Abstract
Cyclodextrins are able to form host–guest complexes with hydrophobic molecules to result in the formation of inclusion complexes. The complex formation between norfloxacin form A and β-cyclodextrin was studied by exploring its structure affinity relationship in an aqueous solution and in the solid state. Kneading, freeze-drying, and physical mixture methods were employed to prepare solid complexes of norfloxacin and β-cyclodextrin. The solubility of norfloxacin significantly increased upon complexation with β-cyclodextrin as demonstrated by a solubility isotherm of the AL type along with the results of an intrinsic dissolution study. The complexes were also characterized in the solid stated by differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), Fourier-transform infrared (FT-IR) spectroscopy, X-ray diffractometry, scanning electron microscopy (SEM), and solid-state nuclear magnetic resonance (ssNMR) spectrometry. The thermal analysis showed that the thermal stability of the drug is enhanced in the presence of β-cyclodextrin. Finally, the microbiological studies showed that the complexes have better potency when compared with pure drug.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Norfloxacin (NFLX), a second-generation fluoroquinolone antimicrobial agent, has been widely used in human and veterinary medicine and demonstrates a wide spectrum of activity against aminoglycoside-resistant Pseudomonas aeruginosa and beta-lactamase-producing organisms (1). This drug inhibits DNA gyrase by interfering with the DNA-rejoining reaction (2). It is effective in the treatment of urinary tract infections, gonococcal urethritis, and infections diarrhea (3).
Chemically, it is 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline carboxylic acid (Fig. 1) (4). It is freely soluble in glacial acetic acid and very slightly soluble in methanol, ethanol, and water at a pH of 6–10, and it has a relatively slow rate of dissolution (2,5). NFLX exists in several solid forms: three anhydrous polymorphs (forms A, B, and C), an amorphous form, a methanol solvate, several hydrate forms, salts, and cocrystals (6–8).
Cyclodextrins (CDs) are water-soluble cyclic oligosaccharides composed of 6, 7, or 8 glucopyranose units (α-, β-, or γ-CD) with a relatively hydrophobic central cavity and a hydrophilic outer surface, which differ in their ring size and solubility according to the number of glucopyranose units (9,10). One of the most important applications of CDs in the pharmaceutical sciences is the enhancement of the aqueous solubility of drugs through complexation. Hydrophilic CDs can increase the release rate of poorly water-soluble drugs and ultimately enhance drug absorption across biological barriers (11). β-cyclodextrin (β-CD) has been widely used in the early stages of pharmaceutical development due to its ease of availability and a cavity size which is suitable for the widest range of drugs (Fig. 1) (12,13).
The hydrophobic nature of the internal cavity of CDs is the key feature which is responsible for its ability to form complexes with guest molecules. The CD inclusion is a stoichiometric molecular phenomenon in which usually only one molecule interacts and gets entrapped inside the cavity of the CD (14). In the pharmaceutical industry, CDs are used as complexing agents to increase the aqueous solubility of poorly soluble drugs and subsequently to enhance bioavailability and stability (15–17).
The solubility of NFLX in water is pH dependent, increasing sharply either with a decrease in pH below 5 or with an increase in pH above 10. This is why only 35–45% of the drug is absorbed when orally administered. It is therefore to improve the aqueous solubility of NFLX to enhance its extent of absorption (18–20). One study on inclusion complexes of NFLX investigated the influence of dispersion in PEG 6000 and complexation with hydroxypropyl-β-cyclodextrin (HP-β-CD) in systems obtained by freeze-drying (21). The effect of solid dispersions and CD complexation on the bioavailability of NFLX was reported- by Fawaz and coworkers (22). Dua and coworkers investigated the possibility of improving the solubility and dissolution rate of NFLX in the presence of solubilizing agents such as ascorbic acid and citric acid incorporated into β-CD (23). Recently, Chattah and coworkers investigated novel complexes of β-CD and two different solid forms of NFLX at the molecular level (24).
