Abstract
Background
Wetlands are transition zones between aquatic and terrestrial environments of Opa Reservoir Obafemi Awolowo University, Ile-Ife, Nigeria, threatened by increase in agricultural practices and urbanization within the catchment area. A critical appraisal of the current ecological integrity of the wetlands using planktonic composition and community structure which are formidable to sustaining wetlands is essential, hence this study.
Result
A total of 104 plankton species comprises of 68 species of phytoplankton and 36 species of zooplankton were recorded from the wetlands. In each sampled location and during the sampling period, Bacillariophyta had the highest occurrence among the phytoplankton, while Rotifera recorded the highest occurrence among the zooplankton. Generally, Synedra ulna and copepod (cyclopoid) Nauplius, respectively, were the most common phytoplankton and zooplankton recorded during the period of study. Analyses also showed that the phytoplankton had a higher species occurrence and abundance (65.3% and 98.18%, respectively) than the zooplankton (34.6% and 1.82%, respectively). Diversity indices (Shannon–Wiener, Margalef, and Simpson) analyses of both plankton groups revealed that the wetlands were generally polluted.
Conclusion
The study concluded that the ability of the wetlands to support and maintain a balanced adaptive community of plankton with species composition and diversity is being eroded due to anthropogenic activities in the wetland areas. This could have some ecological and socioeconomic implications considering the fact that they are intrinsically a part of the adjoining reservoir which provides many ecosystem services.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Wetlands a marshy, fen, peatland or water area, whether natural or artificial, permanent or temporary, with water that is stationary or flowing, which may be fresh, brackish, or salty, with a depth at low tide not exceed six meters, have provided a wide range of ecosystem services for humankind in all continents (Junk, 2002; Millennium Ecosystem Assessment, 2005). They are characterized by a large number of ecological niches and harbour a significant percentage of world’s biological diversity. Wetlands are among the most productive ecosystems in the world comparable to rainforests and coral reefs (Thomas and Deviprasad, 2007). They host a considerable biological diversity including planktonic assemblage, which makes them one of the most productive life-supporting systems in the world (WWF, 1999). Vegetative matters that release by wetlands into rivers also help to boost fish trophic plasticity in the rivers and lakes (Orie, 2017). Values associated with biological productivity of wetlands also include water quality and flood control, erosion control, community structure and wild life support, recreation, and esthetics (Leck et al., 2012).
However, various anthropogenic activities or human-induced stress such as burgeoning human population, rapid urbanization, deforestation, siltation, overharvesting, domestic and industrial wastes, and introduction of invasive alien species have contributed to the decline of the quality and quantity of wetlands (Fonge et al., 2012). Consequently, these direct and indirect drivers of wetland degradation are changing their ecosystem functions and undermine their capacity to sustain ecosystem services in their respective basins (Millennium Ecosystem Assessment, 2005; Ribaudo et al., 2001).
Plankton which are primary producers that contribute immensely to water body productivity and food web chain are one of the formidable community structure that sustains wetlands (WWF, 1999). They are also serve as bioindicators of water quality because they respond very quickly to changes in environmental stress and this could result in changes in their composition and community structure (Akindele & Adeniyi, 2013a; Dalu et al., 2014; Harley et al., 2006; Wu et al., 2012). Thus, the composition and community structure of plankton are useful in assessing the biological integrity and functioning of wetland ecosystem (Akindele & Adeniyi, 2013b; Brettum & Andersen, 2005).
Studies of wetland assessment in Nigeria have identified habitat changes caused by anthropogenic activities as the major contributing factor to alteration of wetland characteristics. However, Opa Reservoir wetland in Obafemi Awolowo University, Ile-Ife, which provides a wide range of ecosystem services such as habitat for a wide range of animals, flood control, water abstraction for irrigation, and provision of potable water receives little or no attention to its fair share of habitat degradation and decline in biodiversity due to deforestation, increase in cultivated lands, and construction of structures. In view of the importance of Opa Reservoir wetlands which are being threatened by various human-induced stressors, a critical appraisal of the current ecological integrity of the wetlands using planktonic composition and community structure is essential, hence this study.
