Abstract
Corticosteroid resistance poses a major challenge to effective treatment of chronic obstructive pulmonary diseases. However, corticosteroid resistance can be overcome by co-administration of theophylline. The aim of this study was to formulate the corticosteroid budesonide with theophylline into inhalable dry powders intended for pulmonary combination therapy. Four types of spray-dried powders were prepared: (i) budesonide and theophylline co-dissolved and processed using a 2-fluid nozzle spray drier, (ii) budesonide nanocrystals and dissolved theophylline co-dispersed and processed using a 2-fluid nozzle spray drier, (iii) dissolved budesonide and dissolved theophylline processed using a 3-fluid nozzle spray drier, and (iv) budesonide nanocrystals and dissolved theophylline processed using a 3-fluid nozzle spray drier. Spray drying from the solutions resulted in co-amorphous (i) and partially amorphous powders (iii), whereas spray drying of the nanosuspensions resulted in crystalline products (ii and iv). Even though budesonide was amorphous in (i) and (iii), it failed to exhibit any dissolution advantage over the unprocessed budesonide. In contrast, the dissolution of budesonide from its nanocrystalline formulations, i.e., (ii) and (iv), was significantly higher compared to a physical mixture or unprocessed budesonide. Furthermore, the spray-dried powders obtained from the 2-fluid nozzle spray drier, i.e., (i) and (ii), exhibited co-deposition of budesonide and theophylline at the same weight ratio in the aerodynamic assessment using the New Generation Impactor. In contrast, the depositions of budesonide and theophylline deviated from the starting weight ratio in the aerodynamic assessment of spray-dried powders obtained from the 3-fluid nozzle spray drier, i.e., (iii) and (iv). Based on these results, the powders spray-dried from the suspension by using the 2-fluid nozzle spray drier, i.e., (ii), offered the best formulation properties given the physically stable crystalline solid-state properties and the co-deposition profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In recent years, there has been a trend towards pulmonary delivery of multiple active pharmaceutical ingredients (APIs) in combination for the treatment of lung diseases due to improved therapeutic efficacy, safety, and patient compliance (1). Pharmaceutical combination therapy can be achieved by using separate inhalers sequentially. However, fixed combination dosage forms are more attractive from a patient compliance point of view, as compared to the sequential administration of separate dosage forms (2,3,4). Examples of fixed combination dosage forms include the combination of beclomethasone dipropionate and salbutamol, salmeterol xinafoate and fluticasone propionate, and budesonide and formoterol, respectively (5).
Chronic obstructive pulmonary disease (COPD) is a complex lung disease that involves multiple metabolic and intracellular signaling pathways. Corticosteroids (e.g., budesonide, which was used as a model corticosteroid in this study) have been commonly used in the treatment of COPD-associated inflammation (6). Corticosteroids can reverse histone acetylation induced by nuclear factor-κB and switch off the expression of inflammatory proteins by binding to glucocorticoid receptors and recruiting histone deacetylase-2 (HDAC-2) (7). However, corticosteroids have limited efficacy, which may be due to reduced activity of HDAC-2 in COPD patients (8). A promising approach to overcome the limited efficacy of corticosteroids in the treatment of COPD-associated inflammation is to co-deliver corticosteroids with an API, which can restore the activity of HDAC-2, using fixed-dose combination inhalers (9). Theophylline is known to restore the activity of HDAC-2 (10). Hence, combination of corticosteroids with theophylline in theory has the potential of overcoming corticosteroid resistance, which otherwise limits the clinical efficacy of anti-inflammatory corticosteroids in the treatment of COPD (9).
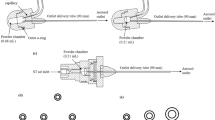
Dry powder-based dosage forms for pulmonary combination therapy are attractive because they display improved physicochemical stability, compared to solution- or suspension-based combination dosage forms (11). Commonly applied methods for manufacturing of inhalable dry powders include milling, spray drying, spray freeze drying, and the use of supercritical fluid technologies (12). Among these approaches, spray drying is a cost-effective method, which offers the possibility to engineer particles with customized properties (12,13). Inhalable dry powder dosage forms of API combinations have been formulated containing, e.g., doxycycline and ciprofloxacin (14), ciprofloxacin hydrochloride and gatifloxacin hydrochloride (15), and colistin and rifampicin (16), respectively. For the spray drying of these API combinations, a conventional 2-fluid (2-F) nozzle spray drier is used to prepare the inhalable dry powders. The two APIs are prepared as a single solution containing both APIs and fed into the 2-F nozzle, followed by atomization and drying to solid particles. An alternative to the use of the 2-F nozzle spray drier, a novel 3-fluid (3-F) nozzle spray drier can also be used to prepare inhalable dry powders of API combinations, where the two different APIs are prepared in two separate feed samples. The two APIs can in theory be dissolved in two different solvents with optimized solubilization properties for each API. The two different feed samples are pumped through two separate liquid passages, and they meet at the tip of the nozzle, where they are atomized by the compressed gas provided by the third passage (17). The feed sample used for spray drying can be in form of a solution or suspension (18). In addition, the solvents used for preparing the feed sample can be water or organic solvent, or co-solvent.
