100.25% for SD composites and 99.49–100.27% for physical mixtures). FT-IR analysis revealed chemical interactions between IND and Q-CS in SD composites. DSC and PXRD results indicated the conversion of IND into an amorphous state, confirmed by SEM micrographs showing the disappearance of IND crystals, particularly with higher Q-CS content. The morphologies of Q-CS appeared as hollow spheres in some composites, potentially enhancing carrier surface area and improving water wettability. These enhancements significantly improved IND solubility and dissolution rate. Spray drying techniques for the preparation of SD composites are particularly effective, attributed to increased drug wettability, surface area, and reduced particle size. Thus, these findings highlight the utility of Q-CS based SD composites as a potential strategy for enhancing drug solubility, dissolution, and bioavailability.
Data Availability
The data will be made available on request.
References
Renukuntla J, Vadlapudi AD, Patel A, Boddu SHS, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm. 2013;447(1–2):75–93. https://doi.org/10.1016/j.ijpharm.2013.02.030.
Tong P, Zografi G. A study of amorphous molecular dispersions of indomethacin and its sodium salt. J Pharm Sci. 2001;90(12):1991–2004. https://doi.org/10.1002/jps.1150.
Warnken Z, Smyth HDC, Williams RO. Route-specific challenges in the delivery of poorly water-soluble drugs. In: Williams RO, Watts AB, Miller DA, editors. Formulating Poorly Water Soluble Drugs. 2nd ed; 2016. p. 1–39.
Warnken Z, Smyth HDC, Williams RO. Route-specific challenges in the delivery of poorly water-soluble drugs. In: Williams Iii RO, Davis DA, Miller DA, editors. Formulating Poorly Water Soluble Drugs. Cham: Springer International Publishing; 2022. p. 1–31.
Bellantone RA, Patel P, Sandhu H, Choi DS, Singhal D, Chokshi H, et al. A method to predict the equilibrium solubility of drugs in solid polymers near room temperature using thermal analysis. J Pharm Sci. 2012;101(12):4549–58. https://doi.org/10.1002/jps.23319.
Ma Q, Sun H, Che E, Zheng X, Jiang T, Sun C, et al. Uniform nano-sized valsartan for dissolution and bioavailability enhancement: influence of particle size and crystalline state. Int J Pharm. 2012;441(1–2):75–81. https://doi.org/10.1016/j.ijpharm.2012.12.025.
Patel K, Shah S, Patel J. Solid dispersion technology as a formulation strategy for the fabrication of modified release dosage forms: a comprehensive review. DARU J Pharm Sci. 2022;30(1):165–89.
El-Badry M, Fetih G, Fathy M. Improvement of solubility and dissolution rate of indomethacin by solid dispersions in Gelucire 50/13 and PEG4000. Saudi Pharm J. 2009;17(3):217–25.
Zhang W, Zhang C-n, He Y, Duan B-y, Yang G-y, Ma W-d, et al. Factors affecting the dissolution of indomethacin solid dispersions. AAPS PharmSciTech. 2017;18:3258–73.
Mennini N, Furlanetto S, Bragagni M, Ghelardini C, Di Cesare Mannelli L, Mura P. Development of a chitosan-derivative micellar formulation to improve celecoxib solubility and bioavailability. Drug Dev Ind Pharm. 2014;40(11):1494–502.
Inada A, Oshima T, Takahashi H, Baba Y. Enhancement of water solubility of indomethacin by complexation with protein hydrolysate. Int J Pharm. 2013;453(2):587–93.
Jain S, Patel N, Lin S. Solubility and dissolution enhancement strategies: current understanding and recent trends. Drug Dev Ind Pharm. 2015;41(6):875–87.
Janssens S, Van den Mooter G. Review: physical chemistry of solid dispersions. J Pharm Pharmacol. 2009;61(12):1571–86. https://doi.org/10.1211/jpp.61.12.0001.
Yadav PS, Kumar V, Singh UP, Bhat HR, Mazumder B. Physicochemical characterization and in vitro dissolution studies of solid dispersions of ketoprofen with PVP K30 and d-mannitol. Saudi Pharm J. 2013;21(1):77–84. https://doi.org/10.1016/j.jsps.2011.12.007.
Ghosh I, Snyder J, Vippagunta R, Alvine M, Vakil R, Tong W-Q, et al. Comparison of HPMC based polymers performance as carriers for manufacture of solid dispersions using the melt extruder. Int J Pharm. 2012;419(1–2):12–9. https://doi.org/10.1016/j.ijpharm.2011.05.073.
