Abstract
The aim of this study was to investigate the influence of the production method and the polymeric carrier on the ability to generate and maintain the supersaturation of a poorly soluble drug in biorelevant medium. The amorphous solid dispersion of sulfamethoxazole, an antibacterial drug, was produced using two different polymers by spray-drying or hot melt extrusion methods. When Eudragit EPO was used, supersaturation was maintained up to 24 h for both techniques at all drug-polymer proportions. However, when Soluplus was employed in hot melt extrusion, a smaller amount of drug was dissolved when compared to the amorphous drug. The proportion of 3:7 drug-Eudragit EPO (w/w) produced by spray-drying presented a higher amount of drug dissolved in supersaturation studies and it was able to maintain the physical stability under different storage conditions throughout the 90-day evaluation. Supersaturation generation and system stability were found to be related to more effective chemical interaction between the polymer and the drug provided by the production method, as revealed by the 1D ROESY NMR experiment. Investigation of drug-polymer interaction is critical in supersaturating drug delivery systems to avoid crystallization of the drug and to predict the effectiveness of the system. Chemical compounds studied in this article: Sulfamethoxazole (PubChem CID: 4539) and Methacrylate copolymer — Eudragit EPO (PubChem CID: 65358)
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Solubility is a prerequisite for proper drug absorption and, consequently, the therapeutical efficacy of orally administered drugs. Supersaturating Drug Delivery Systems (SDDSs) are a promising model aiming to deliver drugs that present limited oral bioavailability due to their low aqueous solubility. A supersaturated solution of a drug is metastable; however, if this metastable solution could be maintained for enough time to allow drug absorption, the higher drug intraluminal concentration could result in a higher amount able to permeate through the intestine. SDDSs provide a drug solution above its saturation due to the action of precipitation inhibitors, which are effective in stabilizing the high energy of these supersaturated systems, which are thermodynamically unstable and tend to precipitate (1,2,3). Selected polymers have demonstrated significant crystallization inhibition due to the intermolecular interactions between the polymer and the drug, which reduce drug-drug interactions and avoid nucleation and crystal growth, both in solid state and after contact with the gastrointestinal medium (4,5,6).
Amorphous solid dispersions (ASDs) have been developed as a potentially viable commercial process able to form SDDSs made by spray-drying (SD) or hot melt extrusion (HME). Both techniques enable a continuous process that can be easily scaled up from a small-scale laboratory to production-scale equipment. SD is a solvent method that can increase the interactions between the drug and the polymer due to the liquid medium (7). However, HME is a solvent-free method where the solid materials melt and mix in the barrel, generating an extrudate that quickly solidifies. The difference in the ASD production method may result in a different drug-polymer interaction pattern, which could result in a different ASD supersaturation performance (8,9,10).
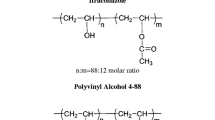
Sulfamethoxazole (SLM, Fig. 1) is a sulfonamide antibiotic extensively used due to its broad spectrum against microorganisms, including resistant strains. It belongs to class II of the biopharmaceutical classification system, which covers drugs where dissolution is the limiting process to oral absorption. Due to SLM’s low aqueous solubility, high dosages of this drug are necessary for its therapeutic effectiveness and this could lead to the development of some resistant strains (11,12,13,14). The rational development of ASD, which could improve poorly soluble drugs’ biopharmaceutical characteristics, includes the appropriate polymer choice (15). Recently, our group designed a rational selection of polymeric carriers, evaluating the miscibility between them and the SLM by theoretical and practical methods. This parameter evaluated revealed Soluplus and Eudragit EPO (Fig. 1) as promising carriers; following the Flory-Huggins theory, negative values were observed for both polymers, which means that there is a stronger interaction between drug-polymer than drug-drug or polymer-polymer. Furthermore, these polymers presented the same or improved antibacterial activity by the microdilution method. Soluplus is an amorphous polymer recently developed for HME due to its low Tg of 72°C (16), and Eudragit EPO (Tg of 54°C) is a type of methacrylate polymer with many different applications in the pharmaceutical industry, including the production of ASD. In this context, the aim of this study was to evaluate the interactions between SLM and the polymers (Eudragit EPO and Soluplus) in ASD obtained by SD and HME methods. Drug-polymer interactions were evaluated by several solid-state characterization techniques, including nuclear magnetic resonance (NMR) and in vitro supersaturation studies. Stability studies were also conducted for the formulation that presented the best in vitro supersaturation performance. For the first time, a complete study, ranging from drug-polymer interactions until supersaturation and stability studies, of one SDDS containing SLM was performed.