In this study, we aimed to formulate a combination of budesonide and theophylline into inhalable dry powders intended for the treatment of COPD. For this purpose, two types of spray driers (i.e., 2-F nozzle and 3-F nozzle spray driers) were employed to prepare the inhalable dry powders, and the feed samples were in two forms (i.e., solution and suspension). Four types of inhalable dry powders were obtained, and the morphology, solid-state properties, moisture content, dissolution profile, and aerosolization performance of these dual API-containing dry powders were characterized.
MATERIALS AND METHODS
Materials
Budesonide (Mw = 430.5 g/mol, > 98%) was a gift from Hubei Gedian Renfu Pharmaceuticals (Wuhan, Hubei, China). Theophylline (Mw = 180.2 g/mol, anhydrous, ≥ 99%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Poloxamer 188 and polyethylene glycol sorbitan monolaurate were acquired from Sigma-Aldrich. Ethanol (> 99.7%, HPLC grade) was purchased from VWR International (Leuven, Belgium) and acetonitrile (> 99.9%, HPLC grade) was from Chemsolute (Roskilde, Denmark). Water used for the preparation of all solutions was prepared using a MilliQ water system from LabWater (Los Angeles, CA, USA).
Preparation of Nanosuspension by Wet Ball Milling
The nanosuspension of budesonide was prepared by wet ball milling as previously reported (19,20). Briefly, glass beads (Sigma-Aldrich, St. Louis, MO, USA) with a diameter of 0.5 and 1.0 mm, respectively, mixed at a weight ratio of 1:3 were used for the wet ball milling. A volume of 10 ml of aqueous suspension containing 300 mg of budesonide, 100 mg of poloxamer 188, and 10 g of glass beads was stirred by using a magnetic stirrer (IKA RCT basic, IKA-Werke GmbH & Co. KG, Staufen, Germany) at dial 7 (dial 1–10 equals a speed range from 50 to 1100 min−1) at room temperature (rt) for 5 h. The milled particles were ultracentrifuged to remove the liquid phase using gradient centrifugation, i.e., 6000g for 5 min, 12,000g for 5 min, 21,000g for 5 min, 34,000g for 5 min, and 48,000g for 10 min at 25°C (Optima™ Max-XP Ultracentrifuge, Beckman Coulter, CA, USA). The supernatant was discarded, and the pellet containing the nano-sized budesonide particles was collected and re-dispersed in water. The particle size and morphology as well as the stability of the nanosuspension have been reported in a previous publication (21).
Preparation of Inhalable Dry Powders by Spray Drying
Inhalable dry powders of budesonide and theophylline were prepared by using a Büchi B-290 spray dryer (Büchi Labortechnik AG, Falwil, Switzerland) applying the process conditions listed in Table I and formulation compositions listed in Table II. The 2-F nozzle consists of one gas passage and one liquid passage (Fig. 1). The feed solution containing a mixture of budesonide and theophylline dissolved in a water-ethanol mixture (20:80, v/v) was spray-dried into a powder formulation. In addition, an aqueous feed suspension containing the nanosuspension of budesonide and dissolved theophylline was spray-dried into a nanocomposite powder. The 3-F nozzle is equipped with one gas passage and two liquid passages (Fig. 1). Budesonide dissolved in a water-ethanol mixture (20:80, v/v) and theophylline dissolved in a water-ethanol mixture (20:80, v/v) were supplied from separate passages and spray-dried using the spray drier equipped with the 3-F nozzle (Büchi Labortechnik AG, Flawil, Switzerland). In addition, the nanosuspension of budesonide and the aqueous solution of theophylline were fed separately into two different liquid passages and spray-dried into a nanocomposite powder. An inert loop B-295 (Büchi Labortechnik AG) and a dehumidifier (Büchi Labortechnik AG) were used when ethanol was included in the feed solution.
Morphology of Spray-Dried Powders
The morphology of unprocessed budesonide and theophylline was assessed by using a Hitachi TM3030 Tabletop scanning electron microscope (SEM, Hitachi High-Technologies Corporation, Tokyo, Japan). The unprocessed materials were sputter-coated with a gold layer and imaged at an accelerating voltage of 15 kV. The morphology of the spray-dried powders was examined by using a FEI Quanta 3D FEG scanning electron microscopy (FEI, Hillsboro, OR, USA). The samples were sputter-coated with gold (6 nm) using a Leica EM ACE 200 (Vienna, Austria), and images were acquired at × 20,000 magnification.
