Abstract
Current methods to assess risk in infants exposed to maternal medication through breast milk do not specifically account for infants most vulnerable to high drug exposure. A workflow applied to lamotrigine incorporated variability in infant anatomy and physiology, milk intake volume, and milk concentration to predict infant exposure. An adult physiologically based pharmacokinetic model of lamotrigine was developed and evaluated. The model was scaled to account for growth and maturation of a virtual infant population (n=100). Daily infant doses were simulated using milk intake volume and concentration models described by a nonlinear equation of weight-normalized intake across infant age and a linear function on the relationship of observed milk concentrations and maternal doses, respectively. Average infant plasma concentration at steady state was obtained through simulation. Models were evaluated by comparing observed to simulated infant plasma concentrations from breastfeeding infants based on a 90% prediction interval (PI). Upper AUC ratio (UAR) was defined as a novel risk metric. Twenty-five paired (milk concentrations measured) and 18 unpaired (milk concentrations unknown) infant plasma samples were retrieved from the literature. Forty-four percent and 11% of the paired and unpaired infant plasma concentrations were outside of the 90% PI, respectively. Over all ages (0–7 months), unpaired predictions captured more observed infant plasma concentrations within 90% PI than paired. UAR was 0.18–0.44 when mothers received 200 mg lamotrigine, suggesting that infants can receive 18–44% of the exposure per dose as compared to adults. UARs determined for further medications could reveal trends to better classify at-risk mother-infant pairs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the past decade, the US Food and Drug Administration (FDA) has been increasingly seeing the value of evidence-based decisions for lactating women and their health care providers on drug treatment and breastfeeding during therapy. As part of the FDA’s initiative to provide recommendations and guidance on conducting clinical lactation studies to inform breastfeeding with medication use, a draft industry guidance was published in May 2019 (1). The draft guidance outlined key considerations, including those involved in study design and measurement of infant milk intake. Several of these considerations emphasized the need to collect and understand the variability that exists for the mother and breastfed infant (1). First, maternal and infant factors (weight, age, ethnicity, race, etc.) should be accounted for. Second, pharmacokinetic (PK) variability and the variability in lactation physiology should be considered. Finally, depending on the design and primary objective of the study, inter- and intra-subject variability for the mother and breastfed infant would also be of interest. The role of variability in the measurement of infant milk intake volume was highlighted. The typically applied 150 mL/kg/day milk intake by the infant was acknowledged as a reasonable assumption, however, estimates based on a 200 mL/kg/day milk intake were encouraged. This recommendation reflects the idea that, although an intake of 150 mL/kg/day is often applied in measures of drug exposure risk to the infant, such as the relative infant dose (RID), feeding volumes are not constant across postnatal ages and between infants (2, 3). RID as an example, is measured by infant dose of drug in milk per body weight divided by weight-adjusted maternal dose, expressed as a percentage. As a result, if only a standard milk volume intake (together with maternal milk concentrations) is used to determine the daily dose of drug in milk to an infant, the variability in infant exposure to the drug will not be accounted for. Consequently, drug exposure through breast milk might be under- or over-estimated. Without accounting for this variability, the infants who are at the highest risk for dose-dependent toxic effects of the drug (e.g., outliers consuming >150 mg/kg/day), are not considered in the risk assessment. There is a clear importance of incorporating variability of both daily infant milk intake volume and drug in maternal milk levels in this assessment.
PK variability can be accounted for using physiologically-based pharmacokinetic (PBPK) models. These are mechanistic representations of drug disposition in the body with the ability to provide in silico estimates of drug exposure given the proper parameterization of host physiology and drug properties (4). Current metrics such as the RID, focus on assessing risk directly from maternal and infant dose. These metrics do not specifically account for how infants handle drugs in milk, age dependent factors, nor the variability in infant exposure that would identify outliers who may be most vulnerable to drug toxicity. In contrast, integrating the infant dose with a PBPK model can lead to a metric of exposure that, when linked to a measure of safety, can serve as the basis for risk assessment. Thus, PBPK models are able to account for how an infant uniquely handles the drug. With a study framework that incorporates variability in milk intake volume and drug in milk concentration, these models can improve risk assessment.