The aim of the current research was to prepare complexes of the commercial form of NFLX (form A) and β-CD (NFLX-β-CD) by a variety of methods and fully characterize the resulting complexes using thermal analysis, solid-state nuclear magnetic resonance (ssNMR), powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), and Fourier-transform infrared (FT-IR) spectroscopy. In addition, in vitro intrinsic dissolution was investigated, and the antibacterial activity against Staphylococcus epidermidis species was assessed in vitro.
MATERIAL AND METHODS
Reagents and Chemicals
NFLX form A (purity 100%) was obtained from Galena Química Farmacêutica LTDA (Campinas, Brazil). β-CD was supplied by Ferromet agent of Roquette (France) in Argentina. All reagents used were of analytical grade. Eluents and standard solutions were prepared with double-distilled water obtained from a Milli-Q system (Millipore, Milford, MA, USA).
Phase Solubility Studies
The solubility measurements were performed according to the method of Higuchi and Connors (25). An excess of NFLX was suspended in aqueous buffer solutions (pH 6.0 and 8.0) containing different concentrations of β-CD ranging from 2.5 to 14.7 mM. NFLX in the absence of β-CD was used to determine intrinsic solubility (S 0). Suspensions were sonicated for 15 min (ULTRASONIC LC 30 H Elma) and then placed in a 25.0 ± 0.1°C constant temperature water bath (Haake DC10 thermostat) for 72 h. These suspensions were sonicated at several time intervals. After equilibrium was reached, the excess NFLX was removed by filtration using a 0.45-μm polyvinylidene difluoride membrane (Millipore, USA). The clear solutions were suitably diluted and analyzed by UV–vis spectrophotometry (SHIMADZU UV-160A spectrophotometer). Absorbance was measured at 277 nm the wavelength (λ) maxima of NFLX.
Preparation of Solid Systems
Solid-state systems of NFLX-β-CD in a 1:1 molar ratio were prepared by kneading, freeze-drying, and physical mixture.
Kneading Method
The solid-state system of NFLX-β-CD was prepared by weighing accurately appropriate amounts of β-CD and then transferring them to a mortar. An acetone-water (50:50 v/v) mixture was added to the powder, and the resultant mixture was kneaded for about 10 min. Then, NFLX was added in small portions with the simultaneous addition of solvent in order to maintain a suitable consistency. This mixture was further kneaded for about 45 min, and the resultant paste was dried under a vacuum at 40°C for 48 h protected from light.
Freeze-drying Method
Appropriate amounts of β-CD and NFLX were suspended in water and sonicated at 25.0 ± 0.1°C constant water temperature until the drug was dissolved completely. Solutions were frozen at −40°C for 24 h to ensure complete solidification before freeze-drying (Freeze Dye 4.5 Labconco corp., Kansas City, MO).
Physical Mixture
Physical binary mixtures of β-CD and NFLX were prepared by blending the components uniformly in a porcelain mortar.
Characterization of NFLX-β-CD Complexes
Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
The DSC analysis of the complexes was performed using a DSC TA 2920, and the TGA curves were recorded on a TG TA 2920. The complexes were placed in aluminum hermetic pans, and the analysis was carried out under nitrogen gas flow at a heating rate of 10°C/min over a temperature range of 25–300°C.
IR Spectra (FT-IR)
The FT-IR spectra of the complexes were recorded on a Nicolet 5 SXC FT-IR Spectrophotometer (Madison, WI, USA). Potassium bromide disks were prepared by compressing the powder.
X-Ray Diffractometry (XRD)
X-Ray diffraction (Diffractor Siemens D5000, Diffrac Plus XRD commander, USA) analysis was performed using the following conditions: 40 kV; 30 mA; between 4° and 70° (2 theta); and 0.05 step and 1 s step time.