Methods
The study area
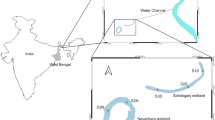
The study was carried out on the wetlands of Opa Reservoir, Obafemi Awolowo University, Ile-Ife, Southwestern, Nigeria. The reservoir has a catchment area of about 116 km2 and extends from longitude 004° 31′ E to 004° 32′ E and latitude 07° 30′ N to 07° 31′ (Komolafe & Arawomo, 2001). The reservoir, which was impounded in 1978, takes its source from Oke Opa hills (Akinbuwa & Adeniyi, 1996) and has a surface area of 0.95 km2 with the maximum capacity of about 675 m3 at impoundment (Komolafe & Arawomo, 2001). However, the reservoir was recently reported to have shrunk in size due to siltation and aquatic weed infestations (Adesakin et al., 2017). The catchment area is characterized by annual dry and wet seasons with the wet season extending from April to September while the dry season extends from October to March (Komolafe & Arawomo, 2001). The mean annual rainfall over the catchment area ranges from 1000 to 1237 mm while the average temperature had a peak of 28.8 °C in February and about 24.5 °C in August (Bayowa et al., 2011).
Selection of the sampling points
Random selection of the proposed sampling locations was done using ecological assessments survey design, commonly applied in a variety of research fields (Levin, 2006). Six sampling locations were established in the Opa Reservoir wetlands. The established sampling points were denoted as Sites A, B, C, D, E, and F (Fig. 1). The coordinates of the sampling points were established using a handheld Global Positioning System (GPS) for subsequent samplings. The characteristics of the sampled sites are shown in Table 1.
Sample collection
Plankton samples were collected by straining 30 L of water collected from each sampling site with a plankton net (45 μm mesh size) and concentrating it to 100 ml. The plankton samples were preserved in-situ with 5% formalin to ensure the integrity of the samples.
Laboratory analyses
Further laboratory analysis was carried out on 1.5 mL concentrate plankton sample in a Sedgewick Rafter counting chamber viewed under an Olympus compound light microscope at scanning power (× 40) and low power (× 100). Each observed plankton was enumerated, and their scaled photomicrograph was taken. Identification of the recorded plankton species was based on phytoplankton and zooplankton guides such as American Public Health Association (1998), Kutikova (2002), Victor (2002), and Opute and Kadiri (2013), in addition to other works of Akinbuwa (1999), Fernando (2002), Suthers and Rissik (2009), Bellinger and Sigee (2010), and Ekhator et al. (2014). The species diversity, abundance, and evenness were determined using appropriate indices. The abundance of each plankton taxon per unit volume of the water was estimated based on count records obtained. The abundance of each species was determined by the following equation:
where A is the abundance of species per liter of original water source; a is the abundance of species in the counting chamber; b is the total concentrate volume of water used (1.5 mL); c is the original volume of water (30 L) (Bellinger & Sigee, 2010).
Statistical analyses
The data on flora and fauna collected from Opa Wetlands were analyzed using a combination of standard statistical procedures as follows:
Shannon–Wiener diversity index (H)
Shannon–Wiener index (H) (1984), a measure of species richness, was calculated as follows:
where N is the total number of individuals sampled; fi is the number of individual species sampled.
A standard of Shannon–Wiener index for interpreting the ecological condition of an ecosystem as reported by Reiss and Kröncke (2005) is shown in Table 2.
Simpson’s diversity index (SID)
Simpson’s diversity index (1 − D) was calculated as follows:
where D is the measure of diversity, computed as follows: N is the total number of all species counted; n is the count of species.
Margalef’s diversity index (D)
Margalef’s diversity index was calculated using the formula:
where D is the Margalef’s diversity index value; S is the number of species; N is the number of individuals.
Species evenness indices
Pieolu’s Evenness index, which describes the relative distribution of the species, is expressed as:
where H′ is the Shannon–Weiner diversity; ln S is the natural log of the total number of species recorded.