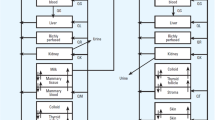
To date, few PBPK models have been developed to simulate breast milk exposure to maternal drug therapy. The abstracts by Cibert et al. (5) and Guedat et al. (6) report PBPK models developed to predict breastfeeding exposure of lamotrigine and clonidine, respectively. Both studies used workflows involving a mammary gland compartment and parameters to describe kinetics into the milk and infant (e.g., milk fat fraction and sucked milk flow) with resulting infant plasma concentrations simulated. Cibert et al. (5) predicted median (90th and 10th percentiles) lamotrigine infant plasma levels of 1 μg/mL (0.75 and 1.2 μg/mL; 200 mg maternal daily dose). This narrow band is however unlikely with one, a case report showing lamotrigine plasma concentrations >1.5 μg/mL in the infant breastfed by a mother taking the same daily dose (7). Cibert et al. (5) and Guedat et al. (6) used predicted rather than observed drug in milk concentrations to estimate infant exposure. Using measured drug concentrations obtained from this easily accessible media would have greatly reduced uncertainty in the models. Garessus, Mielke and Gundert-Remy (8) and Willmann et al. (9) used pediatric PBPK models to predict infant exposure to breastfeeding mothers taking isoniazid and codeine, respectively. The strength of their workflows included accounting for metabolizing enzyme polymorphisms. Olagunju et al. (10) report on a PBPK model to predict breastfed infant exposure to efarvirenz. Simulated infant exposure showed good agreement with observed values for mothers taking a standard daily dose of 600 mg, although the lower end of infant plasma concentrations tended to be underestimated (10). The last study of interest is a pediatric PBPK model for escitalopram published by our group (11). A mean daily milk intake of 153 mL/kg/day based on measured values by Kent (12) was employed across all age groups. Additionally, our group recently quantified the mean weight-normalized milk infant intake across postnatal age for full-term exclusively breastfeeding infants from a comprehensive literature review (13). This current study uses the variability of daily infant breast milk intake quantified by our literature review (13) to expand on our work with escitalopram (11). The feasibility and utility of incorporating variability from anatomy and physiology of breastfed infants, volume of milk intake, and maternal milk concentrations, were assessed with lamotrigine. Finally, a novel drug in milk risk assessment metric incorporating breastfeeding-related variability to account for potential infant outliers who may be at-risk of adverse reactions was developed.
MATERIALS AND METHODS
Modeling Strategy
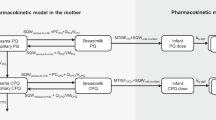
A PBPK model was first developed to describe the disposition of lamotrigine in adults. The adult PBPK model was then scaled to simulate lamotrigine exposure in a population of virtual infants. The adult and pediatric PBPK models were validated using multiple dose administration datasets and PK parameters of children directly administered lamotrigine, respectively. Model evaluation was quantitatively assessed by calculating the average fold error (AFE; bias) and absolute AFE (AAFE; precision) of drug plasma concentrations. Twofold error was deemed reasonable. The mean and variability in volume of milk intake and maternal milk lamotrigine concentrations were obtained from the literature and used to calculate daily infant dose. Daily infant dose was used as an input to the virtual infants to provide predicted lamotrigine exposure levels.
Evaluation of the simulated infant exposures were performed with two types of datasets from the literature: 1) lamotrigine concentrations measured in maternal milk and infant plasma at similar time points (paired mother-infants), and 2) lamotrigine concentrations measured in infant plasma only (unpaired infants).
Software Used
PBPK modeling was performed using the open-source PBPK modeling platform, PK-Sim version 8 (Open Systems Pharmacology Suite). Published PK profiles were digitized with Plot Digitizer (v2.6.8 by Joseph Huwaldt) to obtain concentration-time data. Model fitting and simulation to define ontogeny profiles, variability assessment in milk volume intake and milk lamotrigine concentrations, simulation of infant daily doses, calculation of exposure metrics, predictive performance evaluation, and creation all graphical plots were performed with R (R Core Team, 2019, Vienna, Austria).
Development and Evaluation of Adult and Pediatric PBPK Models
The workflow of Maharaj, Barrett and Edginton (14) was followed for development of the pediatric PBPK model. Briefly, a PBPK model in adults following intravenous (IV) administration was constructed based on lamotrigine physicochemical properties, knowledge of distribution, metabolism, and excretion, and IV PK datasets for model building. The study by Yuen and Peck (15) was used in the IV model construction. Lipophilicity and non-specific enzyme clearance were optimized to the IV dataset. The Rodgers and Rowland (16, 17) and PK-Sim standard methods were used to predict partition coefficient and cellular permeability, respectively. The glomerular filtration rate fraction was optimized using the fraction excreted unchanged in urine with the resulting value of 0.05. Two main lamotrigine metabolizing enzymes, UGT1A4 and UGT1A3, were incorporated and their relative contributions to clearance were allocated accordingly. Following optimization of the IV model, a PBPK model using 15 studies with lamotrigine single-dose oral administration ranging from 25-300 mg in adults (15, 18,19,20,21,22,23,24,25,26,27,28,29,30,31) was built. Optimization of absorption-specific parameters (specific intestinal permeability, dissolution half-time) provided a final model.
The adult population PBPK model was evaluated with PK data following multiple-dose administration of 50-200 mg. The appropriateness of PK variability following single dose oral administration was assessed by creating 100 virtual adults with an age, weight, and height range similar to the participant pool of the respective observed study used for evaluation (n=4). Appendix A provides a complete description of the model development and evaluation process.
The adult oral PBPK model was scaled to simulate drug exposure in virtual breastfeeding infants. The anatomy and physiology were scaled to that of infants at different ages. Growth and maturation of relevant processes (metabolic capacity, glomerular filtration rate, protein binding, and body composition) were accounted for (32, 33), and realistic variability around anatomy and physiology were applied (34) to produce a virtual infant population. The ontogeny of the two main enzymes involved in lamotrigine metabolism, UGT1A4 and UGT1A3, were based on in vitro studies by Badée et al. (35) and Miyagi and Collier (36). Appendix A outlines the maturation functions used for each enzyme. After normalizing enzyme activity levels to adult activity (between 0 and 1), a Hill and linear function were fitted for UGT1A4 and UGT1A3, respectively and included in the model. The pediatric PBPK models were evaluated against observed PK studies in children who were directly administered lamotrigine in oral dosage form.