MATERIAL AND METHODS
Materials
Sulfamethoxazole (SLM, Mw 253.28 g mol−1) was kindly donated by Elofar (Florianópolis–Brazil). Methacrylate copolymer (EUDRAGIT EPO, Mw 47,000 g mol−1) was donated by Evonik Brasil (São Paulo, Brazil) and polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer trade name Soluplus (90,000 g mol−1 ≤ Mw ≤ 140,000 g mol−1) was donated by BASF (São Paulo, Brazil). The biorelevant medium used to simulate the intestinal conditions was FaSSIF (Fasted State Simulated Intestinal Fluid) acquired from Biorelevant.com Ltd. (London, UK). All other analytical reagents were of analytical grade.
Preparation of Amorphous Solid Dispersion
Two different methods were used to produce ASD in three different drug polymer proportions (3:7, 5:5 e 7:3 w/w), from which Eudragit EPO and Soluplus were the polymers selected in the previous study. (i) SD method: SLM pure crystalline drug and polymers at the different drug-polymer proportions were weighed, dissolved in acetone, and then diluted with water to a final concentration of 20% of acetone (v/v). The resultant solution was spray-dried in a Mini Spray Dryer (Buchi B-290, Germany) under the following conditions: inlet temperature 140°C, outlet temperature 45°C, air flow rate 30 mL/min, aspiration capacity of 15%, and atomizing air pressure 1.5 bar. A solution of SLM pure crystalline drug was produced in the same way for the comparison. (ii) HME method: SLM pure raw material drug and polymers at the different drug polymer proportions were extruded using a co-rotating twin screw extruder Max (Custom Scientific Instruments, EUA) at 175°C. The extrudates were milled using a knife mill MA 048 (Forlab, Portugal) fitted with a stainless steel 3 mesh.
Solid-State Characterization
The amorphous nature of the samples was confirmed through DSC and X-ray powder diffraction (XRPD) analysis. DSC thermograms of SLM, polymers, and ASD samples were obtained on a Shimadzu DSC-50 cell (Kyoto, Japan) using aluminum crucibles with around 5.0 mg of sample, temperature ranging from 0 to 200°C, with a heating rate of 5°C/min, and nitrogen flow rate of 50 mL/min. A pre-heating treatment was carried out for all samples to avoid interference of any residual solvent. XRPD patterns were obtained on a Bruker D2 Phaser θ–θ X-ray diffractometer, with a tube of CuKa, a voltage of 30 kV, and a current of 10 mA, in the range of 5–40 (2θ) with a pass time of 5 s. Detection was performed on a scintillation counter one-dimensional LYNXEYE detector.
The chemical interactions between the SLM and the polymers were also investigated by diffuse reflectance Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR, item 2.5). The SLM, polymer, and ASD selected were prepared by weighing equivalent amounts of SLM and polymers (1:1 w/w) mixed with potassium bromide. The spectra were recorded using a Shimadzu IR Prestige 21 FTIR (Japan) within a scan range of 400–4000 cm−1 and an average of over 20 scans at a spectral resolution of 1 cm−1. A background spectrum was obtained for each experimental condition.
Morphological characteristics were observed by a scanning electron microscope (SEM) on a Jeol JSM-6390LV. Samples were mounted onto metal stubs using double-sided adhesive tape, vacuum coated with gold (350 Å) in a Leica EM SCD 500, and directly analyzed by SEM. An accelerating voltage of 10 kV was used.
Disintegration Test
Nova Ética (São Paulo–Brazil) disintegrator equipment was used in order to evaluate the disintegration time of the extrudates. One extrudate strand (unmilled) of approximately 200 mg was placed in each of the six glass tubes of the basket containing fasted state simulated intestinal fluid (FaSSIF, pH 6.5). The disintegration time was recorded when all the six extrudates were completely disintegrated.
