Abstract
The Adyar estuary is one of the highly productive transitional zones of southeast India, situated in the southern part of Chennai city and acts as a nursery ground for several endemic flora and fauna. Since few decades, due to anthropogenic activities,indiscriminate dumping and discharge of domestic and industrial wastes and pollutants has environmentally damaged the Adyar estuary lowering many of its ecological and socio-economic attributes. In order to mitigate further environmental damage and to restore it to its earlier pristine condition, the Government and several non-Governmental agencies have undertaken ecological restoration measures to enhance its ecology and diversity. In order to evaluate the restoration process, our present study attempts to assess the diversity and abundance of zooplankton population in the restored and non-restored parts of the Adyar creek and estuary. 34 species belonging to 12 zooplankton groups from 4 stations of the Adyar creek and estuary were recorded. Copepods and rotifers were the dominant groups of zooplankton in Adyar estuary. The overall density of zooplankton from 4 stations ranged between 11.5 ± 4.39 and 23,046.67 ± 2872.68 Ind l−1. In general, Adyar estuary recorded higher zooplankton abundance than creek. The relative percentage composition was maximum for rotifers with 85.67% at Station-IV. Copepods dominated Station-I, whereas rotifers dominated the other 3 stations. Overall results indicate that the restoration activities had beneficial effect on the hydrological parameters and in increasing the diversity of zooplankton in the restored part of the Adyar creek and estuary compared to the non-restored part. Ecological indices have been used to assess the present status of the restored and non-restored parts in the Adyar creek and estuary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries are characterized by wide fluctuations in hydrodynamic, physio-chemical and biological properties, making them the most highly productive zones in terms of both ecology and economy (Pritchard 1967; Day 1980; Costanza et al. 1997; Jassby and CloernJE 2002; Mumby and Hastings 2008). They constitute ideal breeding and nursery grounds for many finfishes and shellfishes (Beck et al. 2001; Hughes et al. 2014; Nagelkerken et al. 2015; Sheaves et al. 2015). Excessive anthropogenic activities due to industrialization and urbanization along with consequent eutrophication has led to pollution and decline in water quality of estuaries resulting in hypoxia and growth of undesirable algal blooms (Pihl et al. 1991; Alpine and Cloern 1992; Cloern 2001; Duarte 2009; Switzer et al. 2009; Tucker et al. 2014). Pollution enhances decline in biodiversity and water quality, which in-turn influences the structural and functional aspects of an ecosystem (Balmford and Bond 2005; Tapia-González et al. 2008; Schneiders et al. 2012). Negative impact on estuarine ecosystems by the anthropogenic activity triggers attention towards the need for monitoring and managing ecological integrity of an estuary to promote their long-term sustainability (Kennish 2002; Borja et al. 2008). Relationship between species distribution and environmental characteristics are major forces driving the distribution of species to community level (McLusky and Elliot 2004; Peres-Neto et al. 2006). Planktonic communities and their populations can be considered as biological indicators for monitoring aquatic pollution (Buskey 1993; Leandro et al. 2007; Moreno et al. 2008; Aboul Ezz et al. 2014), wherein their presence or absence acts as an indication on the level of disturbance in an ecosystem (Chapman 1989). Further, zooplankton community responds promptly and quickly to any environmental change in terms of nutrient recycling and production (Rocha et al. 1997).
Adyar estuary is one of the economically important and highly productive transition zone connecting the Chennai city (130 01’N 800 27′ E), a metropolitan city in the southern state of Tamilnadu, India with Bay of Bengal, Southeast India. Before being an estuary and entering the Bay of Bengal, a part of Adyar River, about 40 km long,takes northward and then westward as a tidal creek with tidal influence up to 5 km long. Present day Adyar creek and estuary lost their previous pristine condition and are now under acute ecological stress, since few decades, due to urbanization, encroachment, release of untreated domestic sewage, solid waste disposal, industrial effluents and storm water drains from in and around the Chennai city (Janakiraman et al. 2017). Further, frequent formation of sand bar across the mouth of this estuary prevents tidal exchange of water, consequently leading to stagnation of water column, and in altering the physio-chemical parameters affecting the zooplankton community structure.
