Abstract
To ensure optimal, reliable treatment, it is necessary to investigate the efficacy, safety and the optimal dose of drug substances and to develop suitable age-specific pharmaceutical formulations for the different paediatric age groups due to a lack of evidence-based therapeutic options for children. While WHO recommends the use of solid dosage forms in general, European Medicines Agency (EMA) requires evidence for the suitability of these dosage forms in the targeted age group. This review aims to summarize and discuss the data obtained in acceptability studies on the suitability of coated and uncoated mini-tablets in children of different ages in comparison to a sweet syrup considered as gold standard. The predefined outcome parameters ‘acceptability’ and ‘capability to swallow’ of the two different mini-tablet formulations (uncoated and film-coated) were statistically significantly higher than that of the syrup.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The lack of sufficient evidence-based therapeutic options for children currently leads to the administration of potentially inadequate substances or dosages in the paediatric population (1).
The preferred route for administering drugs in the paediatric population is the oral one. For young children, liquid formulations are most frequently used, because tablets are widely considered not to be applicable, at least up to the age of 6 years (2). But the application of medicines in form of liquids or syrups results in surprisingly inaccurate dosing with the risk of substantial under- or over-dosing and has major disadvantages, such as chemical, physical or microbial instability, taste issues, lack of controlled release properties, limited number of safe excipients and unreliable dosing because of incomplete swallowing (3).
Therefore, it is not only necessary to investigate the efficacy and the optimal dose of pharmaceutical substances for the different paediatric age groups but also to develop and investigate suitable age-specific drug dosage forms.
The lack of approved medicines and adequate drug formulations for children led to global regulatory initiatives. According to the European Regulation on Paediatric Medicines (1), suitable dosage forms for children, particularly for the very young children, have to be developed by a pharmaceutical company as part of their paediatric investigation plan (PIP) (4). While WHO recommends the use of solid dosage forms in all age groups (5), the European Medicines Agency (EMA) has previously questioned general applicability of solid dosage forms to children aged below 2 years (6). In a recent EMA guideline, the applicability is assumed to be a function of children’s age and size of tablet, and EMA requires evidence for the suitability of solid dosage forms in the respective age groups (7).
So far, only few scientifically valid data on applicability and swallowability of mini-tablets in small children has been available.
Thomson et al. administered one drug-free uncoated mini-tablets with 3-mm diameter to 100 children aged 2 to 6 years (8). Only 46% of the 2-year-old children were able to swallow the mini-tablets, whereas up to 86% of the oldest children were capable of swallowing. The authors therefore concluded that it was safe to use 3-mm mini-tablets in children aged 4–6 years. There was no comparative formulation administered.
In 2011, Van de Vijver et al. (9) published the result of a randomized phase II study in 16 children, aged 6 to 30 months, with cystic fibrosis, administering four different doses of pancrelipase via 1 to 4 enteric coated, 2-mm-diameter mini-tablets over 5 days. The primary endpoint was the effect of pancrelipase, palatability of the mini-tablets as a secondary parameter: ‘Palatability was scored fair to good by the parents in each of the treatment groups’ (9).
Van Riet-Nales et al. (10) tested the acceptability of and the preference among four oral placebo formulations (4-mm tablets, powder, suspension and syrup) in 148 domiciliary infants and preschool children aged 1 to 4 years. The parents were asked to report the child’s acceptability. At the end of the study, they were asked to report the preference of the child and of themselves. Results showed that the acceptability was significantly higher for the tablet than that for the suspension. The number of intakes fully swallowed was significantly higher for the tablet than that for the other formulations. Children and parents preferred the tablet and the syrup over the suspension and the suspension over the powder.
The first clinical trial in sixty 2- to 3-year-old children testing the acceptability of several mini-tablets administered at once (11) showed that most of these children were able to swallow five to ten 2-and 3-mm mini-tablets with jelly food.
OBJECTIVES
The overall aim of the three recently performed studies (12–14) in 517 children was the generation of valid data on acceptability of uncoated and coated mini-tablets of 2-mm diameter in children below the age of 6 years.