NMR Analysis
The chemical interactions between the SLM and the polymers, obtained from two different ASDs, were evaluated by NMR spectroscopy analysis. For this, 1H and 1D ROESY NMR experiments were performed at 30°C in acetone-d6 on a Bruker AVANCE III 600 NMR spectrometer operating at 14.1 T, observing 1H at 600.13 MHz, equipped with a 5-mm four-channel (2H, 1H, 13C, and 15N) inverse detection probe with z-gradient. 1H NMR spectra were acquired with a spectral width of ≅ 11.5 ppm and 64 k data points, providing a digital resolution of 0.05 Hz. The 1D ROESY experiments were obtained with the same conditions as in 1H NMR spectra, although using selective 180° pulse excitation and selective refocusing with shaped pulse through the gradient selroegp from the Bruker library pulse sequence, 300 ms for ROESY spin-lock, recycle delay of 2.0 s, and 512 transients. The 1H and 1D ROESY NMR spectra were processed by applying a Lorentz exponential multiplication of the FIDs by a factor of 0.3 and 1.0 Hz for 1H and 1D ROESY, respectively, followed by Fourier transform with zero-filling to 128 k data points. All 1H NMR chemical shifts are given in ppm in relation to the TMS signal at 0.00 ppm as an internal reference and all pulse programs were supplied by Bruker.
Contact Angle Measurements
Wettability of ASD was investigated by measuring contact angle, performed at room temperature with a Ramé Hart goniometer model 250 (New Jersey, USA). ASD samples were compressed into a pellet by a hydraulic press at 400 psi for 1 min. Water or diiodomethane (20 μL) was dropped from a microsyringe on the pellet surface. Images of the drop were photographed; the contact angle could be measured directly from the photograph by the software DROPimage Standard. Triplicate studies were carried out.
Supersaturation Studies
The supersaturated solutions of SLM were prepared by adding an excess amount of amorphous powder of SLM or equivalent of ASD in 20 mL of FaSSIF (pH 6.5). SLM concentration was evaluated on the solution concentration-time profile. The flasks were maintained at 37.0 ± 1.0°C in a shaker incubator (Nova Tecnica, Brazil) at 240 rpm and aliquots of the resulting suspensions were withdrawn and filtered through polyamide syringe filters (pore size of 0.45 μm, Chromafil Xtra) at specified time intervals over 24 h. The filtered solution was diluted with methanol to prevent any further precipitation. SLM concentration was evaluated by high-performance liquid chromatography (HPLC) as described in the “High-performance Liquid Chromatography” section. All measurements were carried out in triplicate.
High-performance Liquid Chromatography
The drug content was determined by HPLC based on the official method (17). The chromatographic analysis was performed in a Shimadzu liquid chromatographer (Shimadzu, Kyoto, Japan) with a LC-10A VP quaternary pump equipped with a Phenomenex® Gemini 110A (Torrance, CA, USA) C18 reversed-phase column (250 mm × 4.6 mm; 5 μm particle size) at 25°C and UV detector set at 254 nm. The column was eluted in an isocratic mode by using a mobile phase of 0.1% of triethylamine (pH 6.0 adjusted with acetic acid) and acetonitrile (70:30 v/v) at a flow rate of 1.0 mL/min and an injection volume of 20 μL. The method was validated according to the International Conference on Harmonization (ICH) considering specificity, linearity, precision, accuracy, robustness, limit of detection, and quantification (18). Validation parameters were within the acceptable range.
Stability Studies
SLM amorphous drug and selected formulations were subjected to stability studies by placing the solid samples in an open glass vial at 40°C/75% R.H. or in a sealed glass vial at 40°C and 4°C for 3 months and analyzed by XRPD.
Statistical Analysis
Results are expressed as the mean ± standard deviation of triplicate. The graphs were traced and the statistical analysis was performed using GraphPad Prism, version 6.0 for Windows (GraphPad Software, San Diego, CA, USA).