Zhou X, Hu Y, Tian Y, Hu X. Effect of N-trimethyl chitosan enhancing the dissolution properties of the lipophilic drug cyclosporin A. Carbohydr Polym. 2009;76(2):285–90.
Aranaz I, Mengibar M, Harris R, Panos I, Miralles B, Acosta N, et al. Functional characterization of chitin and Chitosan. Curr Chem Biol. 2009;3(2):203–30.
Xu T, Xin M, Li M, Huang H, Zhou S. Synthesis, characteristic and antibacterial activity of N,N,N-trimethyl chitosan and its carboxymethyl derivatives. Carbohydr Polym. 2010;81(4):931–6.
Ruihua H, Bingchao Y, Zheng D, Wang B. Preparation and characterization of a quaternized chitosan. J Mater Sci. 2012;47(2):845–51. https://doi.org/10.1007/s10853-011-5862-4.
Jonker C, Hamman JH, Kotzè AF. Intestinal paracellular permeation enhancement with quaternised chitosan: in situ and in vitro evaluation. Int J Pharm. 2002;238(1–2):205–13. https://doi.org/10.1016/S0378-5173(02)00068-6.
Kotze AF, Thanou MM, Luessen HL, De Boer ABG, Verhoef JC, Junginger HE. Effect of the degree of quaternization of N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). Eur J Pharm Biopharm. 1999;47(3):269–74.
Ma G, Yang D, Zhou Y, Xiao M, Kennedy JF, Nie J. Preparation and characterization of water-soluble N-alkylated chitosan. Carbohydr Polym. 2008;74(1):121–6.
Cai J, Dang Q, Liu C, Fan B, Yan J, Xu Y, et al. Preparation and characterization of N-benzoyl-O-acetyl-chitosan. Int J Biol Macromol. 2015;77:52–8.
Mohammadi E, Daraei H, Ghanbari R, Athar SD, Zandsalimi Y, Ziaee A, et al. Synthesis of carboxylated chitosan modified with ferromagnetic nanoparticles for adsorptive removal of fluoride, nitrate, and phosphate anions from aqueous solutions. J Mol Liq. 2019;273:116–24.
Jia Z, Xu W. Synthesis and antibacterial activities of quaternary ammonium salt of Chitosan. Carbohydr Res. 2001;333(1):1–6.
Pathak K, Misra SK, Sehgal A, Singh S, Bungau S, Najda A, et al. Biomedical applications of quaternized chitosan. Polymers. 2021;13(15):2514.
Mourya VK, Inamdar N. Trimethyl Chitosan and its applications in drug delivery. J Mater Science: Mater Med. 2009;20(5):1057–79. https://doi.org/10.1007/s10856-008-3659-z.
Opanasopit P, Sajomsang W, Ruktanonchai U, Mayen V, Rojanarata T, Ngawhirunpat T, Methylated. N-(4-pyridinylmethyl) chitosan as a novel effective safe gene carrier. Int J Pharm. 2008;364(1):127–34.
Opanasopit P, Petchsangsai M, Rojanarata T, Ngawhirunpat T, Sajomsang W, Ruktanonchai U. Methylated N-(4-N,N-dimethylaminobenzyl) chitosan as effective gene carriers: Effect of degree of substitution. Carbohydr Polym. 2009;75(1):143–9. https://doi.org/10.1016/j.carbpol.2008.07.012.
Guo Z, Xing R, Liu S, Zhong Z, Ji X, Wang L, et al. The influence of the cationic of quaternized chitosan on antifungal activity. Int J Food Microbiol. 2007;118(2):214–7. https://doi.org/10.1016/j.ijfoodmicro.2007.07.003.
Gonil P, Sajomsang W, Ruktanonchai UR, Pimpha N, Sramala I, Nuchuchua O, et al. Novel quaternized chitosan containing β-cyclodextrin moiety: synthesis, characterization and antimicrobial activity. Carbohydr Polym. 2011;83(2):905–13. https://doi.org/10.1016/j.carbpol.2010.08.080.
Zhu Y-a, Sun P, Duan C, Cao Y, Kong B, Wang H, et al. Improving stability and bioavailability of curcumin by quaternized chitosan coated nanoemulsion. Food Res Int. 2023;174:113634.