Solid-State Properties of Spray-Dried Powders
X-ray powder diffraction (XRPD) measurements were performed using an X’Pert PRO X-ray diffractometer (PANalytical, Almelo, The Netherlands). The samples were exposed to Cu Kα radiation (λ = 1.54187 Å) generated from a current of 40 mA and a voltage of 45 kV, respectively. Data were collected and analyzed using the X’Pert Data Collector software (PANalytical). The samples were scanned from 5 to 35° 2θ with a step size of 0.026° 2θ and a scanning speed of 0.067° 2θ/s. The thermal behavior of the unprocessed materials and the spray-dried powders was characterized by using a Discovery differential scanning calorimeter (DSC, TA Instruments, New Castle, USA) under a nitrogen flow of 50 ml/min. An amount of 2–4 mg of the samples was filled into a Tzero aluminum pan and sealed with a Tzero lid. The melting points (Tm) of the unprocessed materials and the spray-dried powders were determined at a heating rate of 10°C/min. For the spray-dried powders, a modulated temperature mode was used to determine the glass transition temperature (Tg), where samples were heated at a linear heating rate of 2°C/min, an amplitude of 0.2120°C and a period of 40 s. Analysis of the Tm (onset temperature) and the Tg (midpoint) from the reversing heat flow was performed by using the Trios software (TA Instruments). Determination of the Tg was based on three independent samples of each spray-dried powder.
Moisture Content of Spray-Dried Powders
A Discovery thermogravimetric analyzer (TGA, TA Instruments, New Castle, USA) was used to determine the moisture content of the spray-dried powders. The samples were placed in 100-μl platinum pans and heated from rt to 150°C at a heating rate of 10°C/min. The moisture content is expressed as the percentage of the weight loss between rt and 140°C. All measurements were performed on three independent samples from one batch of each spray-dried powder.
Dissolution Testing
The dissolution profiles of unprocessed theophylline, a physical mixture of unprocessed budesonide and theophylline, and the spray-dried powders were evaluated using a USP type II apparatus (Erweka DT70 dissolution tester, Erweka GmbH, Heusenstamm, Germany) in a modified, custom-made set-up with a rotating mini-paddle at a rotation speed of 50 rpm with 200 ml of 10-mM phosphate buffer (pH 7.4 ± 0.1) at 37°C. A hydroxypropyl methylcellulose capsule (Size 3; Capsugel, West Ryde, Australia) loaded with a weighed amount of powder equivalent to an amount of 4 mg of budesonide or approximately 1.67 mg of theophylline was placed in a custom-made metal sinker, which was subsequently submerged in the dissolution medium. At specific time points (5, 10, 20, 30, 45, and 60 min), a volume of 3 ml of the dissolution medium was withdrawn and replaced immediately with pre-warmed medium. The samples were filtered using 0.22-μm syringe filters (Q-Max Nylon, Frisenette ApS, Knebel, Denmark), and the concentrations of budesonide and theophylline were determined by high-performance liquid chromatography (HPLC), as described below. The experiments were conducted using two independent samples. The average percentages of cumulative budesonide and theophylline release, respectively, were determined as a function of time.
Assessment of Aerodynamic Properties
A Next Generation Impactor (NGI, Copley Scientific, Nottingham, UK) was used to evaluate the aerodynamic performance of the spray-dried powders. All NGI stages were coated with a polyethylene glycol sorbitan monolaurate-ethanol mixture (10:90, v/v) to minimize particle bouncing after deposition. Approximately 10 mg of powder sample was loaded in a hydroxypropyl methylcellulose capsule (Size 3; Capsugel) and placed in an RS01 Monodose dry powder inhaler device (Plastiape, Osnago, Italy). The aerodynamic performance was assessed at a flow rate of 95 l/min for 2.4 s. After actuation, the inhaler, the capsule, the throat, all stages, and the micro-orifice collector (MOC) were washed separately with a water-ethanol mixture (60:40, v/v). The budesonide and theophylline concentrations were quantified by HPLC, as described below. All experiments were conducted using at least three independent samples of one batch of each spray-dried powder. The fine particle fraction (FPF) of loaded powder (FPF loaded), the FPF of recovered powder (FPF recovered), and the FPF of emitted powder (FPF emitted) were defined as the mass fraction of particles < 5.0 μm of aerodynamic diameter with respect to the loaded mass, recovered mass, and emitted mass, respectively.
Quantification of Budesonide and Theophylline by HPLC
The concentrations of budesonide and theophylline in the samples obtained from the dissolution testing and the assessment of aerodynamic properties were quantified by HPLC using an Agilent 1260 infinity HPLC system (Agilent, Santa Clara, USA) equipped with an Agilent 1290 Diode Array Detector. A volume of 20 μl of sample was injected into an Agilent 5 TC-C18 column (250 × 4.6 mm, 5 μm, Agilent) at a flow rate of 1.0 ml/min at rt, and the APIs were detected using an ultraviolet detector. Quantification of budesonide was conducted using a mixture of 35% (v/v) acetonitrile and 65% (v/v) monobasic sodium phosphate buffer (3.17 mg/ml, pH 3.2 ± 0.05) as the mobile phase at a detection wavelength of 254 nm. Linearity, the limit of detection, and the limit of quantification have been reported previously (21). Quantification of theophylline was performed at a detection wavelength of 280 nm using a gradient of monobasic sodium phosphate buffer (3.17 mg/ml, pH 3.2 ± 0.05) and acetonitrile (Supplementary data, Table SI). The standard curve was linear in the range of 0.15–100 μg/ml (R2 > 0.999). The limit of detection and the limit of quantification were 5 and 20 ng/ml, respectively.