RESULTS AND DISCUSSION
Supersaturation Studies
In our previous work, Soluplus and Eudragit EPO were selected as the polymers miscible with SLM that present the same or improved antibacterial activity. ASDs at three different proportions of each polymer were produced by SD. The raw material is named here as crystalline SLM, which was used for comparison purposes. Pure SLM was also spray-dried and the resultant sample is named amorphous SLM. SD is the technique that could reduce the crystallinity to a great extent, and the resultant solid material presented the glass transition temperature (Tg) expected for amorphous systems (supplementary material, Figure S1). The supersaturation study was performed at FaSSIF (pH 6.5), the biorelevant medium used to mimic the intestinal condition. SLM has two different pka values, pka1 = 1.85 ± 0.30 and pka2 = 5.60 ± 0.04 (19,20); therefore, at the pH of the intestinal fluid, above both pKa values, the drug is negatively charged. Concerning the polymers, Soluplus is a non-ionic polymer used in a broader pH range without compromising solubilization (21). Eudragit EPO, on the other hand, is a cationic polymer, and its ionization at pH less than 5.1 is known to form salts with drugs due to strong ionic interactions between the cationic nitrogen of the polymer with carboxylic groups of the drugs. In FaSSIF, the majority of the polymer is in the non-ionic form and only a small part of the polymer is in the cationic form (pKa = 8.5) (22,23,24). Therefore, the results observed in the supersaturation study were considered related to chemical interaction between the drug and the polymer rather than an ionic interaction, which has a limited effect on the dissolution of the ASD.
Figure 2 shows the supersaturation study results obtained for ASD 3:7, 5:5, and 7:3 SLM:Eudragit EPO or SLM:Soluplus in FaSSIF. Both polymers were able to maintain the drug supersaturation during all the evaluated period (24 h). Eudragit EPO achieved greater supersaturation extension than Soluplus, demonstrated by the apparently higher drug solubility in FaSSIF when using systems composed by this polymer. This is probably due to the stronger interaction between Eudragit EPO and SLM, as already demonstrated by different techniques in our previous work. For better visualization and comparison, the areas under the curve (AUC) were calculated for each graphic. The AUC obtained for formulation 3:7 SLM:Eudragit EPO was different (p < 0.05) when compared with formulations 5:5 or 7:3 of the same polymer, and it was the formulation that promoted the highest SLM supersaturation extension. Formulations produced with Soluplus as carrier did not present a statistical difference between the different drug-polymer proportions tested. Due to the higher content of SLM dissolved presented by 3:7 SLM-Eudragit EPO, this proportion was selected to be produced by HME. Since Soluplus did not present a difference between the proportions tested, the same 3:7 SLM-Soluplus was selected to HME study. In this way, using the same proportion for both polymers in the HME method enables the comparison of the formulations to be restricted to the polymer type, excluding the proportion factor.
Apparent dissolution profiles and AUC of amorphous SLM (white circle), crystalline SLM (white triangle), and amorphous solid dispersions 3:7 SLM-Soluplus (white square), 5:5 SLM-Soluplus (white diamond), 7:3 SLM-Soluplus (x), 3:7 SLM-Eudragit EPO (black square), 5:5 SLM- Eudragit EPO (black circle), 7:3 SLM- Eudragit EPO (black triangle) produced by spray-drying (a and b) or produced by hot-melt extrusion (c, HME) 3:7 SLM-Soluplus (white square), 3:7 SLM-Eudragit EPO (black square) at 37°C. Error bars represent 1 standard deviation (n = 3). Some error bars are smaller than the symbols. *Represents statistical difference of the formulation when compared with other groups of the same graphic (p < 0.05)
It is important to note that the proportion of 5:5 and 7:3 would reduce the pill burden in the final product, since the polymer has a higher molar mass than the drug. Systems that present improved antibacterial activity could also reduce the amount of the drug required to produce the same effect. A previous work by our group indicates that the mixture of the drug and the polymer results in improved antibacterial activity in vitro. Therefore, the proportion selected to continue the study was based on the result of the best supersaturation performance, since it is the main concern of this work. However, other proportions that could stabilize high drug loading and improve the antibacterial activity should be considered in future studies.
ASD produced by HME with the carrier Eudragit EPO followed a similar dissolution profile of the SD formulation. On the other hand, 3:7 SLM:Soluplus revealed, unexpectedly, a smaller SLM dissolved amount than amorphous SLM. This behavior was already observed in the literature (25,26). Thiry and coworkers (2016) observed a slow release rate of itraconazole from ASD produced by HME using Soluplus. The group attributed this to the “gel effect” that Soluplus presents when it comes in contact with water (27). This property was investigated by the disintegration test as explained in the “Disintegration Test” section. All the six extrudates of 3:7 (w/w) SLM-Soluplus evaluated needed around 26 h to disintegrate completely, which creates a barrier to the release of the drug. As already reported in the literature, the mechanism of disintegration of the extrudates is bulk erosion, because water is not able to diffuse through the polymer matrix, as observed with immediate-release tablets that disintegrate faster. This slow disintegration time could be observed in the milled extrudates used in the supersaturation study; due to the “gel effect,” the SLM remains attached to the system, resulting in a very low amount released from the system.