Quantifying Infant Milk Intake and Drug in Milk Concentrations
Mean weight-normalized human milk intake (WHMI) increases from birth until reaching a maximum of 152.6 ml/kg/day at 19.7 days of age, and then declines thereafter (13) (Eq. 1).
where WHMI is in mL/kg/day and t is infant age in days. The variability in milk intake by breastfeeding infants was obtained by averaging the coefficient of variation (CV) using study data obtained from the literature by Yeung et al. (13). Studies with reported mean and standard deviation (SD) in weight-normalized human milk intake were grouped by age: by day from >0 to 7 days, by week from >1 to 4 weeks, and by month from >1 to 12 months old. If an age group consisted of one study, that study’s CV was used to represent the age group. If an age group consisted of multiple studies, an average of the study CVs weighted by study sample size was used to represent the age group. Since the resulting CVs for each of the age groups did not differ greatly across all groups (i.e., 1–2 weeks: 20.5%, 2–3 weeks: 15.5%, and 3–4 weeks: 17.1%), the age groups were further simplified. The >0 to 1 days old group CV was first compared with the next age group of >1 to 2 days old. If the percent change in CV was <30%, the age group CVs were averaged and classified under a single age bin. This procedure was repeated until a change in CV was ≥30% as compared to the previous age group, leading to the start of a new age bin. The mean WHMI equation together with the created bin CVs were referred to as the “milk intake model”.
The literature was searched for studies where milk concentrations of lamotrigine were obtained from mothers. Daily dose of the mother and resulting milk concentrations were recorded. Lamotrigine transfer into milk was assumed to be a passive process. Concentrations of lamotrigine in plasma have also been reported to be proportional to dose when administered over 50-400 mg as single doses (37). Therefore, a linear function was used to describe the relationship of mean drug in milk concentrations and maternal doses. Variability in drug in milk concentrations was determined by taking the average and SD of dose-normalized drug concentrations to derive the CV across all samples. The mean drug in milk concentration equation together with the overall CV was referred to as the “milk concentration model.”
Evaluation of Models Incorporating Variability on Predicting Breastfed Infant Exposure
Evaluation of the ability of the model to accurately recapitulate infant exposure was completed by comparing simulated exposure in virtual infants to observed infant plasma lamotrigine concentrations following exposure through breastfeeding. The following paragraphs describe the evaluation steps.
Data from mother-infant pairs with recorded maternal milk concentrations (i.e., before a feed) and infant plasma levels (i.e., after a feed), hereafter referred to as “paired data,” were used to evaluate the milk intake model. These samples were taken 2–15 h after a maternal dose (7, 38, 39); however, most of the studies did not report time sampled after dose. Infant plasma lamotrigine values from the literature were excluded if infant age was not reported and if there were clear indications of partial breastfeeding.
For evaluation using the paired data, infant populations of 100 individuals were simulated from the evaluated PBPK model for each age bin in days: >0 to 7, >7 to 14, >14 to 30, >30 to 60, and >60 to 84. The virtual infant populations used the International Commission on Radiological Protection (ICRP) population (40) (50% female) available in PK-Sim. Each virtual infant was assigned a single oral dose of 1 mg/kg lamotrigine as a solution (i.e., dissolved in breast milk) to obtain the area under the plasma concentration vs time curve from time zero to infinity (AUC∞). The average concentration at steady state (Cavg,ss) over 24 h was then calculated. Next, the weight-normalized dose received by each infant was calculated by multiplying daily milk intake volume (mL/kg) by the observed drug in milk concentration (μg/mL). The milk intake volume was either informed by the typical 150 mL/kg (no variability applied) or through use of the milk intake model. For the milk intake model, a WHMI was drawn from a normal distribution with mean derived from equation 1 and SD specific to the age bin of each infant.
Predictions of infant plasma lamotrigine concentrations were considered successful if observed data were within 90% prediction interval (PI) of simulated Cavg,ss plasma levels. Sensitivity analyses were conducted in the instance where the model consistently failed to correctly predict infant plasma levels at certain age bins to assess those model parameters most likely to be influential.
A similar method was applied to the evaluation using “unpaired data,” where only infant plasma concentrations after breastfeeding were reported. Virtual infant populations of 100 individuals were created for each age bin in days: >0 to 7, >7 to 14, >14 to 30, >30 to 60, and >60 to 213. The paired maternal drug in milk concentrations were not known for these infants. Therefore, the milk concentration model built from literature lamotrigine in milk values was used to give a concentration for each virtual infant. Evaluation used the same method as for the paired data.
Determining Measures of Exposure: RID, Predicted AUC∞, and the Novel Metric
The RID was calculated by taking the mean of lamotrigine in milk concentrations from the literature multiplied by the standard 150 mL/kg/day, divided by maternal dose. A dose of 200 mg was selected as it is the recommended maximum dose in the manufacturer’s label for bipolar disorder for maintenance treatment (labeled use), and acute bipolar major depression (off label) (37). The mean maternal dose on a per body weight basis was estimated by using a value of 70 kg for the mother. Equation 2 provides a single value for lamotrigine risk assessment in breastfeeding infants.
