Abstract
Background
The use of a distal cerebral protection device during extracranial carotid artery stenting is still a matter of debate. The aim of this work was to evaluate the safety of performing carotid artery stenting procedure without the use of cerebral protection device in patients with symptomatic carotid stenosis. A retrospective study was performed during the period from September 2015 till March 2020 including 91 patients with symptomatic carotid artery stenosis. All patients were treated with a single stent type (Wall stent® - Boston scientific) without the use of cerebral protection device. Pre- and post-procedural clinical assessment with the national institute of health stroke scale (NIHSS). Post procedure brain diffusion-weighted magnetic resonance imaging (DW-MRI) if clinically indicated within 24 h was used to determine periprocedural cerebral embolization.
Results
A low complication rate was found as only one case (1%) showed mild deterioration in NIHSS and new acute cerebral emboli were detected with brain DW-MRI.
Conclusion
Carotid artery stenting can be performed safely without the use of cerebral protection device.
Similar content being viewed by others
Background
Carotid artery stenting (CAS) is used as an alternative to carotid endarterectomy in treatment of carotid artery stenosis, especially in patients with high risk for surgery [1].
The most feared hassle of the stenting technique is cerebral embolism due to dislodgement of atherosclerotic materials during the procedure. Several cerebral protection devices (CPDs) had been developed to keep away from or lessen the risk of periprocedural complications. Nowadays, the usage of those devices has broadly increased and is routinely used in clinical practice; however, these protection devices are too expensive and represent about 40% of the whole fee of the procedure.
There are two main types of CPDs which include proximal devices with occlusion balloon and distal devices with occlusion balloon or a filter, but the most commonly used are those based on distal filter placement to seize emboli dislodged from the atherosclerotic plaque with preservation of the flow within the artery. The filter catches fragments larger than its pore size and permits the passage of smaller debris [2].
Many reviews and meta-analyses showed that the rate of ipsilateral stroke and death was less in patients treated with CAS and protection devices [3, 4]. On the other hand, several uncontrolled studies showed fantastic results in patients treated without the use of protection devices [5,6,7,8,9]. In a recent meta-analysis, the usage of CPD reduced the rate of symptomatic stroke after CAS; however, in patients with symptomatic lesions, its efficacy was not much obvious and therefore, its routine usage during the procedure should be assessed well before mandatory usage [10].
Methods
A retrospectively analysis was done to our carotid stenting data registry from September 2015 to March 2020. The study is retrospective, so ethical committee approval is not required as the patient’s acceptance of hospital admission includes consent to retrospective use of their data according to the Egyptian law. Ninety-one patients with moderate and severe internal carotid artery stenosis were included and underwent ninety-one CAS procedures with a single stent type with closed-cell design (Wall stent®-Boston scientific) without the use of CPDs. Indication for CAS was the presence of symptomatic carotid stenosis. The patients were chosen according to the carotid duplex findings if the stenosis is greater than 50% with confirmation of degree of stenosis during the diagnostic phase of the digital subtraction angiography as it should be greater than 50% to proceed for the stenting procedure. Measurement of the degree of angiographic carotid stenosis was performed using North American Symptomatic Carotid Endarterectomy trial (NASCET) methodology. Exclusion criteria included the presence of a source for cardiac embolization, occlusion of the ipsilateral intracranial portion of the internal carotid artery, severe disabling stroke that makes a great difficulty to participate in the study, presence of intraluminal carotid thrombus, contraindication to anticoagulation or antiplatelet therapy, and history of contrast nephropathy.
Neurological assessment was performed using the NIHSS by a well-trained neurologist just before the procedure, immediately after and at 24 h later before discharge. All CAS procedures were performed under local anesthesia through the common femoral artery. All patients received dual anti-platelet therapy with aspirin 150 mg once daily and clopidogrel 75 mg once daily, 1 week before the procedure or aspirin 150 mg and clopidogrel 300 mg the day before or at least 6 h before the procedure and continued post procedure on aspirin 150 mg per day for life and clopidogrel 75 mg per day for 6 months. All procedures were completely performed by a well-trained interventional neurologist at the interventional neurology unit of Ain Shams University Hospital using a monoplane neurovascular machine (Siemens, Germany). Pre-dilatation was selectively done using 2.5 × 20 mm balloons. Post-dilatation was also selectively done using 5.5 × 20 mm balloons if the degree of residual stenosis after stent placement was greater than 30%. Cervical and cerebral angiographic views were routinely obtained before and after stent deployment. Successful stenting is defined as covering the whole lesion with a single stent and achieving less than 30% residual stenosis.