ASD Solid-State Characterization
ASDs obtained were characterized regarding their thermal properties (DSC) and structural properties (XRPD, SEM, and FTIR). Figure 3a presents DSC curves obtained for ASDs produced by SD and HME, and a tabular representation of the DSC data is presented in the supplementary material (Table S1). All DSC curves showed a single Tg value, which indicates the formation of a glassy solution for each ASD, confirming the amorphous identity of the sample. All the DSC curves of ASD produced with Eudragit EPO revealed an exothermic event after Tg, with the exception of 3:7 proportion produced by SD. This event is probably due to the recrystallization of the SLM with a later endothermic event around 168°C, characteristic of SLM melting point (supplementary material). DSC curves of ASD SLM-Eudragit EPO 5:5 and 7:3 obtained by SD presented similar ∆H values of the exothermic and endothermic events, suggesting that all the recrystallized SLMs melted with the increase in temperature. On the other hand, the endothermic event obtained for the 3:7 HME SLM-Eudragit EPO system was more pronounced than the recrystallization event, which indicates that some crystalline SLM was already present in the sample before recrystallization. In this case, the recrystallization probably occurs due to the presence of a small amount of residual SLM crystals that induce the nucleation of drug molecules in the recrystallization process. This small amount of crystals was not enough to be detected by the XRPD technique.
The presence of small amounts of crystals in the ASD can be attributed to the differences between ASD production methods. The SD technique requires previous drug solubilization that enables greater interaction between the drug polymers than the solid-state mixture of HME. Due to this lower interaction attributed to the HME method, some SLM melts during the process but cannot chemically interact with the polymer and recrystallize at room temperature inside the extrudate. As already presented by our group, the quench cooling method was not able to transform the crystalline SLM completely in its amorphous form. These findings reveal that it is necessary for the polymer to interact with the drug and generate an amorphous system, characterized by a single Tg value.
The amorphous state of the ASD obtained was confirmed by the gold standard technique XRPD (Supplementary material, Figure S2). For all XRPD patterns, it is not possible to observe any crystalline event of SLM. This result is in accordance with Tg observed in DSC analysis, confirming its amorphous identity.
The morphological aspect of ASDs was visualized by SEM (Supplementary material Figure S3). Pure crystalline SLM shows the characteristic crystal structure while amorphous SLM produced by SD reveals a small round-shaped particle size, characteristic of the drying process. Soluplus formulations produced by SD in 3:7 and 5:5 proportions were found to be very similar to amorphous SLM, unlike the 7:3 proportion, which is more similar to crystalline SLM. This is probably due to the excess of the drug, where the content of the polymer was not enough to stabilize the high content of SLM and the obtained ASD present remained as SLM crystals. All ASD produced with Eudragit EPO by SD presented the same amorphous characteristic with no difference between the proportions tested. These findings are in accordance with our previous work that revealed Eudragit EPO to be the polymer with the highest capacity to solubilize the drug, even in a lower proportion (7:3 SLM-Eudragit EPO); the polymer was able to stabilize the total content of SLM. Both formulations obtained by HME presented the milled characteristic with no similarity with the amorphous drug or polymers. The particle size of ASD did not influence the supersaturation studies, as already described in “Supersaturation Studies” section.
ASDs were analyzed by FTIR and the spectra are presented in Fig. 3b. Spectra of ASD produced with Soluplus showed the vibrational bands between 3600 and 3200 cm−1 corresponding to –OH of the polymer. In all ASDs produced with Soluplus at different proportions, ester stretching at 1739 cm−1 presented a displacement to 1732 cm−1, which indicates the possible interaction of the ester group with SLM. ASD formulations containing Eudragit EPO show displacement of groups between 3000 and 3500 cm−1, characteristic of –NH group from SLM, and it is possible to observe a spectral band at 1724 cm−1, which is probably related to the displacement of a vibrational band at 1730 cm−1 from C=O of esterified carboxyl presented in Eudragit EPO molecule. These findings are indicative of hydrogen bonding formation between the groups that present the displacement of Eudragit EPO and SLM. Chemical interactions between the drug and the polymer increase the activation energy required for drug nucleation, delaying recrystallization occurrence, ensuring the maintenance of supersaturation promoted by the amorphous drug.