To account for variability in the risk assessment process, the predicted infant plasma AUC∞ of lamotrigine across the five age bins were calculated by creating 100 infants per age bin. The virtual infants were provided doses from the milk intake volume and milk concentration models and assuming a maternal dose of 200 mg. The resulting AUC∞ distribution was used to calculate an upper AUC ratio (UAR) defined as the 95th percentile of simulated pediatric AUC∞ divided by the median adult therapeutic AUC∞ (Eq. 3):
The median adult therapeutic AUC∞ was calculated by using the adult oral PBPK model to simulate concentration-time profiles of 100 women (25–34 years old, using the ICRP population) administered 200 mg lamotrigine.
RESULTS
The development and evaluation of the adult and pediatric PBPK models are reported in Appendix A. The adult IV and oral datasets used in model optimization produced an AFE of 0.95 and AAFE of 1.27. Model performance was successfully evaluated in three pharmacokinetic datasets with adult subjects administered multiple doses of lamotrigine (41,42,43). The evaluation produced acceptable AFE and AAFE values, 1.04 and 1.13, respectively. Adult virtual populations of 100 individuals were created in PK-Sim using four studies (19, 22, 28, 29) and PK variability was successfully captured. The oral adult models were scaled to children and predicted PK parameters were comparable to two studies where a single dose of 2 mg/kg lamotrigine was administered to infants and children (44, 45).
The results of capturing variability in infant WHMI using the CV method are presented in Table I. The final age bins in days were >0 to 1, >1 to 2, >2 to 3, >3 to 182.4, >182.4 to 212.8, >212.8 to 365. SDs were calculated at every infant age using the relevant bin CV and mean WHMI equation, resulting in the plot depicted in Fig. 1.
Mean and variability of WHMI up to 1-year postnatal age of exclusively breastfed term infants. The solid line represents the mean WHMI derived by fitting a nonlinear regression to the mean study WHMI (grey circles) weighted by sample size. The dashed line represents +SD and -SD from the fitted mean WHMI line as determined by the age bin CV (Table I). Sample-size weighted mean ± SD for each age bin (by day up to 7 days old, by week up to 4 weeks old, and by month up to 12 months old) are described by the black squares and associated error bars
Next, the workflow incorporating variability in infant anatomy and physiology, maternal milk lamotrigine levels, and infant WHMI was evaluated. Figure 2 shows the lamotrigine in milk levels retrieved from the literature. Most of the studies did not report the timing of sample collection, however, when reported, these were 2–15 h after maternal dose (7, 38, 39). Paired data collected from the literature are presented in Table II. The results of the evaluation in predicting the observed infant plasma levels are shown in Table III. The milk intake model predicted only 20–30% of the samples outside of the 90% PI for infants 7 to 60 days old. However, the model underpredicted in the youngest (n=2) and oldest (n=4) age groups, which showed 100% of the observed plasma levels outside of the 90% PIs. The lack of predictive ability at these ages were shared with using the standard 150 mL/kg/day milk intake value (Table III). A sensitivity analysis in PK-Sim revealed that UGT1A4 activity level was one of the most important parameters of the model (Appendix B). Over all ages, the model incorporating variability in milk intake performed slightly better or similar compared to without, where 44% and 48% of the paired samples fell outside the 90% PI across infants, respectively (Table III).
The studies retrieved from the literature and used in the unpaired dataset evaluation are shown in Table IV. Mean drug in milk concentrations were determined by using the known lamotrigine dose the mother took into the linear function describing their relationship in Fig. 2 (Eq. 4).
where lamotrigine in milk concentration is in μg/mL, β0 is 0.274 μg/mL, β1 is 0.00921 μg/mL/mg, and maternal dose is in mg. A drug in milk concentration was then drawn from a normal distribution with the lamotrigine in milk concentration mean and SD calculated from the dose-normalized drug concentration CV of 69.3%. Both workflows incorporating the milk intake standard and milk intake model were comparable and performed well, with 0–75% of samples outside the 90% PI (Table V). Over all ages, the standard and milk intake model resulted in 28% and 11% of the unpaired samples falling outside the 90% PI, respectively. As with the paired evaluation, the youngest age group had lower predictability. The single unpaired infant in the oldest age group was successfully captured within the 90% PI (Table V).
For determining RID, the mean lamotrigine in milk concentration from studies in Fig. 2 was 3.67 μg/mL. The estimated RID was determined as 19.3%. Predicted AUC∞ of infant lamotrigine plasma levels using the variability-incorporated workflow are presented in Fig. 3. Infant plasma AUC∞ did not appear to widely differ across age bins; however, the youngest and oldest age group had lower median AUC∞ as compared to 7 to 60 day olds (Fig. 3). Several outliers with relatively high predicted plasma AUC∞ are evident across all age groups. Table VI presents the 95th percentile of simulated pediatric AUC∞ across the five age groups that was divided by the median adult therapeutic AUC∞ to produce age-dependent UAR values. The median simulated maternal AUC∞ of women taking 200 mg was 109 μg∙h/mL, which is similar to values observed in PK studies (28,29,30).