Xie Y, Gong X, Jin Z, Xu W, Zhao K. Curcumin encapsulation in self-assembled nanoparticles based on amphiphilic palmitic acid-grafted-quaternized chitosan with enhanced cytotoxic, antimicrobial and antioxidant properties. Int J Biol Macromol. 2022;222:2855–67.
Fazal T, Murtaza BN, Shah M, Iqbal S, Rehman M-u, Jaber F, et al. Recent developments in natural biopolymer based drug delivery systems. RSC Adv. 2023;13(33):23087–121.
Sripetthong S, Nalinbenjapun S, Basit A, Surassmo S, Sajomsang W, Ovatlarnporn C. Preparation of Self-Assembled, curcumin-loaded Nano-Micelles using Quarternized Chitosan–Vanillin Imine (QCS-Vani imine) conjugate and evaluation of synergistic Anticancer Effect with Cisplatin. J Funct Biomaterials. 2023;14(10):525.
Anitha A, Divya Rani VV, Krishna R, Sreeja V, Selvamurugan N, Nair SV, et al. Synthesis, characterization, cytotoxicity and antibacterial studies of chitosan, O-carboxymethyl and N,O-carboxymethyl chitosan nanoparticles. Carbohydr Polym. 2009;78(4):672–7.
French DL, Mauger JW. Evaluation of the physicochemical properties and dissolution characteristics of mesalamine: relevance to controlled intestinal drug delivery. Pharm Res. 1993;10(9):1285–90.
Stegemann S, Leveiller F, Franchi D, de Jong H, Lindn H. When poor solubility becomes an issue: from early stage to proof of concept. Eur J Pharm Sci. 2007;31(5):249–61.
Martins AF, Bueno PVA, Follmann HDM, Nocchi SR, Nakamura CV, Rubira AF, et al. Synthesis, characterization, and cytotoxicity of TMC-graft-poly(vinyl alcohol) copolymers. Carbohydr Res. 2012. https://doi.org/10.1016/j.carres.2012.11.014.
Sonia TA, Sharma CP. Chitosan and its derivatives for drug delivery perspective. Adv Polym Sci. 2011;243:23–54.
Mourya VK, Inamdar NN. Chitosan-modifications and applications: opportunities galore. Reactive Funct Polym. 2008;68(6):1013–51. https://doi.org/10.1016/j.reactfunctpolym.2008.03.002.
Sajomsang W, Tantayanon S, Tangpasuthadol V, Daly WH. Synthesis of methylated chitosan containing aromatic moieties: Chemoselectivity and effect on molecular weight. Carbohydr Polym. 2008;72(4):740–50. https://doi.org/10.1016/j.carbpol.2007.10.023.
Sajomsang W, Tantayanon S, Tangpasuthadol V, Daly WH. Quaternization of N-aryl chitosan derivatives: synthesis, characterization, and antibacterial activity. Carbohydr Res. 2009;344(18):2502–11. https://doi.org/10.1016/j.carres.2009.09.004.
Ewing AV, Clarke GS, Kazarian SG. Stability of indomethacin with relevance to the release from amorphous solid dispersions studied with ATR-FTIR spectroscopic imaging. Eur J Pharm Sci. 2014;60:64–71.
Xiang T-X, Anderson BD. Molecular dynamics simulation of amorphous indomethacin–poly(vinylpyrrolidone) glasses: solubility and hydrogen bonding interactions. J Pharm Sci. 2012;102(3):876–91. https://doi.org/10.1002/jps.23353.
Fini A, Cavallari C, Ospitali F. Raman and thermal analysis of indomethacin/PVP solid dispersion enteric microparticles. Eur J Pharm Biopharm. 2008;70(1):409–20.
Patel PV, Panchal SS, Mehta TA. Improvement of dissolution rate of tacrolimus by solid dispersion technique. J Pharm Invest. 2013;43:45–53.
Allen D, Foley J, Perepezko J. Nanocrystal development during primary crystallization of amorphous alloys. Acta Mater. 1998;46(2):431–40.
Andrusenko I, Hamilton V, Lanza AE, Hall CL, Mugnaioli E, Potticary J, et al. Structure determination, thermal stability and dissolution rate of δ-indomethacin. Int J Pharm. 2021;608:121067.