Statistical Analysis
All experimental data are presented as mean values ± standard deviation (SD), unless otherwise stated. Statistically significant differences were assessed by a one-way analysis of variance (ANOVA) or a t test at a 0.05 significance level (GraphPad Software, La Jolla, CA, USA).
RESULTS AND DISCUSSION
Morphology
Unprocessed budesonide displayed an irregular shape and a broad size distribution (Fig. 2a), whereas unprocessed theophylline exhibited a flake-like shape (Fig. 2b). The primary particles of budesonide-theophylline 2-F were spherical with a diameter smaller than 5 μm (Fig. 2c). The primary particles of budesonide-theophylline 3-F were also mostly spherical, but the particle size distribution appeared to be broader, compared to the budesonide-theophylline 2-F particles (Fig. 2d). The budesonide nanosuspension-theophylline 2-F and the budesonide nanosuspension-theophylline 3-F (Fig. 2e, f) particles possessed comparable morphological features, which were as expected different from those of the budesonide-theophylline 2-F and budesonide-theophylline 3-F particles. The average hydrodynamic diameter (z-average) of the nanosuspension of budesonide was approximately 300 nm, which was reported in a previously published paper (21). Budesonide nanosuspension-theophylline 2-F and budesonide nanosuspension-theophylline 3-F particles appeared to be composed of nano-sized crystallites that resulted in a rough surface appearance of the particles and flake-like crystals embedded throughout the matrix composed of nano-sized crystallites. The flake-like characteristics of unprocessed theophylline and the use of the nanosuspension of budesonide in the feed formulation suggest that the flake-like crystals displayed in the particles were from theophylline, and the nano-sized particles were from budesonide.
Solid-State Analysis
The distinct diffraction peaks present in the diffractograms of unprocessed budesonide and theophylline confirm the crystalline state of the unprocessed APIs as seen in Fig. 3. The diffractogram of budesonide-theophylline 2-F powder displays a halo, which suggests an amorphous form of budesonide and theophylline. The diffractogram of budesonide-theophylline 3-F powder indicated partial crystallinity mainly due to the presence of diffraction peaks of the anhydrous crystalline form of theophylline (7.2° and 12.7° 2θ) (22); however, the underlying halo pattern suggests that at least some amorphous content of theophylline has been obtained using the 3-F nozzle approach. In contrast, the diffractograms of budesonide nanosuspension-theophylline 2-F and budesonide nanosuspension-theophylline 3-F powders display distinct diffraction peaks of crystalline budesonide (6.2°, 11.5°, 14.6°, 15.5°, and 16.2° 2θ) and the anhydrous crystalline form of theophylline (7.2° and 12.7° 2θ) (21), suggesting a crystalline state. The similarity of the diffractograms to the diffractogram of unprocessed physical mixtures suggests that these two samples are indeed a crystalline physical mixture of both budesonide and theophylline. This was expected since budesonide was present in crystalline form prior to spray drying in the suspension, and a pure theophylline solution cannot be prepared as an amorphous material using spray drying but also results in a crystalline product.
Representative X-ray diffractograms of powders, including unprocessed budesonide (a), unprocessed theophylline (b), a physical mixture of unprocessed budesonide and theophylline (c), budesonide-theophylline 2-F (d), budesonide-theophylline 3-F (e), budesonide nanosuspension-theophylline 2-F (f), and budesonide nanosuspension-theophylline 3-F (g)
The sample that displayed amorphous properties was subjected to Tg analysis by DSC. The budesonide-theophylline 2-F powder displayed a single Tg of 71.3 ± 0.6°C, which confirms the X-ray data suggesting successful co-amorphous drug-drug formation (23,24). To further investigate if the Tg is representative of a co-amorphous drug-drug formulation formed after spray drying, neat amorphous budesonide was prepared via spray drying at an inlet temperature of 100°C using a water-ethanol mixture (20:80, v/v) as the solvent (Supplementary data, Fig. S1). Neat amorphous budesonide displayed a Tg of 89.4 ± 1.0°C. Theophylline cannot be made amorphous via spray drying (25) nor via any other approach (26), and hence an experimental Tg analysis cannot be determined for neat amorphous theophylline.