DISCUSSION
This study demonstrated the feasibility of incorporating breastfeeding-related variability into a workflow to predict infant exposure to maternal medications through breast milk. The model using variability from infant anatomy and physiology, daily WHMI, and lamotrigine in milk concentrations performed slightly better than using the standard 150 mL/kg/day in exclusively breastfed infants up to approximately 7 months of age. However, the improvement offered by the milk intake model did not appear significantly greater than the standard intake in our workflow example with lamotrigine. The addition of milk concentration variability allowed for the capture of more observed infant plasma levels indicating the sensitivity of the milk concentration to overall exposure. This becomes important when capturing outliers is a goal of the risk assessment.
Although the model was able to predict most observed breastfed infant plasma levels, the concentrations were generally underpredicted. The ontogeny of UGT1A4 in young children was an important model parameter as defined using sensitivity analysis and this was deemed a likely reason for the underprediction. UGT1A4 accounts for about 90% of lamotrigine liver metabolism (51, 52). The UGT1A4 ontogeny model was informed with 17 data points below 1 year of age with the lowest age being 12 days old. There was also high uncertainty with some ages showing in vitro UGT1A4 activity ranging from almost no activity to activity similar to the adult value (Appendix A). Setting a lower activity would have fixed some underprediction however this would not have been evidence-based. While this is clearly a limitation caused by uncertain model inputs, the UAR outcome still suggests that outlier infants would reach exposures similar to those of adults taking lamotrigine. It would be of interest to test this workflow with additional lamotrigine sample data as there were very few samples to test, and in drugs where ontogeny profiles are well established to confirm this workflows’ efficacy.
The model tended to largely underpredict in the youngest age group, >0 to 7 day olds, however, likely due to an alternative rationale. These infants were breastfed by mothers who were taking lamotrigine to treat their condition while pregnant (7, 38, 46, 48). As a result, the higher than expected concentrations observed in infant plasma may be due to placental transfer that was not accounted for in the breastfeeding-variability incorporated model. Three samples of lamotrigine concentrations in neonatal plasma, 7.71 ug/mL (unpaired), 3.30 μg/mL (paired), and 2.79 μg/mL (paired), were relatively high and unable to be captured by the 90% PI windows with the milk intake model (7, 38, 46). Future studies should explore including this placental transfer for neonatal predictions in the first week of life. Understanding its influence can aid in the prediction of different risk scenarios. For instance, mothers taking lamotrigine during pregnancy and breastfeeding versus only during breastfeeding.
To apply the workflow in this study, an assumption on dose was made. In recognizing the complications of assessing infant exposure during multiple daily feeds, the total dose per day was used with no attempt to split the dose by number of feeds. By calculating the estimated average concentration at steady state and comparing to infant plasma level, we assume that the observed plasma levels approximated average steady state concentrations. Timing of the infant plasma level as related to maternal dose timing or timing of feed was largely unknown and this added a layer of uncertainty that was not accounted in the simulations. Additionally, assay development for drugs in this matrix has been known to be an analytical challenge due to the high protein and fat content of breast milk (53). Not all the studies reporting lamotrigine in milk concentrations provided information on the validity of the assays used, thus limiting further insight into this source of variability.
Another source of uncertainty in the workflow was the inclusion of plasma concentrations from infants who were breastfed at an unknown extent (39, 46, 48, 50). Therefore, if these infants were partially rather than exclusively breastfed, the observed infant lamotrigine plasma levels would be lower than expected. It is possible that these observed plasma concentrations would no longer be captured in the 90% PI, thus affecting model performance. However, since infant plasma levels from exclusively and unreported nursing extent were similar, the latter were treated as exclusively breastfed values. Nonetheless, future lactation studies should report breastfeeding extent to reduce potential uncertainties.
Three exposure metrics were examined in this study. Using maternal milk lamotrigine level and dose data reported in the literature, the average RID was calculated as 19.3%. The workflow in this study produced predicted infant plasma AUC∞ across the five unpaired age groups. Relative to each other, the median predicted infant plasma AUC∞ was low in the first age group, rose in line with increased feeding rate across the next age groups and was lower in the highest age group where clearance was greatest. The higher plasma AUC∞ in the very young infants was expected due to low CL capacity and high WHMI in the second week of life. The predicted 95th percentile varied at different ages. Compared to the median predicted maternal AUC∞, infants appear to be at high risk in the first month of age where there is a high likelihood for outliers. This finding is consistent with a review of case reports and studies indicating that breastfed infants of mothers taking medications had most adverse effects reported in the first month of postnatal age (54).
There are several advantages of the UAR over currently used risk metrics. The UAR serves as a metric to identify outlying infants with exposure as demonstrated using the infant predicted 95th percentile in AUC∞. To date, adverse effects were observed in three breastfed infants (12, 16, and 40 day olds) from mothers who were taking lamotrigine (38, 49, 55). In this study, the presented UARs demonstrated that lamotrigine exposure through breastfeeding can reach levels similar to those in mothers taking 200 mg, although the probability is likely to be low. Furthermore, the metric can determine infants at-risk breastfed from mothers taking other doses. For example, lamotrigine is also used to treat focal (partial) onset seizures and generalized onset seizures. Typically, a maintenance dose of 225–375 mg/day is suggested and thus the UAR can be calculated with this range of doses (37). Finally, the UAR is based on a simulated population of mothers and infants whereas metrics such as the RID are unable to account for variation. For example, the RID is limited to use of a single maternal weight value to calculate maternal dose (e.g., 70 kg).