ASD Wettability
ASD wettability was measured by the contact angle with water (black) and diiodomethane (blue) showed in Fig. 4. Crystalline SLM is hydrophobic with a contact angle of 67.1° and 42.9° with water and diiodomethane, respectively. In the amorphous form, SLM showed improved hydrophilicity, as observed in the supersaturation studies (“Supersaturation Studies” section), with a reduction of the contact angle with water to 48.2° and an increase of contact angle with diiodomethane to 47.0°. All ASDs were able to improve the wettability in comparison with the crystalline drug, maintaining the contact angles in the range between the crystalline and the amorphous SLM. Soluplus exhibited the highest wettability for both production methods. Therefore, it might be said that the enhanced dissolution of SLM from ASD presented in the “Supersaturation Studies” section has an influence from the improved wettability of the drug by the polymer; however, it is not the only factor that increases the dissolution profile. In comparison with supersaturation studies, the drug-polymer interaction seems to be the property with more importance than the wettability, because the AUC of amorphous SLM is smaller when compared with all ASD formulations.
Contact angle with water or diiodomethane of crystalline SLM, amorphous SLM, and ASD 3:7 SLM-Soluplus, ASD 5:5 SLM-Soluplus, ASD 7:3 SLM-Soluplus, ASD 3:7 SLM-Eudragit EPO, ASD 5:5 SLM-Eudragit EPO, and ASD 7:3 SLM-Eudragit EPO produced by spray-drying (SD) or hot-melt extrusion (HME) ASD 3:7 HME SLM-Soluplus and 3:7 HME SLM-Eudragit EPO
Determination of Drug-Polymer Interaction
Interaction of SLM and polymers (Soluplus and Eudragit EPO) was first evidenced by comparing the 1H NMR spectra, acquired under the same experimental conditions, regarding only SLM and the ASD 3:7 SLM-Soluplus and ASD 3:7 SLM-Eudragit EPO, obtained through SD and HME. In this way, a severe broadening of the 1H NMR signals was observed for all hydrogen nuclei of SLM in the system of SLM/Soluplus obtained through the HME method (Fig. 5). When an effective interaction of SLM with a polymer takes place, it causes a reduction in its molecular tumbling; thus, the compound experiences several magnetic fields instead of an average field while freely tumbling. In other words, small compounds come to act like macromolecules. This is an indication of intermolecular interaction.
Even broadening of the signals is an indication of intermolecular interaction, Nuclear Overhauser effect experiments are much more effective in measuring intermolecular interactions and, therefore, this effect was investigated. Broadening of the 1H NMR signals of SLM in the SLM/Soluplus system obtained by the HME method was not supported by one-dimensional rotating frame nuclear Overhauser effect (1D ROESY) NMR experiments, which are usually suitable to measure nOe in complexes. On the other hand, 1D ROESY NMR revealed interaction between SLM and Eudragit EPO obtained by the SD method. In this way, the selective excitation of any hydrogen nuclei from the polymer has caused equal nOe enhancement in all hydrogen nuclei of SLM (Fig. 6).
The intensification of all SLM signals in the 1D ROESY NMR spectra, after selective excitation of the resonance frequency of any hydrogen nuclei from the Eudragit EPO polymer, revealed that SLM/Eudragit EPO complexes were formed when produced by SD. This was not observed for the other drug-polymer systems, in which no NOE signal enhancements were observed. These findings revealed that a more effective chemical interaction was obtained between SLM and Eudragit EPO, produced by the SD method, supporting the supersaturation data.
Stability Studies
ASD at 3:7 SLM-Eudragit EPO produced by SD was selected to conduct stability studies because it presented the highest amount of drug dissolved in the supersaturation studies performed, as well as more effective drug-polymer chemical interactions, which was confirmed by the solid-state characterization, contact angle, and NMR analysis. The capacity to maintain the amorphous characteristic for up to 3 months at different storage conditions of the ASD 3:7 SLM-Eudragit EPO was compared with the amorphous SLM, both produced by SD technique. The XRPD diffractograms obtained are shown in Fig. 7.