Scanning Electron Microscopy (SEM)
Morphological structures of the raw materials and complexes prepared by kneading, freeze-drying, and physical mixture were investigated and photographed using a scanning electron microscope (JEOL, Field Emission Scanning Electron Microscope, model JSM-7500F). The samples were fixed on a brass stub using double-sided aluminum tape. To improve conductivity, samples were gold coated under a vacuum employing a sputter coater (Bal-Tec Model SCD 050). The magnification selected was sufficient to study the general morphology of the samples in detail.
Solid-state Nuclear Magnetic Resonance (ssNMR)
High-resolution solid-state 13C NMR spectra of the raw materials and complexes prepared by kneading, freeze-drying, and physical mixture were recorded using the ramp cross polarization/magic angle spinning (CP-MAS) sequence with proton decoupling during acquisition (26). The ssNMR analysis was performed at room temperature in a Bruker Avance II spectrometer operating at 300.13 MHz for protons and equipped with a 4-mm MAS probe. The operating frequency for carbons was 75.46 MHz. Glycine was used as an external reference for the 13C spectra, and spectra were recorded with 1600 scans. The contact time during CP was 1.5 ms, and the recycling time was 5 s. The SPINAL64 pulse sequence was used for decoupling, with a proton field H1H satisfying ω 1H/2π = γ HH1H/2π = 78 kHz (27). The spinning rate for all samples was 10 kHz.
In Vitro Intrinsic Dissolution Study
The intrinsic dissolution studies for NFLX and its complexes with β-CD were performed using the Wood’s apparatus and Vankel dissolution system. An accurately weighed 200 mg of sample of the kneaded and the physical mixture were transferred to the die cavity of the apparatus and compressed at 5500 psi for 2 min. Of the buffer solution [0.26% v/v acetic acid and 0.0125 M NaOH in deionized water (pH 4)], 300 ml was used as the dissolution medium. The equipment was adjusted to 100 rpm, and the dissolution media temperature was maintained at 37.0 ± 0.5°C using a water bath throughout the experiment. Prior to the experiment, the dissolution medium was sonicated while being maintained at 37°C for 15 min to remove dissolved oxygen and avoid bubble formation below the die cavity of the Wood’s apparatus. The aliquots of the dissolution medium (1.0 mL) were withdrawn at 0, 1, 2, 3, 4, 5, 10, 15, 20, 25, 30, 45, 60, 75, 90, 105, and 120 min while replacing the volume with an equivalent volume of fresh dissolution medium at each time point. The samples were analyzed by high-performance liquid chromatography (HPLC) for NFLX dissolution.
To determine the surface area contributed by NFLX in the physical mixture and kneaded system during dissolution, a correction factor of 0.1099 cm2 was employed. The correction factor was calculated based on the following equation: Surface area due to NFLX in the physical mixture and kneaded system = Total surface area × Weight fraction (pi × r 2 × 2 × weight fraction; r is radius = 0.4 cm of the die; weight fraction = 1.96/8.96 = 0.22). The weight fraction was employed since the area, volume, and weight fraction for all sample types were equal. This is because the solid density (2.09 g/mL) of the physical mixture, the kneaded system, pure NFLX, and pure β-CD was the same after compaction in the die of the Wood’s apparatus. The solid density was determined based on weight (g)/volume (mL) [Weight = 0.2 g and Volume = pi × r 2 × h; where r is the radius of the die cavity (0.4 cm); h is the height of the compacted sample (0.2 cm)] for each of the samples studied.
HPLC analysis was performed on a Waters 600E multisolvent delivery system with a Waters 717 autosampler, Shimadzu solvent degasser DGU-14A, and 996 Waters photodiode array detector. A reverse-phase chromatographic column, Phenomenex Prodigy ODS (2) (150 mm × 4.6 mm, 5 μm), was used for the separation. The column temperature was maintained at 45°C. The mobile phase for the isocratic mode was 10 mM ammonium acetate in 0.5% acetic acid (pH 3.8):methanol (70:30% v/v). The flow rate of mobile phase was set to 0.75 mL/min. The sample volume injected was 10 μL. The absorbance detection of samples was performed at 277 nm, the wavelength of maximum absorption for NFLX. The retention time of NFLX under the above chromatographic separation condition was 4.5 min.