Results
The phytoplankton assemblage of Opa Reservoir wetlands is presented in Table 3. A total number of 66 species belonging to six divisions (Cyanophyta, Chlorophyta, Baccillariophyta, Euglenophyta, Ochrophyta, and Charophyta) were recorded during the study period. The order of dominance in relation to species richness was as follows: Bacillariophyta (34 species), Charophyta (12 species), Cyanobacteria (7), Chlorophyta (6 species), Ochrophyta (6 species), and Euglenophyta (one species). In terms of abundance, Bacillariophyceae (56.54%) recorded the highest, followed by Chlorophyceae (38.63%), Charophyceae (3.18%), Cyanophyceae (1.11%), Ochrophyceae (0.48%), and Euglenophyceae (0.07%). Among individual species, Synedra ulna was the most abundant, followed by Oedogonium sp. and Synedra sp. Other phytoplankton species with relatively high abundances were Humidophilia contenta, Closterium sp., and Navicula capitatoradiata.
Stations E and F were remarkably and relatively high in both taxa richness and abundance, while stations A and B, respectively, recorded lower values. One-way ANOVA indicated that there was no significant difference (F = 1.344, df = 5, p = 0.223) among the stations with respect to distribution of the species. Diversity indices were generally low in all the stations with station C recording the lowest values for both Shannon–Wiener and Simpson, while stations B and D recorded the highest values, respectively.
Zooplankton comprised a total of 36 species belonging to three animal phyla Protozoa, Rotifera, and Arthropoda (Table 4). In terms of species richness, Rotifera was the most species-rich (19 species) while Arthropoda and Protozoa recorded 13 and 4 species, respectively. The order of dominance among the groups was as follows: Rotifera (60%), Arthropoda (35.24%), and Protozoa (4.76%). Among the six sampled locations, site F had the highest number of zooplankton species followed by sites C, E, B, A, and site D. Station F again recorded the highest abundance followed by stations C, D, E, B, and A.
One-way analysis of variance indicated that there was a significant difference (F = 6.18, df = 5, p = 0.00003) in the spatial distribution of the organisms among the stations. Diversity indices based on Shannon–Wiener, Simpson and Margalef indicated that station F recorded the highest values, while station D recorded the lowest. However, the lowest evenness was recorded at stations F but increased slightly at site C, while other stations recording the highest possible value or one that is close to it.
Overall, copepod (cyclopoid) nauplius larvae were the most abundant followed by Argonotholca foliacea. In each sampled location and during the sampling period, Bacillariophyta had the highest occurrence among the phytoplankton, while Rotifera recorded the highest occurrence at sites F, C, and D among the zooplankton. Rotifera and Arthropoda were both the highest at site F while Protozoa had the highest occurrence at sites B and C. During the period of study, the phytoplankton had a higher species occurrence (65.3%) than the zooplankton (34.6%). In the same vein, phytoplankton recorded a much higher abundance (98.18%) than the zooplankton (1.82%).
Discussion
Opa Reservoir was considerably rich in phytoplankton, and most of the recorded species have been previously reported in Nigeria and tropical freshwaters (Atobatele, 2013; Sharma et al., 2013; Singh et al., 2013; Peresin et al., 2014; and Akindele & Olutona, 2017). Groups Bacillariophyceae, Charophyceae, Chlorophyceae, and Cyanophyceae which are the dominant phytoplankton in terms of species richness have similarly been reported as the dominant groups in different countries and continents of the world (Rodrigues et al., 2009; Tavernini et al., 2011; Wu et al., 2012; Atobatele, 2013; Peresin et al., 2014, and Akindele & Olutona, 2017). Some indicator species of eutrophication (Anabaena circinalis, Euglena gracilis, and Brachionus falcatus) were recorded in this study; hence, their occurrence could be an indication that the wetlands were organically polluted (Tanimu et al., 2011, and Singh et al., 2013).
Like many of the previous studies in Nigeria, members of the Phylum Rotifera dominated the zooplankton in the current study (Akindele & Adeniyi, 2013b; and Atobatele, 2013). The success of rotifers as plankton has been attributed to the following factors: parthenogenetic reproduction, presence of transparent lorica, and ability to adjust to environmental changes caused by climatic conditions (Rodríguez and Matsumura-Tundisi, 2000; Kutikova, 2002; Roche & Rocha, 2005). It is worth being mentioned that the ratio of phytoplankton to zooplankton in terms of taxonomic composition (~ 2:1) and abundance (~ 6:1) in the wetlands suggests that phytoplankton is autotrophic and is at the base of the food pyramid in an aquatic ecosystem. They are usually greater in numbers than heterotrophic animals up the food chain. A similar observation was observed by Akindele and Olutona (2017) in Osun River (Nigeria), and the relative distributions of phytoplankton and zooplankton in a freshwater system have long been described by Hynes (1970), i.e., phytoplankton > zooplankton in terms of abundance.