Patients with clinical deterioration due to ischemic lesions had a brain DW-MRI within 24 h after the procedure. Brain DW-MRI was obtained using standard head coils on 1.5-Tesla (Achieva and Ingenia, Philips medical system, Eindhoven, Netherlands). Acute cerebral ischemic lesions were defined as hyper-intense areas with diffusion restriction signals, which were confirmed using apparent diffusion coefficient mapping to exclude any artifacts. The DW-MRI studies were assessed by radiologists blinded to the clinical status and outcome of the patients.
The collected data was revised, coded, and introduced to a personal computer using Statistical Package for Social Science (SPSS 25, by IBM: Armonk; New York, USA). A suitable analysis for the data was done according to its type. Quantitative data was summarized by the mean, standard deviation (± SD) while qualitative data was summarized by frequencies and percentages.
Results
The study included ninety-one patients who underwent ninety-one CAS procedures without the use of CPDs .The baseline characteristics and risk factors of the patients are shown in Tables 1 and 2. The clinical presentations and initial brain DW-MRI findings are illustrated in Tables 3 and 4. Both carotid duplex and digital subtraction angiography findings are illustrated in Tables 5 and 6.
As regard the interventional details, twelve cases (13%) underwent early stenting (within 2 weeks of onset of symptoms) and seventy-nine cases (87%) underwent delayed stenting (after 2 weeks of symptoms onset), sixty-one cases (67%) underwent stenting to the right side and thirty cases (33%) underwent left sided stenting, sixty cases (66%) had a smooth surface of the stenotic plaque during angiography while thirty-one cases (34%) had irregular surface, forty-one cases (45%) underwent pre-dilatation angioplasty but post-dilatation angioplasty was done for all patients.
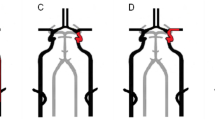
All cases were clinically assessed using the NIHSS as a baseline before CAS. Thirty-nine cases (42.8%) had NIHSS equal to zero, thirty-three cases (36.3%) had NIHSS ranging from 1 to 4 and nineteen cases (20.9%) had NIHSS ranging from 5 to 15. Immediately after the intervention, ninety cases (99%) had no change in their initial NIHSS and one case (1%) showed mild deterioration of the NIHSS. This case with clinical deterioration was a 75-year-old male with left internal carotid artery stenosis about 90%. Baseline NIHSS before intervention was zero. Delayed carotid stenting was done with pre- and post-dilatation angioplasty. The NIHSS changed immediately after intervention to become three. Follow-up brain DW-MRI showed recent two small embolic foci. The patient returned to the initial baseline NIHSS after 1 week. The images of this case are illustrated in Fig. 1.
A Extracranial left internal carotid artery stenosis about 90%. B The extracranial part of the left internal carotid artery after stenting. C The intracranial portion of the left internal carotid artery after intervention. D Brain DW-MRI showing two embolic foci at left frontal and left parietal regions
Discussion
Endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) trial raised an alert as it recommended that CAS procedure should be done only with the use of distal cerebral protection devices with filter to protect against cerebral embolization [11]. The importance of this recommendation was arguable because the patients without cerebral protection was an average of 8 years older and most of the strokes does not occur during the stenting procedure but later in the clinical course, so the non-usage of CPD was not the only cause of embolic complications and hard to blame. Also, the small number of patients in the non CPD group made the difference of a little significance.
In a recent publication by Cho YD and colleagues [10], they analyzed a total of 539 symptomatic CAS procedures from four studies; of these, 345 were done with CPD and 194 were done without protection device. The number of stroke was six (1.7%) in protected CAS and 11 (5.7%) in unprotected CAS which was statistically non-significant (p = 0.160) and so the use of CPD did not significantly decrease the events of stroke after CAS.
The pros of using CPDs with filter are the ability to keep the flow during CAS procedure and to protect the brain from embolization. The cons of those devices are the dislodgement of materials during its deployment which is attributed to its large crossing profile, low flexibility and torquability, and also the danger of cerebral micro embolization after its deployment because of flow around and through the filter, pore size, bad apposition in tortuous vessels, and during its retrieval [12].
Although the usage of CPDs may seem important in brain protection, it remains a debatable topic as the risk of cerebral embolization is present in all stages of CAS: passing the lesion with a wire, pre-dilatation, placement of the protection device, stent deployment, and post dilatation [13, 14].