Microbiological Studies
The antimicrobial activity of the NFLX-β-CD complex was compared with NFLX pure drug against S. epidermidis ATCC 12228 IAL 2150 using a turbidimetric bioassay (5). Brain heart infusion (BHI) broth incubated at 35.0 ± 0.2°C for 24 h was used for the bacterial cultures. The samples were incubated immediately (32 rotations per minute; Shaker-model MA 420, Marconi-Brazil) and maintained at 35.0 ± 0.2°C. Two control samples, without antibiotic and microorganism (negative control) and one with S. epidermidis (positive control), were prepared in inoculated medium. The tubes were incubated for 4 h. After incubation, 0.5 ml of 12% formaldehyde was added to each tube. Negative control was used to set the optical baseline for the turbidimetric measurements. Absorbance with the wavelength set at 530 nm was measured. Potency was then calculated.
RESULTS AND DISCUSSION
Phase Solubility Studies
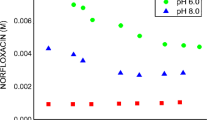
Solubility profiles, obtained by plotting the changes in guest molecule solubility as a function of β-CD concentration, showed an increase in NFLX solubility when the concentration of β-CD was increased. As shown in Fig. 2, the interaction between NFLX and β-CD in water and a buffer solution of pH 6.0 displayed a typical AL curve, according to Higuchi and Connors, indicating the formation of soluble complexes of a 1:1 stoichiometry (25). However, the solubility profile obtained in a buffer solution of pH 8.0 showed a negative intercept deviation resulting in an A-L type profile. Loftsson and coworkers stated that self-association of poorly soluble drugs and excipient interactions can explain this type of behavior (28).
The solubility of NFLX (Fig. 2) in water, buffer solutions of pH 6.0 and 8.0, increased 1.2, 1.9, and 2.4 times, respectively, in the presence of β-CD. However, the highest solubility of NFLX in the presence of β-CD was obtained at pH 6.0. These results demonstrated a solubilizing effect of β-CD indicating that complexation with this molecule can be an effective strategy to enhance the solubility of NFLX.
Apparent stability constant (K C) values were estimated from the slope of the initial linear portion of the profiles and the solubility of NFLX (S 0). The data are shown in Table 1.
Our results are in agreement with those obtained by Dua and coworkers, which described a typical AL type phase solubility curve for NFLX-β-CD in water at 25°C. They have reported a similar K C value to that obtained in this work (23).
Solid-state Studies
Information on solid-state interactions between NFLX and β-CD was obtained using DSC, TGA, FT-IR, XRD, SEM, and ssNMR.
Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
The DSC and TGA profiles of NFLX, β-CD, and the solid-state systems obtained by kneading, freeze-drying, and physical mixture are shown in Fig. 3a, b, respectively.
NFLX demonstrated a sharp endothermic melting peak at 221°C. β-CD exhibited a typical broad endothermic peak, between 70 and 150°C assigned to a dehydration process and its subsequent decomposition above 250°C.
Comparison of the DSC curves of the binary systems prepared by physical mixture with those obtained by kneading and freeze-drying confirms an interaction between the components. In fact, the characteristic events observed for the individual components were found in the physical mixture. While, the disappearance and shift of the melting peak of NFLX in the profiles of the systems obtained by freeze-drying and kneading, respectively, indicated molecular encapsulation of the drug inside the CD cavity. Interesting, the TGA curves showed that the systems obtained by freeze-drying and kneading had a higher thermal stability than NFLX in the solid state.