To improve the ecological conditions and thereby enhance the biodiversity, a restoration plan was initiated in the Adyar creek and estuary by the Chennai River Restoration Trust (CRRT) (Janakiraman et al. 2017). As part of this, an eco-restoration activity in the Adyar creek, covering about 58 acres, commenced during January 2008, which resulted in the excavation of about 2.8 *104 m−3 of accumulated sludge and debris from the water body. The eco-restoration work was carried out till January, 2010 and this activity enhanced the water spread of the creek from 5.53% to 59% and facilitated the normal ecological functioning of the creek. There were seven storm-water drain outfalls entering into the creek. Before the implementation of eco-restoration activity, these storm-water drains were been misused for sewage discharge. During restoration, the sewage was diverted into sewer pipeline to prevent the sewage inflow into the creek. Earthen mounds were created around the water body, supporting coastal vegetation such as intertidal plantations like mangroves, mangrove associated reeds and terrestrial plants. Presently, these mounds serve as a niche for birds and other terrestrial animals of the creek ecosystem and as a sound barrier against vehicular traffic. Presently, the plantations at Adyar creek comprise of six vegetative elements such as trees, shrubs, herbs, grasses, climbers and aquatic plants for planting in various zones such as aquatic zone, core zone, peripheral zone, inland and littoral zone, etc. About 110,161 plants belonging to 172 species were planted to provide a green cover and typical vegetation of the Coromandal coast surrounding the water body. During the restoration process, water bodies were protected from any external source of pollution and from hunting and vandalism in the habitats of birds and reptiles (Janakiraman et al. 2017). Our present study describes the impact of eco-restoration process in the restored and non-restored parts of the Adyar creek and estuary by investigating the zooplankton diversity and abundance as an ecological indicator of favourable conditions.
Materials and methods
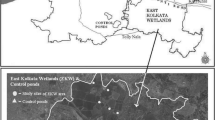
Physico-chemical parameters like atmospheric and surface water temperature were recorded with the help of mercury thermometer. pH was determined with the help of pH papers, salinity was measured by Salinometer (ATAGO-Sigma Scientific Equipments, Chennai, India), and Dissolved oxygen was analysed using Winkler’s methods (Strickland and Parsons 1968). Chemical parameters such as Nitrites, Ammonia and Phosphates were analyzed using Test kits (Aquarium Pharmaceuticals, Canada, Inc.). Monthly zooplankton sampling was carried out at the 4 stations of the Adyar creek and estuary from June 2010 to May 2012. Stations I and II represent the restored parts of the creek, while Station-III represents the non-restored part of the creek and Station-IV the non-restored part of the estuary having its location closer to mouth of the Adyar river (Fig. 1). Zooplankton was sampled using a standard plankton net made up of bolten silk having 50 μm mesh size. At each station, 100 l of subsurface water was filtered through the plankton net during the early hours of the day. Zooplankton samples collected onto the net were fixed immediately and the concentrate was made up to 100 ml with 4% buffered formalin and was stored in 1 l plastic containers until further processing in the laboratory.
In the laboratory, 1 ml of the subsample was taken using a wide mouthed pipette on to a Sedgwick rafter counting chamber and was enumerated under a stereozoom microscope (Model: Olympus Stereo zoom microscope SZ61) for their abundance. Triplicate subsample was enumerated and their mean abundance was expressed as individuals per litre (ind. l−1) (Santhanam et al. 1989). Zooplankton were identified upto species level following standard descriptions of Newell and Newell (1963), Kasturirangan (1963), Rajendran (1973), Coull (1977), Artl (1983), Dussart and Defaye (2001), Huys et al. (1996), Dhanapathi (2000), Perry (2003), Altaff (2004), Varghese et al. (2006) and Al-Yamani et al. (2011).