Amorphous SLM maintained its amorphous characteristic during the first 30 days in all conditions (data not shown). However, after 90 days of storage, the recrystallization could be noticed for all conditions. In contrast, ASD was able to strongly suppress the recrystallization of SLM from the formulation for up to 3 months. This can be attributed to the chemical interaction between SLM and Eudragit EPO, which reduced the recrystallization potential of the drug in solid state; consequently, this avoided the unwanted recrystallization that could result in unpredictable changes in the dissolution profile.
CONCLUSION
ASD method production has been found to be a critical parameter in providing chemical interactions between the drug and the polymer. This study revealed ASD formulations capable of generating SLM supersaturation in FaSSIF biorelevant medium. This supersaturated state maintained for up to 24 h was a result of the wettability improvement and the amorphization of the systems. The SD method was more effective regarding the occurrence of chemical interactions between SLM and Eudragit EPO, which was found to improve the solid-state physical stability of ASD formulation to crystallization.
This new formulation containing SLM as the SDDS may result in dose reduction due to the higher amount of drug able to be absorbed by the gastrointestinal tract. This dose reduction may consequently provide lower side effects, improvement in patient treatment adherence, and decrease the selection of resistant strains to antibiotics. Therefore, the impact of polymer choice and ASD method production on the stability and supersaturation maintenance towards crystallization should be carefully considered benefiting from these systems.
Abbreviations
- API:
-
Active pharmaceutical ingredients
- ASD:
-
Amorphous solid dispersion
- BCS:
-
Biopharmaceutical Classification System
- FaSSIF:
-
Fasted State Simulated Intestinal Fluid
- SDDS:
-
Supersaturating drug delivery systems
- SLM:
-
Sulfamethoxazole
- Soluplus:
-
Polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer
References
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72.
Raina SA, Zhang GGZ, Alonzo DE, Wu J, Zhu D, Catron ND, et al. Enhancements and limits in drug membrane transport using supersaturated solutions of poorly water soluble drugs. J Pharm Sci. 2014;103(9):2736–48.
Taylor LS, Zhang GGZ. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv Drug Deliv Rev. 2016;101:122–42.
Gilley AD, Arca HC, Nichols BLB, Mosquera-Giraldo LI, Taylor LS, Edgar KJ, et al. Novel cellulose-based amorphous solid dispersions enhance quercetin solution concentrations in vitro. Carbohydr Polym. 2017;157:86–93.
Zhou D, He S, Cong Y, Xie Z, Chen X, Jing X, et al. A polymer-(multifunctional single-drug) conjugate for combination therapy. J Mater Chem B. 2015;3(24):4913–21.
Santos A, Sinn Aw M, Bariana M, Kumeria T, Wang Y, Losic D. Drug-releasing implants: current progress, challenges and perspectives. J Mater Chem B. 2014;2:6157–82.
Adebisi AO, Kaialy W, Hussain T, Al-Hamidi H, Nokhodchi A, Conway BR, et al. Solid-state, triboelectrostatic and dissolution characteristics of spray-dried piroxicam-glucosamine solid dispersions. Colloids Surfaces B Biointerfaces [Internet]. 2016 Oct 1 [cited 2018 Sep 3];146:841–51. Available from: https://www.sciencedirect.com/science/article/pii/S0927776516305343
Harmon P, Galipeau K, Xu W, Brown C, Wuelfing WP. Mechanism of dissolution-induced nanoparticle formation from a Copovidone-based amorphous solid dispersion. Mol Pharm. 2016;13(5):1467–81.
Genina N, Hadi B, Löbmann K. Hot melt extrusion as solvent-free technique for a continuous manufacturing of drug-loaded mesoporous silica. J Pharm Sci. 2018;107(1):149–55.
Paudel A, Worku ZA, Meeus J, Guns S, Van Den Mooter G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: formulation and process considerations. Int J Pharm. 2013;453(1):253–84.
Lindenberg M, Kopp S, Dressman JB. Classification of orally administered drugs on the World Health Organization model list of essential medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm. 2004;58(2):265–78.
Eliopoulos GM, Huovinen P. Resistance to trimethoprim-sulfamethoxazole. Clin Infect Dis. 2001;32(11):1608–14.
Alonzo DE, Zhang GGZ, Zhou D, Gao Y, Taylor LS. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharm Res. 2010;27(4):608–18.