In a pilot study, conducted with 60 children, an equal suitability of 2-mm uncoated mini-tablets and glucose syrup for drug administration to young children was assumed (12). The objectives of the confirmatory cross-over trial (13) in 306 children were to investigate the acceptability of 2-mm uncoated drug-free mini-tablets (primary objective) and the acceptability of 2-mm coated drug-free mini-tablets (secondary outcome), as well as the capability to swallow both dosage forms (secondary outcome) compared with glucose syrup in six different age groups in the range of 6 months to 6 years.
After observing that even children aged 6 months to 1 year are able to accept and swallow mini-tablets, the question aroused if neonates were also able to do so. In 151 newborns, a 2-mm uncoated mini-tablet was compared to syrup. The primary objective of this trial was to prove that the acceptability of the uncoated mini-tablet in neonates is not inferior to the acceptability of the syrup. The secondary objectives relating to swallowability included the neonates’ percentage of swallowability, as well as potential differences in the swallowability of the two oral placebo formulations.
METHODS
All three studies were performed according to GCP, had an ethical approval from the University Ethics Committee Düsseldorf, Germany, and were registered in the German Clinical Trial Register. For each child, both parents gave their written informed consent. All in- and exclusion criteria were respected.
The trials had a single-centre, randomized, open cross-over design.
In the pilot study (12), 60 patients between 6 months and 6 years were divided into six age groups (6 months to <1, 1 to <2, 2 to <3, 3 to <4, 4 to <5 and 5 to <6 years). Each child received two oral drug-free formulations (2-mm uncoated mini-tablet and 3 ml 15% glucose syrup) in a randomized order.
In the confirmatory study (13), 306 patients sequentially received three oral drug-free formulations (2-mm uncoated and coated mini-tablets (Fig. 1) and 3 ml 15% glucose syrup) and were randomized to one of six possible sequences. The children were also aged between 6 months and 6 years and were stratified in the same six age groups as in the pilot study.
In both studies, mini-tablets were placed on the child’s tongue, and then the child was asked to swallow the mini-tablet with up to three mouthfuls of a drink of choice. The 15% glucose syrup was either administered via a pipette in a slightly opened mouth or with a spoon, depending on the child’s age. The glucose syrup had to be swallowed without any additional liquid. Each deglutition process was thoroughly observed. After each deglutition, the child’s mouth was inspected by the investigator using a flashlight to assess for residuals of the mini-tablets or leftover of the syrup. As soon as the child was ready for the respective second and in the confirmatory study of the third formulation, the administration and assessment procedures were repeated. All formulations were administered within a maximum of 15 min.
In the third study (14), 151 neonates aged 2 to 28 days were enrolled. Each child received one 2-mm uncoated mini-tablet in comparison to 0.5 ml of 15% glucose syrup in a randomized order. In contrast to the previous studies, the mini-tablet was placed in the cheek pouch of the child lying on the side (as performed for breast feeding), and the child had to swallow the mini-tablet with a drink of the parents’ choice. The glucose syrup was given with a pipette in the slightly opened mouth. The glucose syrup had to be swallowed without any additional liquid. Both formulations were administered within 10 min.
In each trial, the results were assessed according to predefined evaluation criteria, which were identical for the pilot and confirmatory studies (12, 13) and slightly varied for the neonatal study (14).
RESULTS
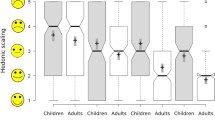
In the pilot study (12), the only age group completely swallowing both mini-tablet and liquid was 5–6 years. In the other age groups, there was no clear difference between the mini-tablets and the glucose syrup: some children chewed on the mini-tablet before swallowing. Interestingly, this was the case for all the age groups from 0.5 to 5 years, but very pronounced from 2 to 3 years (Fig. 2). In this age group, the mean value of the capability to swallow, the first primary endpoint, was slightly higher for the syrup than that for the mini-tablet. Only one child in the group from 1 to 2 years spat out the mini-tablet. For the very young children (0.5 to 1 year), the mean value of the capability to swallow was better for the mini-tablet than that for the syrup. When dosing the liquid formulation, we observed small runlets in three cases in the first age group (0.5 to 1 year). Complete refusal of the administration was observed in all the age categories except the group from 5 to 6 years, and it was surprisingly much higher for the liquid (13) than that for the solid (3) formulation. Almost 40% of the children between 1 and 2 years refused the liquid formulation, but only 10% the mini-tablet. It is important to mention that none of the 60 children choked on either the mini-tablet or the syrup and that no adverse events occurred in the present study.