Asare-Addo K, Alshafiee M, Walton K, Ward A, Totea A-M, Taheri S, et al. Effect of preparation method on the surface properties and UV imaging of indomethacin solid dispersions. Eur J Pharm Biopharm. 2019;137:148–63.
Baird JA, Taylor LS. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv Drug Deliv Rev. 2012;64(5):396–421. https://doi.org/10.1016/j.addr.2011.07.009.
Ohara T, Kitamura S, Kitagawa T, Terada K. Dissolution mechanism of poorly water-soluble drug from extended release solid dispersion system with ethylcellulose and hydroxypropylmethylcellulose. Int J Pharm. 2005;302(1–2):95–102.
Fujii M, Okada H, Shibata Y, Teramachi H, Kondoh M, Watanabe Y. Preparation, characterization, and tableting of a solid dispersion of indomethacin with crospovidone. Int J Pharm. 2005;293(1–2):145–53.
Slavin PA, Sheen DB, Shepherd EE, Sherwood JN, Feeder N, Docherty R, et al. Morphological evaluation of the γ-polymorph of indomethacin. J Cryst Growth. 2002;237:300–5.
Malwade CR, Qu H. Cooling crystallization of indomethacin: effect of supersaturation, temperature, and seeding on polymorphism and crystal size distribution. Org Process Res Dev. 2018;22(6):697–706.
Li M, Ioannidis N, Gogos C, Bilgili E. A comparative assessment of nanocomposites vs. amorphous solid dispersions prepared via nanoextrusion for drug dissolution enhancement. Eur J Pharm Biopharm. 2017;119:68–80.
Janssens S, Annè M, Rombaut P, Van den Mooter G. Spray drying from complex solvent systems broadens the applicability of Kollicoat IR as a carrier in the formulation of solid dispersions. Eur J Pharm Sci. 2009;37(3–4):241–8. https://doi.org/10.1016/j.ejps.2009.02.020.
Comer J, Judge S, Matthews D, Towes L, Falcone B, Goodman J, et al. The intrinsic aqueous solubility of indomethacin. ADMET DMPK. 2014;2(1):18–32.
Newa M, Bhandari KH, Oh DH, Kim YR, Sung JH, Kim JO, et al. Enhanced dissolution of ibuprofen using solid dispersion with poloxamer 407. Arch Pharm Res. 2008;31:1497–507.
Nokhodchi A, Javadzadeh Y, Siahi-Shadbad MR, Barzegar-Jalali M. The effect of type and concentration of vehicles on the dissolution rate of a poorly soluble drug (indomethacin) from liquisolid compacts. J Pharm Pharm Sci. 2005;8(1):18–25.
Sajomsang W. Synthetic methods and applications of chitosan containing pyridylmethyl moiety and its quaternized derivatives: a review. Carbohydr Polym. 2010;80(3):631–47. https://doi.org/10.1016/j.carbpol.2009.12.037.
Fujimoto K, Morisaki D, Yoshida M, Namba T, Hye-Sook K, Wataya Y, et al. Antimalarial effect of bis-pyridinium salts, N,N€-hexamethylenebis(4-carbamoyl-1-alkylpyridinium bromide). Bioorg Med Chem Lett. 2006;16(10):2758–60.
FDA. Giudance for industry: Analytical procidures and methods validation. 2000 [cited August 23, 2006]; http://www.fda.gov/CDER/GUIDANCE/2396dft.pdf.
ICH. Validation of analytical procedures, Proceedings of the International Conference on Harmonization (ICH). Commission of the European Communities. 1996.
Funding
This work was supported by the National Science Research and Innovation Fund and Prince of Songkla University (Grant No. PHA550063S). Miss Sasikarn Sripetthong would like to thank Thailand Research Fund Contract No. MRG-WII525S094 for supporting during master's degree study.
Author information
Authors and Affiliations
Contributions
Sasikarn Sripetthong: Writing an original draft, methodology, and investigation. Sirinporn Nalinbenjapun: Methodology, software, formal analysis. Abdul Basit: Writing-reviewing draft, software, formal analysis, data curation. Chitchamai Ovatlarnporn: Supervision, investigation, conceptualization.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 12.8 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sripetthong, S., Nalinbenjapun, S., Basit, A. et al. Synthesis of Quarternized Chitosans and Their Potential Applications in the Solubility Enhancement of Indomethacin by Solid Dispersion. AAPS PharmSciTech 25, 179 (2024). https://doi.org/10.1208/s12249-024-02893-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02893-9