The thermograms of unprocessed budesonide and theophylline displayed endothermic events at approximately 253°C and 273°C (Fig. 4a, b), which represent the melting points of budesonide and theophylline, respectively (27,28). Based on the melting peak of theophylline at 273°C, the Tg of theophylline was predicted to be approximately 92.8°C, using Eq. 1 (and temperatures in Kelvin) (27):
Representative DSC thermograms of powders, including unprocessed budesonide (a), unprocessed theophylline (b), a physical mixture of unprocessed budesonide and theophylline (c), budesonide-theophylline 2-F (d), budesonide-theophylline 3-F (e), budesonide nanosuspension-theophylline 2-F (f), and budesonide nanosuspension-theophylline 3-F (g)
A theoretical Tg for the budesonide-theophylline 2-F powder can now be calculated using the Flory-Fox equation (28):
where Tg 1,2 is the Tg of the budesonide-theophylline 2-F powder, and Tg 1 and Tg 2 are the Tgs of budesonide (362.4 K) and theophylline (365.8 K, calculated above), respectively; w1 and w2 are the weight fraction of budesonide (0.705) and theophylline (0.295), respectively. Using Eq. 2, the theoretical Tg of the budesonide-theophylline 2-F powder can be calculated (Tg 1,2 = 89.8°C). The Tg of the budesonide-theophylline 2-F power measured by DSC is lower than the theoretical Tg. This may be explained by the presence of residual moisture in the powder (0.5 ± 0.04%) acting as a plasticizer, thus lowering the Tg of the budesonide-theophylline 2-F powder compared to the theoretical Tg (29). The physical mixture displayed a melting point at approximately 224°C (Fig. 4c), which is 29 and 49°C below the melting points of crystalline budesonide and crystalline theophylline, respectively, suggesting that the 1:1 molar ratio represents a eutectic mixture. The DSC analysis of the spray-dried powders was performed in the temperature range from 30 to 240°C due to thermal instability: For example, TGA analysis suggested that the budesonide nanosuspension-theophylline 2-F powder showed approximately 10% weight loss at 250°C (data not shown). The thermograms of budesonide-theophylline 2-F and budesonide-theophylline 3-F powders displayed an exothermic peak at 130 and 115°C, respectively, which implies that the amorphous budesonide and theophylline recrystallize upon heating (Fig. 4d, e) (30,31,32). The recrystallization event of amorphous budesonide and theophylline was followed by a melting endotherm with an onset temperature at approximately 220°C, which is comparable to the melting point of the eutectic mixture of budesonide and theophylline. The thermograms of budesonide nanosuspension-theophylline 2-F and budesonide nanosuspension-theophylline 3-F powders displayed only one endothermic event at approximately 220°C as expected, because these samples are not amorphous (Fig. 4f, g).
Moisture Content
The moisture contents of powders spray-dried from suspensions were significantly lower (p < 0.05) than the moisture contents of powders spray-dried from solutions (Table III). The higher moisture content may be attributed to the fact that budesonide and theophylline are amorphous in the budesonide-theophylline 2-F powder and partially amorphous in the budesonide-theophylline 3-F powder, which results in more hygroscopic materials (33) compared to the moisture contents of the budesonide nanosuspension-theophylline 2-F powder and the budesonide nanosuspension-theophylline 3-F powder.
Dissolution Profiles
Dissolution tests were performed for unprocessed theophylline, a physical mixture of unprocessed budesonide and theophylline, and the spray-dried powders (Fig. 5). As expected, theophylline dissolved relatively fast, as compared to budesonide, from the physical mixture, because theophylline is a water-soluble API (0.85% solubility in water at 25°C) (34). The dissolution of budesonide from unprocessed budesonide and the nanosuspension of budesonide have been reported in a previous study: Less than 10% of budesonide was dissolved from unprocessed budesonide after 60 min and approximately 60% of budesonide was dissolved from the nanosuspension of budesonide after 10 min (21). The dissolution of budesonide from the physical mixture was very slow (Fig. 5): Only approximately 10% of the drug was dissolved after 60 min. The formulations of budesonide nanosuspension-theophylline 2-F and budesonide nanosuspension-theophylline 3-F improved the dissolution rate of budesonide, and approximately 90% of budesonide was dissolved after 60 min. It is apparent from the SEM and XRPD results that the co-spray-dried nanosuspension of budesonide and solubilized theophylline consists of nano-sized particles of budesonide and crystalline theophylline. Hence, it can be assumed that the nano-sized particles of budesonide are re-dispersed during the dissolution process. The increased dissolution rate of budesonide in these formulations can be explained by the small size and thus large surface area of the particles (35).
Cumulative dissolution rate of budesonide in 10 mM phosphate buffer (pH 7.4) (a). A physical mixture of unprocessed budesonide and theophylline (○), budesonide-theophylline 2-F (▲), budesonide-theophylline 3-F (♦), budesonide nanosuspension-theophylline 2-F (▽), budesonide nanosuspension-theophylline 3-F (◁). Cumulative dissolution rate of theophylline in 10 mM phosphate buffer (pH 7.4) (b). Unprocessed theophylline (■), a physical mixture of unprocessed budesonide and theophylline (○), budesonide-theophylline 2-F (▲), budesonide-theophylline 3-F (♦), budesonide nanosuspension-theophylline 2-F (▽), budesonide nanosuspension-theophylline 3-F (◁). Data points represent average values of two independent samples
Converting crystalline APIs into amorphous forms usually enhances the solubility of poorly water-soluble APIs (36). However, in this study, no significant improvement in the dissolution rate of budesonide was observed for the co-amorphous formulation (budesonide-theophylline 2-F powder) (Fig. 5). One reason for this finding could be that the applied dissolution method affects the dissolution behavior of the budesonide-theophylline 2-F powder. In this study, the powder was loaded into a capsule and subsequently placed in a metal sinker to ensure a constant powder dose and to avoid floating of the powder on the surface of the dissolution medium upon exposure to the medium. The capsule shell and the amorphous powder may tend to form a gel-like structure upon contact with the dissolution medium thus preventing the release of budesonide. In addition, the amorphous powder may absorb moisture and form aggregates inside the capsule during dissolution, eventually resulting in poor powder dispersibility (37,38). Furthermore, amorphous powder can recrystallize once exposed to moisture or solvent, which has been found in a previously published study (39). It should be noted, however, that the used dissolution setup cannot reflect the dissolution conditions in the lung; the purpose of the dissolution experiments was simply to compare the dissolution rates of the four types of spray-dried powders.