Future studies should focus on determining the UAR on further medications commonly taken by lactating mothers. Obtaining a range of UAR values would help identify high-risk medications and safety thresholds. This will help to facilitate evidence-based recommendations for breastfeeding when mothers are taking medications. It is possible to produce the UAR for multiple drugs since the workflow relies on easily accessible breast milk sample data. Essentially, application of the workflow to other medications would simply follow a similar process as the example with lamotrigine. First, a developed model describing the drug in milk concentration would be used with the milk volume intake model to calculate daily infant doses. The daily infant doses would serve as inputs to a pediatric PBPK model developed and validated for the drug. Outputs of plasma AUC∞ of infant virtual populations would be used to calculate the 95th percentiles per age bin. These values together with the maternal median plasma AUC∞ would derive the UAR for the drug.
This study showed the feasibility of incorporating variability into a novel drug in milk risk assessment metric and builds the foundation for future studies in this area. This includes examining variability in the maternal population, where drug in milk concentrations can be used to develop population milk-PK models to uncover covariates that contribute to inter-individual variability (e.g., pharmacogenotypes, maternal body weight, maternal age, dose). The covariates will be used to identify mothers in the general population who are at high risk for achieving potentially dangerous drug concentrations in milk. Furthermore, specific groups of infants who would most benefit from use of the UAR to identify outliers would be of interest to study. One such group would be preterm infants, where clearance tends to be relatively low and thus these infants may be highly vulnerable to high drug exposure through breast milk.
CONCLUSION
This study applied a workflow incorporating variability in infant anatomy and physiology, milk intake volume, and milk concentration to predict breastfed infant exposure to lamotrigine as a case example. Pediatric PBPK modeling, and milk lamotrigine concentration and infant intake volume data from the literature, were used to capture sources of variability. The workflow produced a model that showed some improvement over the use of the standard 150 mg/kg/day milk intake volume. Only 11% as compared to 44% of the samples fell outside of a 90% prediction interval for the unpaired (infant plasma samples only; variability in milk lamotrigine concentrations applied) and paired datasets (infant plasma and maternal milk samples available; variability in milk lamotrigine concentrations not applied), respectively. These results demonstrated the importance of incorporating milk concentration variability into the workflow. From the workflow, a novel upper AUC ratio (UAR) metric to identify outlying infants at-risk of high drug exposure through breast milk was applied. The UAR across the examined infant ages ranged from 0.18 to 0.44 for those breastfed by mothers receiving a single 200 mg dose of lamotrigine.
Change history
10 June 2021
A Correction to this paper has been published: https://doi.org/10.1208/s12248-021-00614-9
13 July 2021
A Correction to this paper has been published: https://doi.org/10.1208/s12248-021-00615-8
References
Food and Drug Administration. Clinical lactation studies: Considerations for study design, guidance for industry. 05/01/19.
Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100(1):42–52.
Neville MC, Keller R, Seacat J, Lutes V, Neifert M, Casey C, et al. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988;48(6):1375–86.
Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacometrics Syst Pharmacol. 2014;3(11):e150–e.
Cibert M, Gouraud A, Vial T, Tod M. A physiologically-based pharmacokinetic model to predict neonate exposure to drugs during breast-feeding: application to lamotrigine. Fundam Clin Pharmacol. 2010;24(Suppl. 1):51.
Guedat MG, Gouraud A, Vial T, Tod M. A physiologically-based pharmacokinetic model for predicting neonate dose after breast-feeding by women treated with clonidine. Int J Clin Pharm. 2011;33(2):318.
Rambeck B, Kurlemann G, Stodieck SR, May TW, Jürgens U. Concentrations of lamotrigine in a mother on lamotrigine treatment and her newborn child. Eur J Clin Pharmacol. 1997;51(6):481–4.
Garessus EDG, Mielke H, Gundert-Remy U. Exposure of infants to isoniazid via breast milk after maternal drug intake of recommended doses is clinically insignificant irrespective of metaboliser status. A physiologically-based pharmacokinetic (PBPK) modelling approach to estimate drug exposure of infants via breast-feeding. Frontiers in pharmacology. 2019;10:5.
Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J. Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clin Pharmacol Ther. 2009;86(6):634–43.
Olagunju A, Rajoli R, Atoyebi S, Khoo S, Owen A, Siccardi M. Physiologically-based pharmacokinetic modelling of infant exposure to efavirenz through breastfeeding [version 1; peer review: 2 approved with reservations]. AAS Open Research. 2018;1(16).
Delaney SR, Malik PRV, Stefan C, Edginton AN, Colantonio DA, Ito S. Predicting escitalopram exposure to breastfeeding infants: Integrating analytical and in silico techniques. Clin Pharmacokinet. 2018;57(12):1603–11.
Kent JC, Mitoulas LR, Cregan MD, Ramsay DT. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117(3):e387-ee95.
Yeung CHT, Fong S, Malik PRV, Edginton AN. Quantifying breast milk intake by term and preterm infants for input into paediatric physiologically based pharmacokinetic models. Matern Child Nutr. 2020;16(2):e12938–e.
Maharaj AR, Barrett JS, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013;15(2):455–64.