In the case of mini-tablets, the categories ‘swallowed’ and ‘chewed’ (with subsequent swallowing) can be aggregated to a new category ‘overall acceptance’. For this category, the mean acceptance of the uncoated mini-tablet was higher or at least equal to that of the syrup in all the age categories.
This pilot study provided sufficient data to calculate the sample size of the following confirmatory study (13). Here, we demonstrated the suitability (‘swallowed’ or ‘chewed’) of the uncoated mini-tablet in all the age groups. As primary endpoint of this study, the acceptability of the uncoated 2-mm mini-tablet was significantly higher compared to that of the glucose syrup (difference in proportions 14.8, 95% CI 10.2–19.4; P < 0.0001) for the entire study population. All other results referred to secondary objectives: also, the acceptability of the coated mini-tablet was significantly higher compared to that of the glucose syrup (difference in proportions 14.9; 95% CI 10.4–19.5; P < 0.0001) for the entire study population. In each individual age group, the point estimates for the acceptability of uncoated mini-tablets (78.4–100%) or those of coated mini-tablets (84.3–100%), respectively, were higher than those of the syrup (64.7–90.2%). The capability to swallow for both the uncoated mini-tablet and the coated mini-tablet was superior compared to that for the syrup for the entire study population (uncoated mini-tablet: difference in proportions 12.3, 95% CI 5.4–19.3; P = 0.0008; coated mini-tablet 11.3, 95% CI 4.4–18.3; P = 0.002). In each individual age group, the point estimates for the capability to swallow uncoated mini-tablets (52.9–88.2%) or coated mini-tablets (47.1–84.3%) were higher than that of the syrup (39.2–72.5%). There was no significant difference in acceptability or capability to swallow between the coated or uncoated mini-tablets. All three pharmaceutical formulations were well tolerated: none of the 306 children coughed because of the syrup or the uncoated mini-tablet as a sign of inhaling particles. However, 2 of the 306 children (both in the age group 6 months–1 year) coughed because of the coated mini-tablet, but without any clinical relevance. No serious adverse events occurred.
Surprisingly, the suitability of mini-tablets was even superior to that of the syrup in most of the investigated age groups. As this superiority was also identified in children between 6 and 12 months, the third study (14) with 151 newborns was designed and conducted. The primary objective, the acceptability was defined as an aggregate of the two categories ‘everything swallowed’ and ‘partially swallowed’, and it was 100% for both oral placebo formulations (95% CI 97.6–100.0% for both the groups); thus, no non-inferiority test was performed. The swallowability (secondary objective) was high for mini-tablets (82.2; 95% CI 75.1–87.9%) as well as for syrup (72.2; 95% CI 64.3–79.1%) with a swallowability of mini-tablets non-inferior to syrup (P < 0.0001). Subsequently, in a two-sided test, swallowability of mini-tablets proved to be even higher than syrup (Δ 10.0; 95% CI 1.37–19.34%; P = 0.0315). No serious adverse event was observed in any of the 151 neonates for the two oral placebo formulations. Specifically, no neonate inhaled the formulation or coughed during ingestion of any of the formulations.
DISCUSSION
Based on the significantly higher acceptability and swallowability of the uncoated and coated mini-tablets compared to syrup in these studies, we conclude that the uncoated and coated mini-tablets of 2-mm diameter are a new therapeutic alternative to liquid formulations for neonates, infants and preschool children to facilitate the administration of medicines. Our results strongly support the safe use of coated mini-tablets at least from the age of 1 year on, further enlarging the portfolio of suitable drug dosage forms for children. However, particular care should be given to the use of coated mini-tablets below 1 year of age as two incidences of cough during ingestion were observed during our study, although both without clinical relevance. Additional trials with some more individuals are required for final judgement on the safety of the dosage form.
Due to the unexpected high acceptance of the mini-tablets in comparison to the sweet syrup in all the investigated age groups, we finally extended our study concept to the so far unexplored age group of less than 2 years. There was surprisingly no inferiority of the mini-tablets, even in the very young neonates. The results of our studies led to a change in EMA’s assessment concerning suitability of solid dosage forms for small children since there is no age-limiting recommendation for solid oral dosage forms anymore in the present guideline (7). Our results strongly support WHO’s claim for a shift of paradigm from liquid towards small-sized solid dosage forms (such as 2-mm mini-tablets) for drug administration to young children.