Fourier-transform Infrared Spectroscopy (FT-IR)
Fourier-transformation infrared spectroscopy (FT-IR) was used to evaluate the interaction between CD and NFLX in the solid state. FT-IR spectra of NFLX, β-CD, and the systems obtained by kneading, freeze-drying, and physical mixture are illustrated in Fig. 4. NFLX was characterized by bands at 1730 (carboxylic acid C = O stretch), 1620 (pyridine ring C = C stretch), 1583, 1550, 1522, 1478 (quinolone ring C–C and C–N stretch), and 1251 cm−1 (C–F and carboxylic C–O stretch). It is evident from the spectra of the solid-state systems obtained by kneading and freeze-drying that the characteristic bands of NFLX at 1730, 1550, and 1522 cm−1 had disappeared, while the band at 1251 cm−1 had shifted. In contrast, the spectra of the physical mixture corresponded to the FT-IR spectra of the single individual components. From these observations, we suggest that the pyridine ring of NFLX interacted with β-CD in the solid state. Similar results have been reported for NFLX/CD complexes, that predominant inclusion of the carboxylic portion within the apolar cavity of β-CD (19).
X-Ray Diffraction (XRD)
The XRD patterns of the NFLX, β-CD, the kneaded, and freeze-dried solid-state systems along with the physical mixture are presented in Fig. 5. The XRD pattern of pure drug showed intense peaks indicating the crystalline nature of NFLX. On the other hand, the XRD patterns of the kneaded and freeze-dried binary systems were completely diffuse, with an absence of intense sharp peaks. The disappearance of the NFLX diffraction pattern may have been due to the formation of true complexes of an amorphous nature in which NFLX was trapped in the β-CD cavity. Thus, complexation produced new solid phases that had less crystalline structure as compared to that of the pure drug.
Scanning Electron Microscopy (SEM)
Scanning electron microscopy was used to study the microscopic aspects of the raw materials and the products obtained by the various methods of preparation. Figure 6 illustrates the SEM microphotographs.
The typical crystals of NFLX and freeze-dried NFLX were seen as needle-shaped structures, whereas β-CD and freeze-dried β-CD appeared as a parallelogram shape. The original morphology of the raw materials disappeared in the SEM images of the kneaded and freeze-dried solid-state systems, and it was not possible to differentiate the single components. These changes in the particle shape and aspect are evidence of the formation of new solid phases, as previously supported by the DSC, XRD, and FT-IR analysis. In addition, the characteristic NFLX crystals, which were mixed with β-CD particles, were clearly detectable in the SEM images of the physical mixture, thus confirming the presence of the crystalline drug and the absence of interactions.
Solid-state NMR (ssNMR)
The 13C CP-MAS spectra for NFLX, β-CD, kneaded, freeze-dried systems, and the physical mixture are shown in Fig. 7 together with the carbon assignments. The carbon numbering for NFLX and β-CD molecules is presented in Fig. 1. As shown in their individual spectra, both NFLX and β-CD exhibit different and separate ranges of C chemical shifts. β-CD exhibits a complex spectrum with multiple sharp resonances for each type of carbon atom, typical of a crystalline system. The13C CP-MAS spectrum of NFLX matches well with that previously reported (22).
On comparison of the spectra of the kneaded and freeze-dried systems with that of the physical mixture, it is possible to see several differences which indicate that the kneading and freeze-drying systems are new solid forms. In contrast, the physical mixture signals mostly appear at the same chemical shifts as those in the pure compounds. In particular, the spectrum of the kneaded solid-state system suggested that the more affected signals of NFLX belong to carbons 4, 9, 8, and 3, showing changes between 1 and 3 ppm in their chemical shifts when compared to the physical mixture. The signals belonging to β-CD do not show major changes. In addition, the complete changes in the signals of NFLX indicate that there are no detectable residues of pure NFLX, and all NFLX present interacts with β-CD. The broadening of the peaks of β-CD and the absence of NFLX signals for the freeze-dried solid-state system indicate that the system has an amorphous character.