Univariate statistical analysis was carried out using SPSS v10.0 analytical software. A Spearman’s rank correlation analysis was carried out to test the correlation coefficient between the physico-chemical and biological parameters. Analysis of variance (ANOVA) was performed separately to find out the difference in zooplankton abundance towards different stations sampled during different months. For assessing the current status and to know the species homogeneity among the population, beta ecological diversity indices like Species richness (S), Total individuals (N), Shannon-Wiener diversity index [H′ = Sum(Pi*Log(Pi)] (Shannon and Weaver 1949), Simpson dominance index [D’ = sum(Ni*(Ni-1)/N*(N-1)] (Simpson 1949) and evenness index [J’ = H′/Log(S)] (Pielou 1966) were computed. Prior to analyses for multivariate analysis, the data was pretreated, standardized and then overall transformed using Log(x + 1). Single-linkage Bray-Curtis cluster dendrogram was constructed to determine the similarity in their diversity and abundance distribution using restored and non-restored stations as factors using Primer version 6.0 statistical package (Clarke and Gorley 2006). The relationships between zooplankton abundance under restored and non-restored parts with respect to the physico-chemical parameters were analyzed through correlation based principal component analysis (PCA) using Canoco v5.0 (Lepš and Šmilauer 2003). The graphical package Sigma Plot Version 11.0 was used to construct graphs and general statistics was carried out using Microsoft Excel (MS Office Version 2013).
Results
Physico-chemical parameters
Physicochemical parameters like surface water temperature, pH, DO, salinity, nitrites, ammonia, and phosphates showed a wide range in their variability at all the sampled stations. Atmospheric and surface water temperature ranged between 21 and 32 °C and 24–34 °C, respectively during the study period. Atmospheric temperature showed no marked difference in its range (Fig. 2a), while surface water temperature showed slight variation during the study period (Fig. 2b). Higher surface water temperature was recorded than the atmospheric temperature during early morning hours of sampling period. Salinity ranged between 4 and 39 psu at all the stations, wherein its range varied at different stations according to its distance from the sea i.e. Station-I ranged between 4 and 9 psu, Station-II from 9 to 26 psu, Station-III from 9 to 32 psu and Station- IV from 6 to 39 psu (Fig. 2c), respectively. Higher salinity values were recorded during summer while lower values during monsoon period at all 4 stations, which is mainly due to intrusion of sea water into the estuary and thereby creek influenced by the tides during summer and inflow of freshwater from watersheds during monsoon periods. The pH ranged between 6.0 and 8.8 during the study period, wherein maximum was recorded at Station-II during Jul. 2010 and minimum was recorded at Station-IV during Aug. 2011 (Fig. 1d), respectively.
The dissolved oxygen content of water varied between 0 and 8.45 mg l−1 at all stations, wherein its range varied at different stations, i.e. station I, II, III and IV ranged between 1.91–8.45 mg l−1, 0.67–6 mg l−1, 0–6.8 mg l−1 and 0.5–7.8 mg l−1(Fig. 2e), respectively. The concentration of ammonia during the study period ranged between 0.15–10.0 mg l−1. Maximum levels of ammonia were observed at Station-IV during May 2012 and minimum was observed at Station-I during Oct. 2010(Fig. 2f). The nitrites content ranged between 0.02–0.8 mg l−1 during the study period, wherein maximum was recorded at Station-II, during Oct. 2010 and Jul. 2011, Station-III during Sep. 2010 and Jan. 2011 and at Station-IV during Oct. 2010 and minimum was recorded at Station-1 during Sep. and Nov. 2011 (Fig. 2g), respectively. The phosphates were observed to be in the range between 0.05–9.0 mg l−1, wherein maximum was observed at Station-IV during Jul. 2010 and minimum was observed at Station-I during Jan. 2012 (Fig. 2h). All studied stations showed higher values during the first 6 months of the study period.