Aerodynamic Performance
For inhalable combinations of dry powders, a challenge is that differences in particle properties between the individual APIs may result in segregation phenomena during inhalation, which can induce variability in dose deposition between the two different APIs (40). For the budesonide-theophylline 2-F powder and the budesonide nanosuspension-theophylline 2-F powders, the percentage masses of budesonide and theophylline, respectively, were not significantly different in the stage deposition of the NGI (Fig. 6a, b), suggesting that the two APIs most likely will deposit in the same regions of the lungs. The co-deposition of budesonide and theophylline from the budesonide-theophylline 2-F powder or from the budesonide nanosuspension-theophylline 2-F powder is due to the budesonide feed sample and the theophylline sample being pre-mixed before being subjected to spray drying. For the budesonide-theophylline 3-F powder, there was a significant difference between the budesonide content and the theophylline content in the capsule after actuation (Fig. 6c), and the budesonide and theophylline contents were significantly different in stage 4 of the NGI from the budesonide nanosuspension-theophylline 3-F powder (Fig. 6d). A possible explanation for these differences is that the budesonide liquid-feed and the theophylline feed solution were fed from two separate liquid passages and met at the tip of the 3-F nozzle. Hence, the mixing time may be insufficient for generating a homogeneous mixture the two APIs, eventually resulting in non-identical deposition of budesonide and theophylline.
Aerodynamic assessment of the spray-dried powders, including budesonide-theophylline 2-F (a), budesonide nanosuspension-theophylline 2-F (b), budesonide-theophylline 3-F (c), and budesonide nanosuspension-theophylline 3-F (d). The black bars denote budesonide and the white bars denote theophylline. The bars represent mean values ± SD (n ≥ 3). In c and d, the stars indicate significant differences: *p < 0.05
A significantly higher FPF emitted (p < 0.05) was obtained for the budesonide-theophylline 2-F powder, compared to the other three spray-dried powders (Table III). A high fraction of these three types of spray-dried powders was detected in the throat or on the stages with high cut-off diameters, and only minimal quantities of budesonide and theophylline were collected in the lower stages of the NGI. The surface morphology of budesonide nanosuspension-theophylline 2-F and budesonide nanosuspension-theophylline 3-F powders, i.e., existence of flake-like crystals in the rough particles, may be a reason to explain the low FPFs, as the flake-like crystals may result in a high inter-particulate cohesion. The lower FPF of budesonide-theophylline 3-F powder, compared to the budesonide-theophylline 2-F powder, may be because of the higher moisture contents, which could increase cohesion between particles and decrease the flowability (41).
Based on the results of this study, the pros and the cons of the four types of spray-dried powders are summarized in Table IV. It was observed that powders prepared using the 2-F nozzle and the 3-F nozzle spray driers were different, in particular in the deposition profiles of budesonide and theophylline, even though the spray drying parameters (i.e., feed rate, inlet temperature, drying air flow rate, and atomization air flow rate) and the formulation compositions (i.e., solvent, concentration, solution/suspension form) were kept identical. The feed sample of budesonide and the feed sample of theophylline were pre-mixed into a homogeneous mixture before subjecting them to spray drying, thus the depositions of budesonide and theophylline spray-dried using the 2-F nozzle spray drier were identical. However, this was not the case for the 3-F nozzle spray drier, as the feed samples were only mixed for a short time at the tip of the nozzle instead of being pre-mixed. In this study, the 3-F nozzle spray drying approach showed a limitation in preparing powders containing two APIs with a desired co-deposition profile for APIs, independent of the feed sample being in form of a solution or suspension. Hence, powders prepared using the 2-F nozzle spray drier (i.e., budesonide-theophylline 2-F and budesonide nanosuspension-theophylline 2-F) were superior to the powders prepared using the 3-F nozzle spray drier (i.e., budesonide-theophylline 3-F and budesonide nanosuspension-theophylline 3-F).