Yuen WC, Peck AW. Lamotrigine pharmacokinetics: oral and IV infusion in man. Br J Clin Pharmacol. 1988;26:242P.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Berg M, Welty TE, Gidal BE, Diaz FJ, Krebill R, Szaflarski JP, et al. Bioequivalence Between Generic and Branded Lamotrigine in People With Epilepsy: The EQUIGEN Randomized Clinical Trial. JAMA Neurol. 2017;74(8):919–26.
Ebert U, Thong NQ, Oertel R, Kirch W. Effects of rifampicin and cimetidine on pharmacokinetics and pharmacodynamics of lamotrigine in healthy subjects. Eur J Clin Pharmacol. 2000;56(4):299–304.
Gidal BE, Sheth R, Parnell J, Maloney K, Sale M. Evaluation of VPA dose and concentration effects on lamotrigine pharmacokinetics: implications for conversion to lamotrigine monotherapy. Epilepsy Res. 2003;57(2-3):85–93.
Birnbaum AK, Kriel RL, Burkhardt RT, Remmel RP. Rectal absorption of lamotrigine compressed tablets. Epilepsia. 2000;41(7):850–3.
Birnbaum AK, Kriel RL, Im Y, Remmel RP. Relative bioavailability of lamotrigine chewable dispersible tablets administered rectally. Pharmacotherapy. 2001;21(2):158–62.
Burger DM, Huisman A, Van Ewijk N, Neisingh H, Van Uden P, Rongen GA, et al. The effect of atazanavir and atazanavir/ritonavir on UDP-glucuronosyltransferase using lamotrigine as a phenotypic probe. Clin Pharmacol Ther. 2008;84(6):698–703.
Fillastre JP, Taburet AM, Fialaire A, Etienne I, Bidault R, Singlas E. Pharmacokinetics of lamotrigine in patients with renal impairment: influence of haemodialysis. Drugs Exp Clin Res. 1993;19(1):25–32.
Marcellin P, de Bony F, Garret C, Altman C, Boige V, Castelnau C, et al. Influence of cirrhosis on lamotrigine pharmacokinetics. Br J Clin Pharmacol. 2001;51(5):410–4.
Srichaiya A, Longchoopol C, Oo-Puthinan S, Sayasathid J, Sripalakit P, Viyoch J. Bioequivalence of generic lamotrigine 100-mg tablets in healthy Thai male volunteers: a randomized, single-dose, two-period, two-sequence crossover study. Clin Ther. 2008;30(10):1844–51.
van Luin M, Colbers A, Verwey-van Wissen CP, van Ewijk-Beneken-Kolmer EW, van der Kolk M, Hoitsma A, et al. The effect of raltegravir on the glucuronidation of lamotrigine. J Clin Pharmacol. 2009;49(10):1220–7.
Hermann R, Knebel NG, Niebch G, Richards L, Borlak J, Locher M. Pharmacokinetic interaction between retigabine and lamotrigine in healthy subjects. Eur J Clin Pharmacol. 2003;58(12):795–802.
Incecayir T, Agabeyoglu I, Gucuyener K. Comparison of plasma and saliva concentrations of lamotrigine in healthy volunteers. Arzneimittel-Forschung. 2007;57(8):517–21.
Wootton R, Soul-Lawton J, Rolan PE, Sheung CT, Cooper JD, Posner J. Comparison of the pharmacokinetics of lamotrigine in patients with chronic renal failure and healthy volunteers. Br J Clin Pharmacol. 1997;43(1):23–7.
Depot M, Powell JR, Messenheimer JA Jr, Cloutier G, Dalton MJ. Kinetic effects of multiple oral doses of acetaminophen on a single oral dose of lamotrigine. Clin Pharmacol Ther. 1990;48(4):346–55.
Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45(7):683–704.
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45(10):1013–34.
Willmann S, Höhn K, Edginton A, Sevestre M, Solodenko J, Weiss W, et al. Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn. 2007;34(3):401–31.
Badée J, Qiu N, Collier AC, Takahashi RH, Forrest WF, Parrott N, et al. Characterization of the ontogeny of hepatic UDP-glucuronosyltransferase enzymes based on glucuronidation activity measured in human liver microsomes. J Clin Pharmacol. 2019;59(Suppl 1):S42–s55.
Miyagi SJ, Collier AC. Pediatric development of glucuronidation: the ontogeny of hepatic UGT1A4. Drug Metab Dispos. 2007;35(9):1587–92.
U.S. Food & Drug Administration. LAMICITAL (Lamotrigine) Label. 2015.
Nordmo E, Aronsen L, Wasland K, Småbrekke L, Vorren S. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43(11):1893–7.
Ohman I, Vitols S, Tomson T. Lamotrigine in pregnancy: pharmacokinetics during delivery, in the neonate, and during lactation. Epilepsia. 2000;41(6):709–13.
Basic anatomical and physiological data for use in radiological protection: reference values. A report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals. ICRP Publication 89. 2002. Report No.: 0146-6453 (Print) 0146-6453 Contract No.: 3-4.
Jann MW, Hon YY, Shamsi SA, Zheng J, Awad EA, Spratlin V. Lack of pharmacokinetic interaction between lamotrigine and olanzapine in healthy volunteers. Pharmacotherapy. 2006;26(5):627–33.
Gastrup S, Stage TB, Fruekilde PB, Damkier P. Paracetamol decreases steady-state exposure to lamotrigine by induction of glucuronidation in healthy subjects. Br J Clin Pharmacol. 2016;81(4):735–41.