The lesion load in our study was low, as new cerebral ischemic lesions were noted only in one case (1/91). This result was in keeping with previous studies [15, 16]. The explanations for this could be: first, the use of better materials nowadays concerning the exchange system and the use of flexible guiding catheters instead of the long sheath; second, diminishing the number of device manipulations across the lesion before stent placement by non-use of filter and limited use of pre-stenting balloon dilatation which was selectively done in forty-one cases with tight stenotic lesions to permit passage of the stent across the lesion; third, the use of stent with closed cell design and its placement before angioplasty balloon dilatation in most of our cases. Closed cell designed stents can provide better scaffolding to the carotid lesion and hence decrease the danger of plaque extrusion via the interstices of the stent during its deployment, post dilatation, and after finishing the procedure [17]. Two previous studies showed a trend of better outcome with closed cell stents [18, 19]; fourth, our study focus was on clinical periprocedural complications which is related to the use or non-use of CPD in contrast to most of other studies which did not evaluate the efficacy of the CPD according to the symptomaticity and also there were great differences in the primary endpoints (stroke versus stroke or death versus stroke and death) and the duration of follow-up and its impact on the study conclusion [10].
The only case in our study which experienced a clinical deterioration was a left carotid artery stenting. This finding was similar to what was reported by Naggara and his colleagues. They found that CAS performed for left carotid stenosis was associated with higher ipsilateral strokes than with right-sided stenting [20]. This higher rate of periprocedural complications may be explained by difficulty in access to the left common carotid artery which takes more time to reach stenotic segment and hence more complications are likely to occur. Also, during stenting of the right side, the occurrence of strokes in the non-eloquent right hemisphere may pass asymptomatic.
Conclusion
Carotid artery stenting can be done safely without the use of cerebral protection devices as it does not provide significant additional benefits to prevent cerebral ischemic events. Therefore, in low-resource settings, as a matter of cost benefit ratio, cerebral protection devices should not be used routinely.
Availability of data and materials
Dataset is available as master sheet in Excel format and publicly available in Neurology Department, Ain Shams University, through communicating to the corresponding author.
Abbreviations
- CAS:
-
Carotid artery stenting
- CPD:
-
Cerebral protection device
- DW-MRI:
-
Diffusion-weighted magnetic resonance imaging
- NIHSS:
-
National Institute of Health Stroke Scale
References
Werner N, Zeymer U, Mark B, Hochadel M, Hauptmann KE, Jung J, et al. Carotid artery stenting in clinical practice: does sex matter? Results from the carotid artery stenting registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK). Clin Cardiol. 2012;35(2):111–8. https://doi.org/10.1002/clc.21015.
Müller-Hüllsbeck S, Jahnke T, Liees C, Glass C, Paulsen F, Grimmet J, et al. In vitro comparison of four cerebral protection filters for preventing human plaque embolization during carotid interventions. J Endovasc Ther. 2002;9(6):793–802. https://doi.org/10.1177/152660280200900612.
Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34(3):813–9. https://doi.org/10.1161/01.STR.0000058160.53040.5F.
Garg N, Kargoirgos N, Pismiss GT, Sohal DP, Longo GM, Johanning JM, et al. Cerebral protection devices reduce periprocedural strokes during carotid angioplasty and stenting: a systematic review of the current literature. J Endovasc Ther. 2009;16(4):412–27. https://doi.org/10.1583/09-2713.1.
Maynard M, Baldi S, Rostagno R, Zander T, Rabellino M, Llorens R, et al. Carotid stenting without the use of balloon angioplasty and distal protection devices: preliminary experience in 100 cases. AJNR Am J Neuroradiol. 2007;28(7):1378–83. https://doi.org/10.3174/ajnr.A0543.
Tietke MW, Kerby T, Alfke K, Riedel C, Rohr A, Jensenet U, et al. Complication rate in unprotected carotid artery stenting with closed cell stents. Neuroradiology. 2010;52(7):611–8. https://doi.org/10.1007/s00234-010-0672-y.
Mohammadian R, Sohrabi B, Mansourizadeh R, Mohammadian F, Nasiri B, Haririan S. Unprotected carotid artery stenting: complications in 6 month follow up. Neuroradiology. 2012;54(3):225–30. https://doi.org/10.1007/s00234-011-0867-x.
Baldi S, Zander T, Rabellinio M, González G, Maynar M. Carotid artery stenting without angioplasty and cerebral protection: a single center experience with up to 7 years follow-up. AJNR Am J Neuroradiol. 2011;32(4):759–63. https://doi.org/10.3174/ajnr.A2375.