Zooplankton diversity and abundance
Thirty-four species of zooplankton were recorded from the four stations at Adyar creek and estuary during the study period. Higher number of species were recorded at Station-I (23 species), followed by Station-IV (21), Station-II (19) and Station-III (18) (Table 1), respectively. Apart from decapod and mysid larvae, the foraminiferan (Discobis sp.), nematode (Halalaimus sp.), polychaete (Lopadorhynchus sp.) rotifer (Brachionus plicatilis), cladocera (Diaphanosoma excisum and D. sarsi), ostrocod (Hemicypris sp.), and harpacticoid Onychocamptus bengalensis and Cletocamptus albuquerquensis were recorded from all the four stations. The zooplankton diversity from the four stations of the Adyar estuary and creek was represented by limnetic (Lecaneblachei, L. hornemanni, Dunhevedia crassa crassa, Mesocyclops aspericornis, M. thermocyclopoides and Thermocyclops decipiens), estuarine (Apocyclops dengizicus and A. royi) and marine (Oithona brevicornis and O. rigida) species during the study period.
Copepods and rotifers dominated the zooplankton community in the Adyar estuary and creek. The average zooplankton abundance ranged from a minimum of 11.5 ± 4.39 ind. l−1 to a maximum of 23,046.67 ± 2872.68 ind. l−1 at Station-IV during the study period, whereas a minimum of 17.5 ± 5.0, 75.5 ± 21.86 and 13 ± 4.33 ind. l−1 and a maximum of 171.66 ± 58.21, 4550.51 ± 1947.62, and 12,100 ± 1627.09 ind. l−1 was observed at Station-1, Station-II and Station-III (Fig. 3), respectively. The maximum relative percentage composition of individual zooplankton groups during the study period was dominated by rotifers at Station-IV (85.67%), followed by Station-III (69.88%), and Station-II (51.63%), and by cyclopoid copepods at Station-I (41.18%) (Table 2, Fig. 4), respectively. Cyclopoid copepods dominated at Station-I, whereas rotifers were dominant at all 3 stations depicting the polluted nature of the stations with more resistant rotifer species (Fig. 4).
Results of the one-way ANOVA between zooplankton groups and their abundance during the study period at four stations showed significant differences with dominant groups, i.e. tintinids (F = 2.96, p < 0.05), polychaetes (F = 3.12, p < 0.05), rotifers (F = 3.59, p < 0.05), cladocerans (F = 3.49, p < 0.05), ostracods (F = 2.78, p < 0.05), calanoids (F = 7.68, p < 0.05) and cyclopoids (F = 5.85, p < 0.05). The one-way analysis of Similarity percentages (SIMPER) on the overall abundance showed that average dissimilarity was maximum for restored stations (75.59%) and minimum for the non-restored stations (65.19%), whereas maximum similarity was observed within Station-I (76.25%) followed by Station-II (74.92%) and least was observed at Station-IV (61.47%), respectively. While in the multivariate analysis, the results obtained by the single-linkage Bray-Curtis cluster dendrogram based on the zooplankton abundance at different stations showed that there are 2 distinct groups with Cnidaria, Nematoda, Polychaeta and Calanoida as out-groups in the restored sites (Fig. 5a), whereas 2 distinct groups with Ostracoda and Mysidaceabeing a complete out-group based on the increased linkage distances of their abundances (Fig. 5b).
Correlations between environmental data and zooplankton results were evaluated using Principal Component Analysis in Canoco v5.0 (Fig. 6). Each solid line represented by a solid filled arrow points in the direction of the steepest increase of zooplankton group values and the dotted line with open arrow points in the direction of the steepest increase of the values for corresponding physico-chemical variable, showing that the approximated correlation is positive when the angle is sharp and negative when the angle is larger than 90 degrees. The angle between arrows (alpha) indicates the correlation between individual groups. A similar, but less precise interpretation can be based on the angle between the two compared arrows.
Ecological indices showed monthly variations with respect to their Shannon’s diversity, Simpson’s dominance and Pielou’s evenness for all the 4 stations of the Adyar creek and estuary during the study period. Shannon’s diversity showed higher diversity at Station-I than others, but with almost similar diversity values at all four stations, while higher Simpson’s dominance was observed at non-restored parts (Station III and IV) than at restored parts (Station I and II), and Pielou’s evenness was even at restored parts (Station I and II) than at non-restored parts (Station III and IV) (Fig. 7), respectively.