Formation of amorphous or partially amorphous materials was observed in powders spray-dried from solutions, as budesonide and theophylline were molecularly dispersed in the feed sample, and the fast evaporation of solvent during spray drying prevented the nucleation and/or growth of crystalline materials. In contrast, crystalline states of budesonide and theophylline were preserved or formed in the powders spray-dried from suspension, as budesonide crystallites were already present in the feed sample and theophylline was not able to transform to an amorphous form by itself during spray drying. A crystalline state of APIs is advantageous, as it ensures long-term physical stability of spray-dried powders during storage. At this point, it should be highlighted that the budesonide nanosuspension-theophylline 2-F powder and the budesonide nanosuspension-theophylline 3-F powder were superior to the budesonide-theophylline 2-F and the budesonide-theophylline 3-F powders. The two APIs were in crystalline state in the budesonide nanosuspension-theophylline 2-F powder and the budesonide nanosuspension-theophylline 3-F powder, whereas, the two APIs were amorphous and partially amorphous in the budesonide-theophylline 2-F powder and the budesonide-theophylline 3-F powder, respectively. Considering the solid-state properties and deposition profile in the aerodynamic assessment, the budesonide-theophylline 2-F powder was the best formulation among the four types of spray-dried powders.
CONCLUSIONS
Four types of inhalable powders containing budesonide and theophylline were formulated by applying different spray drying approaches. The powder prepared by spray drying the suspension using the 2-F nozzle spray drier is the best formulation compared to the powder prepared by spray drying the solution using the 2-F nozzle spray drier and the powders prepared by spray drying the solution and the suspension using the 3-F nozzle spray drier, as the crystalline states of budesonide and theophylline were preserved in the spray-dried powder and it displayed identical deposition profiles for both, budesonide and theophylline. In addition, this study has demonstrated the limitations of a 3-F nozzle spray drier in the preparation of two-API combinations with a desired co-deposition profile for the APIs.
References
Toews ML, Bylund DB. Pharmacologic principles for combination therapy. Proc Am Thorac Soc. 2005;2:282–9.
Huchon G, Magnussen H, Chuchalin A, Dymek L, Gonod FB, Bousquet J. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med. 2009;103(1):41–9.
Barnes PJ, Nicolini G, Bizzi A, Spinola M, Singh D. Do inhaled corticosteroid/long-acting beta(2)-agonist fixed combinations provide superior clinical benefits compared with separate inhalers? A literature reappraisal. Allergy Asthma Proc. 2012;33(2):140–4.
Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard ML, et al. Budesonide/formoterol in a single inhaler versus inhaled corticosteroids alone in the treatment of asthma. Pediatr Pulmonol. 2002;34(5):342–50.
Traini D, Adi H, Valet OK, Young PM. Preparation and evaluation of single and co-engineered combination inhalation carrier formulations for the treatment of asthma. J Pharm Sci. 2012;101(11):4267–76.
Cazzola M, Dahl R. Inhaled combination therapy with long-acting beta 2-agonists and corticosteroids in stable COPD. Chest. 2004;126(1):220–37.
Barnes PJ. Alveolar macrophages as orchestrators of COPD. Copd. 2004;1(1):59–70.
Barnes PJ. Theophylline. Am J Respir Crit Care. 2013;188(8):901–6.
Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. 2013;12(7):543–59.
Barnes PJ. Theophylline - new perspectives for an old drug. Am J Respir Crit Care. 2003;167(6):813–8.
Parumasivam T, Chang RY, Abdelghany S, Ye TT, Britton WJ, Chan HK. Dry powder inhalable formulations for anti-tubercular therapy. Adv Drug Deliv Rev. 2016;102:83–101.
Chow AH, Tong HH, Chattopadhyay P, Shekunov BY. Particle engineering for pulmonary drug delivery. Pharm Res. 2007;24(3):411–37.
Nandiyanto ABD, Okuyama K. Progress in developing spray-drying methods for the production of controlled morphology particles: from the nanometer to submicrometer size ranges. Adv Powder Technol. 2011;22(1):1–19.
Adi H, Young PM, Chan HK, Stewart P, Agus H, Traini D. Cospray dried antibiotics for dry powder lung delivery. J Pharm Sci. 2008;97(8):3356–66.
Lee SH, Teo J, Heng D, Ng WK, Chan HK, Tan RB. Synergistic combination dry powders for inhaled antimicrobial therapy: formulation, characterization and in vitro evaluation. Eur J Pharm Biopharm. 2013;83(2):275–84.
Zhou QT, Gengenbach T, Denman JA, Yu HH, Li J, Chan HK. Synergistic antibiotic combination powders of colistin and rifampicin provide high aerosolization efficiency and moisture protection. AAPS J. 2014;16(1):37–47.
Pabari RM, Sunderland T, Ramtoola Z. Investigation of a novel 3-fluid nozzle spray drying technology for the engineering of multifunctional layered microparticles. Expert Opin Drug Deliv. 2012;9(12):1463–74.
Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R. Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int. 2007;40(9):1107–21.
Liu T, Han M, Tian F, Cun D, Rantanen J, Yang M. Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: in vitro and in vivo evaluation. Carbohydr Polym. 2018;181:1143–52.
Yang JZ, Young AL, Chiang PC, Thurston A, Pretzer DK. Fluticasone and budesonide nanosuspensions for pulmonary delivery: preparation, characterization, and pharmacokinetic studies. J Pharm Sci. 2008;97(11):4869–78.