Theis JG, Sidhu J, Palmer J, Job S, Bullman J, Ascher J. Lack of pharmacokinetic interaction between oxcarbazepine and lamotrigine. Neuropsychopharmacology. 2005;30(12):2269–74.
Vauzelle-Kervroëdan F, Rey E, Cieuta C, Pariente-Khayat A, Pons G. D′Athis P, et al. Influence of concurrent antiepileptic medication on the pharmacokinetics of lamotrigine as add-on therapy in epileptic children. Br J Clin Pharmacol. 1996;41(4):325–30.
Chen C, Casale EJ, Duncan B, Culverhouse EH, Gilman J. Pharmacokinetics of lamotrigine in children in the absence of other antiepileptic drugs. Pharmacotherapy. 1999;19(4):437–41.
Fotopoulou C, Kretz R, Bauer S, Schefold JC, Schmitz B, Dudenhausen JW, et al. Prospectively assessed changes in lamotrigine-concentration in women with epilepsy during pregnancy, lactation and the neonatal period. Epilepsy Res. 2009;85(1):60–4.
Newport DJ, Pennell PB, Calamaras MR, Ritchie JC, Newman M, Knight B, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122(1):e223–31.
Tomson T, Ohman I, Vitols S. Lamotrigine in pregnancy and lactation: a case report. Epilepsia. 1997;38(9):1039–41.
Bedussi F, Relli V, Faraoni L, Eleftheriou G, Giampreti A, Gallo M, et al. Normocytic Normochromic anaemia and asymptomatic neutropenia in a 40-Day-old infant breastfed by an epileptic mother treated with lamotrigine: Infant’s adverse drug reaction. J Paediatr Child Health. 2018;54(1):104–5.
Liporace J, Kao A, D'Abreu A. Concerns regarding lamotrigine and breast-feeding. Epilepsy Behav. 2004;5(1):102–5.
Argikar UA, Remmel RP. Variation in glucuronidation of lamotrigine in human liver microsomes. Xenobiotica. 2009;39(5):355–63.
Ladumor MK, Thakur A, Sharma S, Rachapally A, Mishra S, Bobe P, et al. A repository of protein abundance data of drug metabolizing enzymes and transporters for applications in physiologically based pharmacokinetic (PBPK) modelling and simulation. Sci Rep. 2019;9(1):9709.
Fríguls B, Joya X, García-Algar O, Pallás CR, Vall O, Pichini S. A comprehensive review of assay methods to determine drugs in breast milk and the safety of breastfeeding when taking drugs. Anal Bioanal Chem. 2010;397(3):1157–79.
Anderson PO, Manoguerra AS, Valdés V. A Review of Adverse Reactions in Infants From Medications in Breastmilk. Clin Pediatr. 2016;55(3):236–44.
Morin C, Chevalier I. Severe hypernatremic dehydration and lower limb gangrene in an infant exposed to lamotrigine, aripiprazole, and sertraline in breast milk. Breastfeed Med. 2017;12(6):377–80.
Acknowledgements
• Canadian Institutes of Health Research (CIHR); Project Grant; Award Number: PJT-159782
• Canadian Institutes of Health Research (CIHR); Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award (CGS-D), a Canada Graduate Scholarship to Honour Nelson Mandela; Award Number: DF2-171445
Author information
Authors and Affiliations
Contributions
I, Andrea Edginton, am contributing this section to highlight the work of the authors in this research that directly relates to the health of women and children. This important work has been championed by women as well as men. Authorship of this manuscript highlights our combined efforts to bring clarity to breastfeeding mothers and clinicians during an exceptionally vulnerable time in women’s lives. Our male co-author Shinya Ito, has spent a career focused on discovery and education in pediatric clinical pharmacology including lactation and infant exposure to medications and we most certainly consider him a champion of the cause. Julie Autmizguine, a newer independent investigator, is bringing equity to children through her research that will improve health outcomes through investigation of appropriate medication use in this population. My role in this work, and throughout my career, is in integrating the vast body of knowledge discovered by others to answer relevant clinical questions through the use of modeling and simulation. My role is also to inspire and train the next generation of knowledge translators and the first author, Cindy Yeung, is one such trainee. She brings an expertise in systematic review and clinical care guideline development from her MSc degree and we are privileged to have her extend her skill set to PBPK modeling and risk assessment. While my and Cindy’s expertise differs from that of our clinical collaborators, the strength of our Canadian team is indeed in our divergent expertise. Our varied skills, motivations, backgrounds, and beliefs allow for a problem assessment that is far richer than any one of us alone could achieve.
Corresponding author
Additional information
Guest Editors: Diane Burgess, Marilyn Morris and Meena Subramanyam
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
"The original online version of this article was revised to update Table I, and to delete a paragraph from the “Results” section." plus the same explanatory text of the problem as in the erratum/correction article.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yeung, C.H.T., Ito, S., Autmizguine, J. et al. Incorporating Breastfeeding-Related Variability with Physiologically Based Pharmacokinetic Modeling to Predict Infant Exposure to Maternal Medication Through Breast Milk: a Workflow Applied to Lamotrigine. AAPS J 23, 70 (2021). https://doi.org/10.1208/s12248-021-00599-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00599-5