Discussion
In an estuarine habitat where organic enrichment is higher, hypoxia is a common phenomenon (Villate et al. 2013), even in well-mixed estuaries. More nutrient inputs affect the zooplankton constituents with respect to their community structure, abundance, growth and development (Mukherjee et al. 2010), as well as being toxic to aquatic life (Anderson et al. 2002). Monitoring the zooplankton diversity and abundance helps to bring out the quality of the environmental status of any aquatic habitat. Fluctuations in the environment, influences the species diversity in an estuary, due to the extremities in their hydrological parameters, sediment characteristics and pollution (Ansari and Parulekar 1998; Janakiraman et al. 2017).
At Stations-III and IV, apart from anthropogenic pollutants, the seasonal rains during the monsoon influence remarkable variations on the zooplankton community structure. Generally, in these stations when the hydrological features were optimal, water flow reduces the level of pollutants promoting higher diversity during monsoon and post- monsoon periods. Whereas high nutrients, temperature, salinity, least water dilution and low dissolved oxygen leads to stressed ecological conditions of this estuary favoring pollution resistant species, thereby showing higher index of dominance during summer and pre-monsoon in our present study at Stations-III and IV.
The Spearman’s correlation analysis revealed the most dominant species showed significant positive correlation with water temperature, pH, salinity and nutrients like nitrites, ammonia and phosphates and negative correlation with dissolved oxygen even in hypoxic condition, implying that the dominant species are well adapted for wide ecological fluctuations and hypoxic conditions. These findings suggest that the dominant species accurately reflect the ecological conditions when the Adyar estuary faces adverse conditions during sand bar formation at the river mouth (Janakiraman et al. 2017).
Of the four stations, Station-I showed higher zooplankton diversity than other stations in representing all the major zooplankton groups, i.e. the occurrence of rotifers, cladocerans, ostracods and copepods and their nauplii and copepodid stages throughout the study period, which is indicative of its rich biodiversity. Such a zooplankton diversity at Station-I might be due to the result of eco-restoration. Species composition at Station-I related to different groups like rotifers (Brachionus urceolaris, B. calyciflorus, B. quadridentatus, B. caudatus and B. rubens), cladocerans (Diaphanosoma excisum, D. sarsi, Pleuroxusbaduncusaduncus, Alona rectagula rectangula and Dunhevedia crassa crassa), cyclopoids (Mesocyclops aspericornis, M. thermocyclopoides and Thermocyclops decipiens) indicates that this Station shows mostly freshwater conditions with low salinity. Station-II which also comes under the part of creek where restoration has been undertaken, shows lower zooplankton diversity, which can be attributed to the mixing up of polluted water from Station-III during most of the periods of the present study. Overall, least zooplankton diversity at Station-III than all other stations might be due to its highly polluted condition and less influence of the tidal action. Higher zooplankton diversity at Station-IV compared to Station-III might be due to the exchange of seawater through tidal action, which replenishes the oxygenated water and clears the pollutants.
Bray-curtis cluster analysis revealed clear differentiation between the two restored stations and non-restored stations of Adyar creek and estuary and nutrient inputs. The abundance of zooplankton suggests that there are wide variations at Stations-III and IV, which can be attributed to the dominance of two species, first a rotifer, Brachionus plicatilis and second a cyclopoid copepod, Apocyclops dengizicus. These two species are highly tolerant to organic pollutants and thrive well in such conditions.
In general, higher diversity and evenness indices, indicate unpolluted conditions of any aquatic habitat (Ismael and Dorgham 2003). Ecological indices like Simpson’s diversity, Shannon’s dominance and Pielou’s evenness are useful in community level investigations to study the ecological role of species at different stages of succession (Pearson and Rosenberg 1978; Xu et al. 2012). In the present study, zooplankton species diversity and evenness indices had their higher values at Station-I and II (restored part) than Station-III and IV (non-restored part), implying that the non-restored part is affected by sewage inputs and industrial effluents through Adyar River from, in and around the Chennai city. This intrusion of effluents consequently had higher dominance of pollution tolerant species of rotifer, Brachionus plicatilis and cyclopoid copepod, Apocyclops dengizicus, which is similar to the reports of Janakiraman et al. (2017) for meiobenthic fauna in the Adyar creek and estuary.