PA Masters, TA O'Bryan, J Zurlo, DQ Miller, N Joshi Trimethoprim-sulfamethoxazole revisited. Arch Intern Med 2003;163(4):402–410. Available from: https://doi.org/10.1001/archinte.163.4.402
Tian Y, Jones DS, Andrews GP. An investigation into the role of polymeric carriers on crystal growth within amorphous solid dispersion systems. Mol Pharm. 2015;12(4):1180–92.
Caron V, Hu Y, Tajber L, Erxleben A, Corrigan OI, McArdle P, et al. Amorphous solid dispersions of sulfonamide/soluplus® and sulfonamide/PVP prepared by ball milling. AAPS PharmSciTech. 2013;14(1):464–74.
Pharmacopieial Convention U. S. USP [Internet]. Estándares de Referencia 2008. Available from: http://www.usp.org/es/estandares-de-referencia/hojas-de-datos-tecnicos
ICH guidelines. Q2(R1): Validation of analytical procedures: text and methodology. Int Conf Harmon. 2005;1994(November 1996):17.
Niu J, Zhang L, Li Y, Zhao J, Lv S, Xiao K. Effects of environmental factors on sulfamethoxazole photodegradation under simulated sunlight irradiation: kinetics and mechanism. J Environ Sci (China). 2013;25(6):1098–106.
Wang P, Zhang D, Zhang H, Li H, Ghosh S, Pan B. Impact of concentration and species of sulfamethoxazole and ofloxacin on their adsorption kinetics on sediments. Chemosphere. 2017;175:123–9.
Hardung H, Djuric D, Ali S. Combining HME & solubilization: Soluplus® - the solid solution. Drug Deliv Technol. 2010;10(3):20–7.
Khachane P, Date AA, Nagarsenker MS. Eudragit EPO nanoparticles: application in improving therapeutic efficacy and reducing ulcerogenicity of meloxicam on oral administration. J Biomed Nanotechnol. 2011;7(4):590–7.
Barbosa JAC, Abdelsadig MSE, Conway BR, Merchant HA. Using zeta potential to study the ionisation behaviour of polymers employed in modified-release dosage forms and estimating their pKa. Int J Pharm X. 2019;1:100024. Available from: https://doi.org/10.1016/j.ijpx.2019.100024.
Xie T, Gao W, Taylor LS. Impact of Eudragit EPO and hydroxypropyl methylcellulose on drug release rate, supersaturation, precipitation outcome and redissolution rate of indomethacin amorphous solid dispersions. Int J Pharm. 2017;531:313–23.
Thiry J, Broze G, Pestieau A, Tatton AS, Baumans F, Damblon C, et al. Investigation of a suitable in vitro dissolution test for itraconazole-based solid dispersions. Eur J Pharm Sci. 2016;85:94–105.
Xia D, Yu H, Tao J, Zeng J, Zhu Q, Zhu C, et al. Supersaturated polymeric micelles for oral cyclosporine A delivery: the role of Soluplus–sodium dodecyl sulfate complex. Colloids Surfaces B Biointerfaces [Internet]. 2016 May 1 [cited 2018 Sep 3];141:301–10. Available from: https://www.sciencedirect.com/science/article/pii/S0927776516300479
Hughey JR, Keen JM, Miller DA, Kolter K, Langley N, McGinity JW. The use of inorganic salts to improve the dissolution characteristics of tablets containing Soluplus??-based solid dispersions. Eur J Pharm Sci 2013;48(4–5):758–766. Available from: https://doi.org/10.1016/j.ejps.2013.01.004
Acknowledgments
The authors would like to thank Dr. Adailton J. Bortoluzzi for technical support during XRPD analyses, Laboratório Central de Microscopia Eletrônica (LCME) for SEM measurements and Post-graduation Program in Chemistry of Universidade Federal de Santa Catarina for the facilities.
Funding
This study was supported by the Coordination for Enhancement of Higher Education Personnel (CAPES) — financial code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 2099 kb)
Rights and permissions
About this article
Cite this article
Mendes, C., Andrzejewski, R.G., Pinto, J.M.O. et al. Impact of Drug-Polymer Interaction in Amorphous Solid Dispersion Aiming for the Supersaturation of Poorly Soluble Drug in Biorelevant Medium. AAPS PharmSciTech 21, 189 (2020). https://doi.org/10.1208/s12249-020-01737-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01737-6