All indices used to determine the community structure of both phytoplankton and zooplankton indicated low biodiversity status at all stations. Shannon–Wiener diversity values increase with the number of species in a community, and the higher the values, the healthier the community (Maryam et al., 2010). Gencer and Nilgun (2010) explained that values above 3.0 show a stable and balanced habitat while values under 1.0 indicate pollution and degradation of habitat structure. Based on the Shannon–Wiener index for phytoplankton community structure, all but one site (site C) could be considered as being a poor wetland (1 < H′ < 2) while all the sites had poor community structure (1 < H′ < 2) based on Shannon–Wiener index for zooplankton. Margalef indices of the phytoplankton in the six stations and those of zooplankton in two stations indicated a moderate pollution, i.e., 1 < D < 3, while the indices for zooplankton at two stations (A, B, D, and E) indicated a severe pollution, i.e., D < 1 according to Lenat et al. (1980). Simpson’s diversity index (1 − D) which is widely used for comparing diversity between various habitats (Simpson, 1949) also alluded to the pollution status of the studied wetlands. Values ranged from 0 to 1; values close to 1 indicate severe pollution and low diversity, while values close to 0 indicate high diversity and/or pristine condition (Kratzer & Batzer, 2007). Based on this, all the sites could be considered polluted considering Simpson’s indices for zooplankton and also for phytoplankton.
Conclusions
The Opa Reservoir Wetlands may be described as having an unbalanced habitat structure based on their plankton compositions. Indicator species and community structure of the plankton also revealed that the wetlands are impacted. This could have some ecological and socioeconomic implications considering the fact that they are intrinsically a part of the adjoining reservoir which provides many ecosystem services. Hence, consistent monitoring of the Opa Reservoir Wetlands is suggested to sustain and/or enhance their biodiversity and ecological condition of the wetlands and the reservoir.
Availability of data and materials
Data are available on request.
References
Adesakin, T. A., Adedeji, A. A., Aduwo, A. I., & Taiwo, Y. F. (2017). Effect of discharges from re-channeled rivers and municipal runoff on water quality of Opa reservoir, Ile-Ife, Southwest Nigeria. African Journal of Environmental Science and Technology, 11(1), 56–70.
Akinbuwa, O. (1999). The Rotifera fauna and Physico-chemical conditions of Erinle Lake and its major inflows at Ede. Nigeria, Obafemi Awolowo University, Ile-Ife.
Akinbuwa, O., & Adeniyi, I. F. (1996). Seasonal variation, distribution, and interrelationships of rotifers in Opa Reservoir, Nigeria. African Journal of Ecology, 34(4), 351–363.
Akindele, E. O., & Adeniyi, I. F. (2013a). Zooplankton composition and community structure in Lake Tiga, Kano, Nigeria. African Journal of Aquatic Science, 38(3), 279–286.
Akindele, E. O., & Adeniyi, I. F. (2013b). A study of the physico-chemical water quality, hydrology and zooplankton fauna of Opa Reservoir catchment area, Ile-Ife, Nigeria. African Journal of Environmental Science and Technology, 7(5), 192–203.
Akindele, E. O., & Olutona, G. O. (2017). Water quality and biodiversity of potamoplankton in an African sacred grove and world heritage site. Zoology and Ecology, 27(3–4), 292–303.
American Public Health Association. (1998). Standard methods for the examination of water and waste water. American Public Health Association.
Atobatele, O. E. (2013). Pelagic phytoplankton succession pattern in a tropical freshwater reservoir (Aiba Reservoir, Iwo, Osun, Nigeria). Bioremediation, Biodiversity and Bioavailability, 7, 81–84.
Bayowa, O. G., Ilufoye, D. T., & Animasaun, A. R. (2011). Geoelectric investigation of Awba earth dam embankment, University of Ibadan, Ibadan, Southwestern Nigeria, for Anomalous seepages. Ife Journal of Science, 13(2), 227–238.