According to Rosenberg (1975) the dominance index is an important component of species diversity used to identify the main species, i.e. whenever the dominance index is maximum, the evenness index will be at its least and vice versa. Similarly, the present study also showed that whenever high dominance index was recorded, the dominant species belonging to rotifer and cyclopoid copepod group occurred in high densities at Stations-II, III and IV. These measures, which helped to assess the eco-restoration of the Stations-I and II at Adyar creek and estuary are reflected in the increasing zooplankton diversity. If eco-restoration of the Stations-III and IV is undertaken, there will be a significant improvement in the biodiversity of the Adyar creek and estuary, which would enhance the productivity and help in establishing an ideal ecosystem. The present study shows that the composition of zooplankton species were mainly limnetic at Station-I, estuarine at Stations-II and III and estuarine to marine at Station-IV.
Conclusion
Zooplankton community of the Adyar creek and estuary of the restored and non-restored parts shows significant variations with respect to diversity, dominance, evenness, species composition and abundance. They are significantly associated with environmental factors and might serve as ecological indicators of the Adyar estuarine ecosystem. Present study shows that the zooplankton species composition was mainly limnetic at Station-I, estuarine at Stations-II and III and estuarine to marine at Station-IV. Besides, providing baseline information on the zooplankton community, this study also provides better understanding of the interrelationship of zooplankton communities with ecological factors of Adyar creek and estuary. High inorganic nutrients with least perturbations can be favorable for some zooplankton groups, particularly those adapted to hypoxic conditions, tolerant to pollution, continuous breeding, fast growing and quick response to environmental changes. If such conditions prolong, then they may affect the existence and may be detrimental to the most sensitive zooplankton species. Regular dredging of Adyar river mouth during sandbar formation can facilitate the tidal action between the sea and Adyar River, thereby enabling native zooplankton species diversity and density. Present study augment’s further monitoring of time-series on the physico-chemical parameters and zooplankton data to better understand the impact of environmental and anthropogenic pollutants on the zooplankton ecology of the Adyar creek and estuary.
References
Aboul Ezz SM, Abdel Aziz NE, Abou Zaid MM, Raey ME, Abo-Taleb HA (2014) Environmental assessment of El-Mex Bay, southeastern Mediterranean by using Rotifera as a plankton bio-indicator. Egyptian J Aq Res 40:43–57
Alpine AE, Cloern JE (1992) Trophic interactions and direct physical effects control phytoplankton biomass and primary production in an estuary. Limnol Oceanogr 37:946–955
Altaff K (2004) A manual of zooplankton. UGC, New Delhi
Al-Yamani FY, Skryabin V, Gubanova A, Khvorov S, Prusova I (2011) Marine zooplankton practical guide for the northwestern Arabian gulf volume 1. Kuwait Institute for Scientific Research, Kuwait
Anderson D, Glibert PM, Burkholder JM (2002) Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries 25:704–726
Ansari ZA, Parulekar AH (1998) Community structure of meiobenthos from tropical estuary. Indian J Mar Sci 27:362–366
Arlt G (1983) Taxonomy and ecology of some harpacticoids (Crustacea, Copepoda) in the Baltic Sea and Kettegat. Zool Jahrbücher Syst 110:45–85
Balmford A, Bond W (2005) Trends in the state of nature and their implications for human well-being. EcolLett 8:1218–1234