A limitation of our studies is the fact that most drugs require a number of mini-tablets per single dose, as the maximum drug load of a 2-mm mini-tablet (6- to 7-mg total mass) is approximately 2.5 mg active pharmaceutical ingredient. As we had administered only one drug-free mini-tablet, ongoing investigations include the administration of up to several hundred mini-tablets in children as a single dose.
CONCLUSION
This review of most recent studies on the acceptability of mini-tablets showed that these dosage forms are a safe in principle and an easy dose approach to administer medicine to young children. We provided the basis for a broad use of this new pharmaceutical dosage form for many different drug classes and treatment options in the near future.
Abbreviations
- EMA:
-
European Medicines Agency
- mm:
-
Millimetres
- ml:
-
Millilitres
- p :
-
Statistical power
- PIP:
-
Paediatric investigation plan
- WHO:
-
World Health Organization
References
European Parliament and the Council. Paediatric Regulation (EC) No 1901/2006 of the European Parliament and of the Council of [12] December 2006, on medicinal products for paediatric use and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. Official Journal of the European Union L 378/1; 2006.
Yeung VW, Wong IC. When do children convert from liquid antiretroviral to solid formulations? Pharm World Sci. 2005;27:399–402.
Breitkreutz J, Wessel T, Boos J. Dosage forms for per oral administration to children. Paediatr Perinatol Drug Ther. 1999;3:25–33.
Breitkreutz J. European perspectives on pediatric formulations. Clin Ther. 2008;30:2146–54.
World Health Organisation (WHO). EB. Campaign “Make medicines child size”. Progress Reports, Reports by the Secrtariat; 2008. http://apps.who.int/gb/ebwha/pdf_files/EB124/B124_33Add1-en.pdf
European Medicines Agency (EMA) CfMPfHU. Reflection paper: formulations of choice for the paediatric population. EMEA/CHMP/PEG/194810/2005; 2006.
European Medicines Agency (EMA) CfMPfHU. Guideline on pharmaceutical development of medicines for paediatric use. EMA/CHMP/QWP/805880/2012 Rev. 2; 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/07/WC500147002.pdf
Thomson SA, Tuleu C. Mini-tablets: new modality to deliver medicines to preschool-aged children. Pediatrics. 2009;123(2):e235–238.
Van de Vijver E, Desager K, Mulberg AE, Staelens S, Verkade HJ, Bodewes FA, et al. Treatment of infants and toddlers with cystic fibrosis-related pancreatic insufficiency and fat malabsorption with pancrelipase MT. J Peadiatr Gastroenterol Nutr. 2011;53(1):61–4.
van Riet-Nales DA et al. Acceptability of different oral formulations in infants and preschool children. Arch Dis Child. 2013;98(9):725–31.
Kluk A, Sznitowska M, Brandt A, Sznurkowska K, Plata-Nazar K, Mysliwiec M, et al. Can preschool-aged children swallow several minitablets at a time? Results from a clinical pilot study. Int J Pharm. 2015;485(1–2):1–6.
Spomer N, Klingmann V, Stoltenberg I, Lerch C, Meissner T, Breitkreutz J. Acceptance of uncoated mini-tablets in young children: results from a prospective exploratory cross-over study. Arch Dis Child. 2012;97:283–6.
Klingmann V, Spomer N, Lerch C, Stoltenberg I, Frömke C, Bosse HM, et al. Favorable acceptance of mini-tablets compared with syrup: a randomized controlled trial in infants and preschool children. J Pediatr. 2013;163(6):1728–32.
Klingmann V, Seitz A, Meissner T, Breitkreutz J, Möltner A, Bosse HM. Acceptability of uncoated mini-tablets in neonates—a randomized controlled trial. J Pediatr. 2015;167:893–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that she has no conflicts of interest.
Additional information
Guest Editors: Maren Preis and Jörg Breitkreutz
Rights and permissions
About this article
Cite this article
Klingmann, V. Acceptability of Mini-Tablets in Young Children: Results from Three Prospective Cross-over Studies. AAPS PharmSciTech 18, 263–266 (2017). https://doi.org/10.1208/s12249-016-0639-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0639-3