Bellinger, E. G., & Sigee, D. C. (2010). Algae as bioindicators. In: Freshwater algae: Identification and use as bioindicators (pp. 99–136).
Brettum, P., & Andersen, T. (2005). The use of phytoplankton as indicators of water quality. In: NIVA report. Society for NeuroOncology (pp. 4818–2004).
Dalu, T., Froneman, P. W., & Richoux, N. B. (2014). Phytoplankton community diversity along a river-estuary continuum. Transactions of the Royal Society of South Africa, 69(2), 107–116.
Ekhator, O., Opute, F. I., & Akoma, O. C. (2014). A checklist of the phytoplankton flora of a southern Nigerian lotic ecosystem. Current Research Journal of Biological Sciences, 6(1), 1–6.
Fernando, C. H. (2002). A guide to tropical freshwater zooplankton: Identification, ecology, and impact on fisheries (p. 291). Backhuys Publishers.
Fonge, B. A., Tening, A. S., Egbe, E. A., Yinda, G. S., Fongod, A. N., & Achu, R. M. (2012). Phytoplankton diversity and abundance in Ndop wetland plain, Cameroon. African Journal of Environmental Science and Technology, 6(6), 247–257.
Gencer, T., & Nilgun, K. (2010). Applications of various diversity indices to benthic macroinverstebrate assemblages in streams of a natural park in Turkey. Review of Hydrobiology, 3(2), 111–125.
Harley, C. D., Randall, H., Hultgren, A., Miner, K. M., Sorte, B. G., Thornber, C. J., & Williams, S. L. (2006). The impacts of climate change in coastal marine systems. Ecology Letters, 9(2), 228–241.
Hynes, H. B. N. (1970). The ecology of running waters. University of Toronto Press.
Junk, W. J. (2002). Long-term environmental trends and the future of tropical wetlands. Environmental Conservation, 29(4), 414–435.
Komolafe, O. O., & Arawomo, G. A. O. (2001). The distribution and feeding habits of a cichlid fish Oreochromis niloticus (Linnaeus) in Opa Reservoir, Ile-Ife. In: Proceedings of the 14th annual conference of the fisheries society of Nigeria (pp. 40–46).
Kratzer, E. B., & Batzer, D. P. (2007). Spatial and temporal variation in aquatic macroinvertebrates in the Okefenokee Swamp, Georgia, USA. Wetlands, 27(1), 127–140.
Kutikova, L. A. (2002). Rotifera. In C. H. Fernando (Ed.), A guide to tropical freshwater zooplankton (pp. 26–38). Backhuys Publishers.
Leck, M. A., Parker, V. T., & Simpson, R. L. (2012). Ecology of soil seed banks (p. 462). Academi Press.
Lenat, D. R., Smock, L. A., & Penrose, D. L. (1980). Use of benthic macroinvertebrates as indicators of environmental quality. In L. W. Douglass (Ed.), Biological monitoring for environmental effects (pp. 97–114). Lexington Books.
Levin, K. A. (2006). Study design VI: Ecological studies. Evidence-Based Dentistry, 7(4), 108.
Maryam, M. R., Seyed, M. B., Parvin, N. F., & Abdolrahman, R. (2010). Studies on the benthic macroinvertebrate diversity species as bioindicators of environmental health in Bahrekan Bay (Northwest of Persian Gulf). African Journal of Biotechnology, 9(39), 16–27.
Millennium Ecosystem Assessment. (2005). Ecosystems and human well-being: Wetlands and water synthesis. World Resources Institute.
Opute, F. I., & Kadiri, M. O. (2013). Phytoplankton algae of Nigeria: A practical and theoretical guide (p. 304). Mindex.
Orie, E. G. (2017). Sustainable wetlands and biodiversity conservation: Nigeria's Lagos Lagoon in focus. In: Biodiversity Routledge (pp. 29–53). India.
Peresin, D., Torgan, L. C., Schulz, U. H., & Crossetti, L. O. (2014). Structure of potamoplankton along a gradient of preservation of riparian vegetation in subtropical streams. Anais Da Academia Brasileira De Ciências, 86(2), 841–853.
Reiss, H., & Kröncke, I. (2005). Seasonal variability of benthic indices: An approach to test the applicability of different indices for ecosystem quality assessment. Marine Pollution Bulletin, 50(12), 1490–1499.