Beck MW, Heck KL Jr, Able KW, Childers DL, Eggleston DB, Gillanders BM, Halpern B, Hays CG, Hoshino K, Minello TJ, Orth RJ, Sheridan PF, Weinstein MP (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51:633–641
Borja A, Bricker SB, Dauer DM, Demetriades NT, Ferreira JG, Forbes AT, Hutchings P, Jia X, Kenchington R, Marques JC, Zhu C (2008) Overview of integrative tools and methods in assessing ecological integrity in estuarine and coastal systems worldwide. Mar Poll Bull 56:1519–1537
Buskey EJ (1993) Annual pattern of micro- and mesozooplankton abundance and biomass in a subtropical estuary. J Plankton Res 15(8):907–924
Chapman PM (1989) Current approaches to develop sediment quality criteria. Environ Toxicol Chem 8:589–599
Clarke KR, Gorley RN (2006) Primer v6: user manual/tutorial. PRIMER-E Ltd, Plymouth
Cloern JE (2001) Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 210:223–253
Costanza R, Dárge R, Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton PM, Van Den Belt D (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260
Coull BC (1977) Marine flora and fauna of the northeastern United States. Copepoda: Harpacticoida.National Oceanic and Atmospheric Administration, Tech Rep National Marine Fisheries Service 399:1–48
Day JH (1980) What is an estuary? S African J Sci 76:198
Dhanapathi MVSSS (2000) Taxonomic notes on the rotifers from India (from 1880–2000). Publ no. IAAB, Hyderabad, p 10
Duarte CM (2009) Coastal eutrophication research: a new awareness. Hydrobiologia 629:263–269
Dussart BH, Defaye D (2001) Introduction to the Copepoda. In: Dumont HJF (ed) Guide to the identification of the microinvertebrates of the continental waters of the world,2nd edn. Backhuys Publishers, Leiden
Hughes BB, Levey MD, Brown JA, Fountain MC, Carlisle AB, Litvin SY, Greene CM, Heady WN, Gleason MG (2014) Nursery Functions of U.S. West Coast Estuaries. In: The State of Knowledge for Juveniles of Focal Invertebrate and Fish Species. The Nature Conservancy, Arlington
Huys R, Gee JM, Moore CG, Hamond R (1996) Marine and brackish water harpacticoid copepods. Part 1. Keys and notes for identification of the species. In: RSK B, Crothers JH (eds) Synopses of the British Fauna, vol 51, pp 1–352
Ismael AA, Dorgham MM (2003) Ecological indices as a tool for assessing pollution in El-DekhailaHarbour (Alexandria, Egypt). Oceanologia 45:121–131
Janakiraman A, Naveed MS, Asrar Sheriff M, Altaff K (2017) Ecological restoration assessment of Adyar creek and estuary using meiofaunal communities as ecological indicators for aquatic pollution. Reg Stud Mar Sci 9:135–144
Jassby AD, CloernJE CBE (2002) Annual primary production: patterns and mechanisms of change in a nutrient-rich tidal ecosystem. LimnolOceanogr 47:698–712
Kasturirangan LR (1963) A key for the identification of the more common planktonic Copepoda of Indian coastal waters. CSIR, New Delhi
Kennish MJ (2002) Environmental threats and environmental future of estuaries. Environ Conserv 29:78–107
Leandro SM, Morgado F, Pereira F, Queiroga H (2007) Temporal changes of abundance, biomass and production of copepod community in a shallow temperate estuary (ria de Aveiro, Portugal). Estuar Coast Shelf Sci 74:215–222
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
McLusky DS, Elliot M (2004) The estuarine ecosystem: ecology, threats and management. Oxford University Press, Oxford
Moreno M, Ferrero TJ, Gallizia I, Vezzuulli L, Albertelli G, Fabiano M (2008) An assessment of the spatial heterogeneity of environmental disturbance within an enclosed harbour through the analysis of meiofauna and nematode assemblages. Estuar Coast Shelf Sci 77:565–576
Mukherjee B, Nivedita M, Mukherjee D (2010) Plankton diversity and dynamics in a polluted eutrophic lake, Ranchi. J Environ Biol 31:827–839
Mumby PJ, Hastings A (2008) The impact of ecosystem connectivity on coral reef resilience. J Appl Ecol 45:854–862