Leng D, Thanki K, Foged C, Yang M. Formulating inhalable dry powders using two-fluid and three-fluid nozzle spray drying. Pharm Res. 2018;35(12):247.
Otsuka M, Kinoshita H. Quantitative determination of hydrate content of theophylline powder by chemometric X-ray powder diffraction analysis. AAPS PharmSciTech. 2010;11(1):204–11.
Kissi EO, Kasten G, Lobmann K, Rades T, Grohganz H. The role of glass transition temperatures in coamorphous drug-amino acid formulations. Mol Pharm. 2018;submitted;15:4247–56.
Dengale SJ, Grohganz H, Rades T, Löbmann K. Recent advances in co-amorphous drug formulations. Adv Drug Deliv Rev. 2016;100:116–25.
Monnier X, Viel Q, Schamme B, Petit S, Delbreilh L, Dupray V, et al. Vitrification of two active pharmaceutical ingredients by fast scanning calorimetry: from structural relaxation to nucleation phenomena. Int J Pharm. 2018;536(1):426–33.
Blaabjerg LI, Lindenberg E, Rades T, Grohganz H, Löbmann K. Influence of preparation pathway on the glass forming ability. Int J Pharm. 2017;521(1–2):232–8.
Alzghoul A, Alhalaweh A, Mahlin D, Bergstrom CA. Experimental and computational prediction of glass transition temperature of drugs. J Chem Inf Model. 2014;54(12):3396–403.
Fox TG, Flory PJ. 2nd-order transition temperatures and related properties of polystyrene. 1. Influence of molecular weight. J Appl Phys. 1950;21(6):581–91.
Hancock BC, Zografi G. The relationship between the glass-transition temperature and the water-content of amorphous pharmaceutical solids. Pharm Res. 1994;11(4):471–7.
Trotta V, Lee WH, Loo CY, Young PM, Traini D, Scalia S. Co-spray dried resveratrol and budesonide inhalation formulation for reducing inflammation and oxidative stress in rat alveolar macrophages. Eur J Pharm Sci. 2016;86:20–8.
Nolan LM, Tajber L, McDonald BF, Barham AS, Corrigan OI, Healy AM. Excipient-free nanoporous microparticles of budesonide for pulmonary delivery. Eur J Pharm Sci. 2009;37(5):593–602.
Rattanupatam T, Srichana T. Budesonide dry powder for inhalation: effects of leucine and mannitol on the efficiency of delivery. Drug Deliv. 2014;21(6):397–405.
Muzaffar K, Kumar P. Parameter optimization for spray drying of tamarind pulp using response surface methodology. Powder Technol. 2015;279:179–84.
Pillay V, Fassihi R. Evaluation and comparison of dissolution data derived from different modified release dosage forms: an alternative method. J Control Release. 1998;55(1):45–55.
Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;19:930–4.
Kanaujia P, Poovizhi P, Ng WK, Tan RBH. Amorphous formulations for dissolution and bioavailability enhancement of poorly soluble APIs. Powder Technol. 2015;285:2–15.
Perrut M, Jung J, Leboeuf F. Enhancement of dissolution rate of poorly-soluble active ingredients by supercritical fluid processes. Part I: micronization of neat particles. Int J Pharm. 2005;288(1):3–10.
Heertjes PM, Witvoet WC. Some aspects of the wetting of powders. Powder Technol. 1969;3(1):339–43.
Alonzo DE, Zhang GG, Zhou D, Gao Y, Taylor LS. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharm Res. 2010;27(4):608–18.
Pilcer G, Rosiere R, Traina K, Sebti T, Vanderbist F, Amighi K. New co-spray-dried tobramycin nanoparticles-clarithromycin inhaled powder systems for lung infection therapy in cystic fibrosis patients. J Pharm Sci. 2013;102(6):1836–46.
Adi S, Adi H, Tang P, Traini D, Chan HK, Young PM. Micro-particle corrugation, adhesion and inhalation aerosol efficiency. Eur J Pharm Sci. 2008;35(1–2):12–8.
Acknowledgments
The study was funded by a PhD stipend of the Faculty of Health and Medical Sciences, University of Copenhagen, Denmark, and the National Natural Science Foundation of China (No. 81573380). We acknowledge the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen, for the morphology study of the spray-dried particles. We thank the Danish Agency for Science, the Technology and Innovation for funding the Zetasizer Nano ZS and Novo Nordisk for supporting the Next Generation Impactor. We gratefully acknowledge PhD students Junwei Wang and Yongquan Li from the University of Copenhagen for valuable scientific discussion and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Philip J. Kuehl and Stephen W. Stein
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 66 kb)
Rights and permissions
About this article
Cite this article
Leng, D., Kissi, E.O., Löbmann, K. et al. Design of Inhalable Solid Dosage Forms of Budesonide and Theophylline for Pulmonary Combination Therapy. AAPS PharmSciTech 20, 137 (2019). https://doi.org/10.1208/s12249-019-1344-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1344-9