Ribaudo, M. O., Heimlich, R., Claassen, R., & Peters, M. (2001). Least-cost management of nonpoint source pollution: Source reduction versus interception strategies for controlling nitrogen loss in the Mississippi Basin. Ecological Economics, 37(2), 183–197.
Roche, K. F., & Rocha, O. (2005). Aspectos de predacao por piexes e, lagos e represas, com enfoque na planctivoria. In: Ecologia trofica de peixes com enfase na planctivoria em embientes lenticos de agua doce no Brasil (pp. 1–24). Rima.
Rodrigues, L. C., Train, S., Bovo-Scomparin, V. M., Jati, S., Borsalli, C. C. J., & Marengoni, E. (2009). Interannual variability of phytoplankton in the main rivers of the upper Parana River floodplain, Brazil: Influence of upstream reservoirs. Brazilian Journal of Biology, 69(2 suppl.), 501–516.
Rodríguez, M. P., & Matsumura-Tundisi, T. (2000). Variation of density, species composition and dominance of rotifers at a shallow tropical reservoir (Broa Reservoir, SP, Brazil) in a short scale time. Revista Brasileira De Biologia, 60(1), 1–9.
Sharma, C., Jindal, R., Singh, U. B., Ahluwalla, A. S., & Thakur, R. K. (2013). Population dynamics and species diversity of plankton in relation to hydrobiological characteristics of river Sutley, Punjab, India. Ecology, Environment and Conservation, 19(3), 717–724.
Simpson, E. (1949). Measurement of diversity. Nature, 163, 688. https://doi.org/10.1038/163688a0.
Singh, U. B., Ahluwalla, A. S., Sharma, C., Jindal, R., & Thakur, R. K. (2013). Planktonic indicators: A promising tool for monitoring water quality (early-warning signals). Ecology, Environment and Conservation, 19(3), 793–800.
Suthers, I. M., & Rissik, D. (Eds.). (2009). Plankton: A guide to their ecology and monitoring for water quality (p. 256). CSIRO Publishing.
Tanimu, Y., Tanimu, J., Bako, S.P. & Adakole, J.A. (2011). Phytoplankton as bioindicators of water quality in Saminaka Reservoir, Northern Nigeria. Science Press USA. In: International symposium on environmental science and technology,1st to 4th June, 2011, Dongguan, Guangdong Province, China (pp. 318–322).
Tavernini, S., Pierobon, E., & Viaroli, P. (2011). Physical factors and dissolved reactive silica affect phytoplankton community structure and dynamics in a lowland eutrophic river (Po River, Italy). Hydrobiologia, 669, 213–225.
Thomas, M., & Deviprasad, A. J. (2007). Phytoplankton diversity in wetlands of Mysore district. Asian Journal of Microbiology, Biotechnology and Environmental Sciences, 9, 385–392.
Victor, R. (2002). Ostracoda. In C. H. Fernando (Ed.), A guide to tropical freshwater zooplankton (pp. 189–233). Backhuys Publishers.
Wu, N., Schmalz, B., & Fohrer, N. (2012). Development and testing of a phytoplankton index of biotic integrity (P-IBI) for a German Lowland River. Ecological Indicators, 13(1), 158–167.
WWF. (1999). The Ramsar convention: A call for regional accession and implementation (p. 8). WWF.
Acknowledgements
This present study is a part of master’s thesis of Adebayo Tolulope written in Graduate School of Zoology Department, Obafemi Awolowo University, Ile-Ife, Nigeria. The authors are grateful to Prof. A. O. Aina for her assistance during the study.
Funding
There was no funding for this research.
Author information
Authors and Affiliations
Contributions
AT performed the practical section, collected the samples, and analyzed the data. HA and VF conceived the idea, helped in designing the study, supervised the preparation of the experiment, and helped in writing the manuscript. EO assisted in analysis of data and proof reading of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable for this kind of research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adebayo, A.T., Adewole, H.A., Akindele, E.O. et al. Planktonic flora and fauna of Opa Reservoir wetlands, Obafemi Awolowo University, Ile-Ife, Nigeria. JoBAZ 82, 40 (2021). https://doi.org/10.1186/s41936-021-00237-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-021-00237-8