Nagelkerken I, Sheaves M, Baker R, Connolly R (2015) The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fisher 16:362–371
Newell GE, Newell RC (1963) Marine plankton- a practical guide. Hutchinson and Co. Ltd., London
Pearson TH, Rosenberg R (1978) Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr Mar Biol Ann Rev 16:229–311
Peres-Neto P, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Perry R (2003) A guide to the marine plankton of Southern California, 3rd edn. UCLA Ocean Globe, California
Pielou EC (1966) The measurement of diversity in different types of biological collection. J TheorBiol 13:131–144
Pihl L, Baden SP, Diaz RJ (1991) Effects of periodic hypoxia on distribution of demersal fish and crustaceans. Mar Biol 108:349–360
Pritchard DW (1967) What is an estuary: physical viewpoint. In: Lauff GH (ed) Estuaries. AAAS, Washington DC, pp 3–5
Rajendran M (1973) Copepoda. In: Michael RG (ed) A guide to the study of freshwater organisms, Supl 1. J Madurai Univ, India, pp 103–151
Rocha O, Matsumura-Tundisi T, Sampaio EV (1997) Phytoplankton and zooplankton community structure and production as related to trophic state in some Brazilian lakes and reservoirs. Verh Int Verein Limnol 26:599–604
Rosenberg R (1975) Stressed tropical benthic faunal communities of Miami, Florida. Ophelia 14:93–112
Santhanam R, Velayutham P, Gegathesan G (1989) A manual of freshwater ecology. DayaPublishingHouse, New Delhi
Schneiders A, Daele TV, Landuyt WV, Reeth WV (2012) Biodiversity and ecosystem services: complementary approaches for ecosystem management? Ecol Indic 21:123–133
Shannon EC, Weaver W (1949) The mathematical theory of communication. University of Illinois
Sheaves M, Baker R, Nagelkerken I, Connolly RM (2015) True value of estuarine and coastal nurseries for fish: incorporating complexity and dynamics. Estuar Coasts 38:401–414
Simpson EH (1949) Measurement of diversity. Nature 163:688–704
Strickland JDH, Parsons TR (1968) Determination of dissolved oxygen: in: a practical handbook of seawater analysis. Fish Res Bd Canada Bull 167:71–75
Switzer TS, Chesney EJ, Baltz DM (2009) Habitat selection by flatfishes in the northern Gulf of Mexico: implications for susceptibility to hypoxia. J Exp Mar Biol Ecol 381:S51–S64
Tapia-González FU, Herrera-Silveira JA, Aguirre-Macedo ML (2008) Water quality variability and eutrophic trends in karstic tropical coastal lagoons of the Yucatán peninsula. Estuar Coast Shelf Sci 76:418–430
Tucker JM, Schwartz MK, Truex RL, Wisely SM, Allendorf FW (2014) Sampling affects the detection of genetic subdivision and conservation implications for fisher in the Sierra Nevada. Conserv Genet 15:123–136
Varghese M, Krishnan L, Kuttyamma VJ (2006) Systematic account on rotifer of the genus Brachionus from Cochin backwater. J Mar Biol Assoc India 48:147–155
Villate F, Iriarte A, Uriarte I, Intxausti L, Sota A (2013) Dissolved oxygen in the rehabilitation phase of an estuary: influence of sewage pollution abatement and hydro-climatic factors. Mar Poll Bull 70:234–246
Xu H, Zhang W, Jiang Y, Zhu M, Al-Rasheid KAS (2012) Influence of sampling sufficiency on biodiversity analysis of microperiphyton communities for marine bioassessment. Environ SciPollut Res 19:540–549
Acknowledgements
Authors are thankful to The Chennai Rivers Restoration Trust, Chennai, Tamil Nadu for their encouragement and also to the Head, P.G. and Research Department of Zoology, The New College, Chennai for providing necessary facilities for conducting this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Altaff, K., Janakiraman, A., Naveed, M.S. et al. Post-restoration ecological assessment on the zooplankton dynamics of the Adyar creek and estuary. J Coast Conserv 23, 473–483 (2019). https://doi.org/10.1007/s11852-018-00676-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11852-018-00676-6