In Vitro Intrinsic Dissolution Study
The intrinsic dissolution rate was defined as the rate of dissolution of NFLX from the solid-state systems (pure NFLX, physical mixture, and kneaded), where the conditions of surface area, temperature, agitation, pH, and ionic strength are all constant.
The intrinsic dissolution of NFLX was faster from the physical mixture (13.21 μg/mL−1/cm−2/min−1) and the system prepared by kneading (15.26 μg/mL−1/cm−2/min−1), when compared to pure NFLX (5.85 μg/mL−1/cm−2/min−1) (Fig. 8).
Microbiological Studies
The complexes were analyzed using absorption spectrophotometry for quantifying the NFLX present before bioassay. The results showed that the physical mixture, kneaded, and freeze-dried systems had potencies of 97.18, 89.52, and 96.57%, respectively.
Results obtained from the in vitro antimicrobial activity investigation showed that the NFLX-β-CD complex had better potency than the pure NFLX (96.38%). The good activity of the complexes was likely due to their increased water solubility and diffusion to a greater extent into the BHI medium. The kneaded complex demonstrated low potency when compared with the other complexed systems.
CONCLUSIONS
The present research demonstrates that the ligand β-CD is capable of interacting with NFLX to forms binary complexes. Furthermore, these complexes can markedly increase NFLX solubility. The DSC, TGA, FT-IR, XRD, SEM, and ssNMR studies showed that the preparation of complexes in the solid state is feasible. The results obtained from these studies indicated potential functional groups that may be involved in the interaction of NFLX and β-CD. The physical mixture and kneaded system of NFLX evaluated by intrinsic dissolution showed an increase in the dissolution rate of NFLX.
Additionally, the results of microbiological assay showed satisfactory potency for the complexes in comparison with the pure drug.
References
Naumann P, Dopp C. Fluoroquinolones—antibacterial activity, pharmacokinetics and indications for a new group of chemotherapeutic drugs. Internist (Berl). 1989;30:20–31.
Sárkozy G. Quinolones: a class of antimicrobial agents. Vet Med. 2001;46:257–74.
Bomma R, Naidu RAS, Yamsani MR, Veerabrahma K. Development and evaluation of gastroretentive norfloxacin floating tablets. Acta Pharm. 2009;59:211–21. doi:10.2478/v10007-009-0019-6.
Deng B, Su C, Kang Y. Determination of norfloxacin in human urine by capillary electrophoresis with electrochemiluminescence detection. Anal Bional Chem. 2006;371(385):1336–41.
United States Pharmacopeia. 35th ed. Rockville: United States Pharmacopoeial Convention, 2012.
Barbas R, Martí F, Prohens R, Puigjaner C. Polymorphism of norfloxacin: evidence of the enantiotropic relationship between polymorphs A and B. Cryst Growth Des. 2006;6:1463–67. doi:10.1021/cg060101u.
Barbas R, Prohens R, Puigjaner C. A new polymorph of norfloxacin. J Therm Anal Calorim. 2007;89:687–92.
Puigjaner C, Barbas B, Portell A, Font-Bardia M, Alcobé X, Prohens R. Revisiting of the solid state of norfloxacin. Cryst Growth Des. 2010;10:2948–53. doi:10.1021/cg9014898.
Szejtli J. Past, present, and future of cyclodextrin. Pure Appl Chem. 2004;76:1825–45.
Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6:329–57. doi:10.1208/pt060243.
Szejtli J. Cyclodextrins properties and applications. Drug Invest. 1990;2:11–21.
Marques HC. Applications of cyclodextrins. Rev Port Farm. 1994;44:85–96.
Ali N, Harikumar SL, Amanpreet K. Cyclodextrin: an excipient tool in drug delivery. Int Res J Pharm. 2012;3:44–50.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–25.