Mansour OY, Weber J, Niesen W, Schumacher M, Berlis A. Carotid angioplasty and stenting without protection devices: safety and efficacy concern-single center experience. Clin Neuroradiol. 2011;21(2):65–73. https://doi.org/10.1007/s00062-011-0057-6.
Cho YD, Kim SE, Lim JW, Choi HJ, Cho YJ, Jeon JP. Protected versus unprotected carotid artery stenting: meta-analysis of the current literature. J Korean Neurosurg Soc. 2018;61(4):458–66. https://doi.org/10.3340/jkns.2017.0202.001.
Jean LM, Ludovic T, Didier L, Jean FA, Herve R, Alain V, et al. Endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) trial: results up to 4 years from a randomized multicenter trial. Lancet Neurol. 2008;7(10):885–92.
Pinero P, Gonzalez A, Mayol A, Martinez E, Gonzalez Marcos JR, Boza F, et al. Silent ischemia after neuroprotected percutaneous carotid stenting: a diffusion weighted MRI study. AJNR Am J Neuroradiol. 2006;27(6):1338–45.
Vos JA, Van Den Berg JC, Ernst SM, Suttorp MJ, Overtoom TT, Mauser HW, et al. Carotid angioplasty and stent placement: comparison of transcranial Doppler US data and clinical outcome with and without filtering cerebral protection devices in 509 patients. Radiology. 2005;234(2):493–9. https://doi.org/10.1148/radiol.2342040119.
Schnaudigel S, Gröschel K, Pilgram SM, Kastrup A. New brain lesions after carotid stenting versus carotid endarterectomy: a systematic review of the literature. Stroke. 2008;39(6):1911–9. https://doi.org/10.1161/STROKEAHA.107.500603.
Ederle J, Bonati LH, Dobson J, Featherstone RL, Gaines PA, Beard JD, et al. Endovascular treatment with angioplasty or stenting versus carotid endarterectomy in patients with carotid artery stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long term follow up of a randomized trial. Lancet Neurol. 2009;8(10):898–907. https://doi.org/10.1016/S1474-4422(09)70228-5.
Khedr H, Eweda A, Hamza M, Salem A, Elshemy W, Tawfik AM. Carotid endarterectomy versus carotid artery stenting without protection devices for the management of carotid artery stenosis. Egypt J Surg. 2016;35:225–30.
Hart JP, Peeters P, Verbist J, Deloose K, Bosiers M. Do device characteristics impact outcome of carotid artery stenting? J Vasc Surg. 2006;44(4):725–30. https://doi.org/10.1016/j.jvs.2006.06.029.
Jim J, Rubin BG, Landis GS, Kenwood CT, Siami FS, Sicard GA, et al. Society for Vascular Surgery Vascular Registry evaluation of stent cell design on carotid artery stenting outcomes. J Vasc Surg. 2011;54(1):71–9. https://doi.org/10.1016/j.jvs.2010.12.054.
Timaran CH, Rosero EB, Higuera A, Ilarraza A, Modrall JG, Clagett GP. Randomized clinical trial of open-cell vs closed-cell stents for carotid stenting and effects of stent design on cerebral embolization. J Vasc Surg. 2011;54(5):1310–6. https://doi.org/10.1016/j.jvs.2011.05.013.
Naggara O, Touzé E, Beyssen B, Trinquart L, Chatellier G, Meder JF, et al. Anatomical and technical factors associated with stroke or death during carotid angioplasty and stenting: results from the endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) trial and systematic review. Stroke. 2011;42(2):380–8. https://doi.org/10.1161/STROKEAHA.110.588772.
Acknowledgements
Not applicable.
Funding
Self-financing
Author information
Authors and Affiliations
Contributions
AH, AE, and SS conceived of the study and participated in its design and coordination and helped to draft the manuscript. AS and RY participated in the design of the study and performed the statistical analysis. All authors have agreed to conditions noted on the Authorship Agreement Form. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by Neurology Department Research Ethical Committee in November 2020 (approval number not available). Written informed consent was obtained from the patients participating in the study, or their first degree relatives if the patient was unable to provide consent, after informing them about the study rationale and their right to withdraw from the study at any time without any consequences.
Consent for publication
Not applicable.
Competing interests
All authors declare that they do not have any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sudany, A.H., Georgy, S.S., Zaki, A.S. et al. Non-protected carotid artery stenting for symptomatic carotid stenosis in low resource settings. Egypt J Neurol Psychiatry Neurosurg 57, 79 (2021). https://doi.org/10.1186/s41983-021-00330-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-021-00330-3