Lee PS, Han JY, Song TW, Sung JH, Kwon OS, Song S, et al. Physicochemical characteristics and bioavailability of a novel intestinal metabolite of ginseng saponin (IH901) complexes with β-cyclodextrin. Int J Pharm. 2006;316:29–36.
Shen YL, Ying W, Yang SH, Wu LM. Determination of inclusion complex between gossypol and β-cyclodextrin. Spectrochim Acta A. 2006;65:169–72.
Loftsson T, Duchêne D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11.
Swanson BN, Boppana VK, Vlasses PH, Rotmensch HH, Ferguson RK. Norfloxacin disposition after sequentially increasing oral doses. Antimicrob Agents Chemother. 1983;23:284–88.
Ross DL, Riley CM. Aqueous solubilities of some variously substituted quinolone antimicrobials. Int J Pharm. 1990;63:237–50. doi:10.1016/0378-5173(90)90130-V.
Tripathi KD. Essentials of medical pharmacology. 40th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 1999.
Guyot M, Fawaz F, Bildet J, Bonini F, Lagueny AM. Physicochemical characterization and dissolution of norfloxacin/cyclodextrin inclusion compounds and PEG solid dispersions. Int J Pharm. 1995;123:53–63. doi:10.1016/0378-5173(95)00039-L.
Fawaz F, Bonini F, Guyot N, Bildet J, Maury M, Lagueny AM. Bioavailability of norfloxacin from PEG 6000 solid dispersion and cyclodextrin inclusion complex in rabbits. Int J Pharm. 1996;132:271–75. doi:10.1016/0378-5173(95)04387-X.
Dua K, Ramana MV, Sara UV, Himaja M, Agrawal A, Garg V, et al. Investigation of enhancement of solubility of norfloxacin β-cyclodextrin in presence of acidic solubilizing additives. Curr Drug Deliv. 2007;4:21–5.
Chattah AK, Mroue KH, Pfund LY, Ramamoorthy A, Longhi MR, Garnero C. Insights into novel supramolecular complexes of two solid forms of norfloxacin and β-cyclodextrin. J Pharm Sci. 2013;102:3717–24. doi:10.1002/jps.23683.
Higuchi T, Connors KA. Phase-solubility techniques. Advances in analytical chemistry and instrumentation. New York: Wiley-Interscience; 1965. p. 117–212.
Harris RK. Nuclear magnetic resonance spectroscopy. London: Logman Scientific and Technical; 1994.
Khitrin AK, Fujiwara T, Akutsub H. Phase-modulated heteronuclear decoupling in NMR of solids. J Magn Reson. 2003;162:46–53. doi:10.1016/S1090-7807(02)00173-8.
Loftsson T, Hreinsdottir D, Masson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005;302:18–28. doi:10.1016/j.ijpharm.2005.05.042.
Acknowledgments
The authors are grateful to PACD-FCFAr-UNESP (Araraquara-Brazil), FAPESP process no 2010/13335-2 (São Paulo-Brazil) and CNPQ (Brasília-Brazil) for the fellowships and União Química (Minas Gerais-Brazil) for the financial support. In addition, the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba (SECyT-UNC), and Ministerio de Ciencia y Tecnología (MinCyT) de la Provincia de Córdoba are grateful for the financial support. We also thank Ferromet S.A. (agent of Roquette in Argentina) for their donation of β-cyclodextrin. The cooperation from colleagues at the School of Pharmacy, Virginia Commonwealth University (VCU) is also highly appreciated. We also are grateful to Professor Peter R. Byron from VCU for his help and guidance with the intrinsic dissolution study.
Conflicts of Interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chierentin, L., Garnero, C., Chattah, A.K. et al. Influence of β-cyclodextrin on the Properties of Norfloxacin Form A. AAPS PharmSciTech 16, 683–691 (2015). https://doi.org/10.1208/s12249-014-0259-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0259-8