Abstract
Purpose
Metabolic changes in cancer, coupled with treatment, can have deleterious effects and impair recovery. However, studies have shown promising results with the supplementation of polyunsaturated fatty acids omega-3 in chemotherapy patients. Therefore, a systematic review of the literature was conducted to explore the potential benefits of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) supplementation in reducing the side effects of breast cancer treatment in women.
Methods
A search was performed for randomized clinical trials in PubMed, Lilacs, Scopus, SciELO, Web of Science, Clinical Trials, and Google Scholar. The paper was developed following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) and registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the number (CRD42023403833).
Results
Out of 1215 publications, five studies were selected to evaluate 463 women undergoing chemotherapy treatment for breast cancer who were supplemented with omega-3 fatty acids in varying doses for up to six months. The studies assessed various outcomes, including quality of life, lipid profile, and inflammatory biomarkers. Omega-3 fatty acids supplementation was found to be beneficial in reducing symptoms that impact the quality of life, such as peripheral neuropathy and xerostomia. However, no significant differences were observed in other toxicities caused by chemotherapy.
Conclusion
Supplementation with EPA and DHA has shown promising benefits for women undergoing breast cancer treatment with chemotherapy. However, further research is necessary to evaluate the effectiveness of using omega-3 in conjunction with other therapies and to more accurately assess the impact on patients' quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer, considered a global public health problem, has become the most diagnosed cancer worldwide with over 2 million new cases in 2020 [1]. Therapeutic modalities for breast cancer have advanced and can be either local (such as surgery or radiotherapy) or systemic, including chemotherapy and hormone therapy [2].
Individuals with cancer often experience metabolic alterations associated with an inflammatory state [3, 4]. In combination with antineoplastic treatment, these alterations can induce deleterious effects and reduce food intake, worsening nutritional status [5]. Common adverse effects of breast cancer treatment include dry mouth, fatigue, neuropathy, mucositis, nausea, vomiting, diarrhea, lack of appetite, increased bone resorption, loss of bone mineral density, increased risk of fractures, and arthralgias [6].
Consumption of eicosapentaenoic (EPA) and docosahexaenoic (DHA) omega-3 polyunsaturated fatty acids (PUFA n-3) has been associated with different mechanisms linked to the modulation of inflammatory response, reduction of cell proliferation, induction of apoptosis, and suppression of angiogenesis [7]. This occurs, in part, due to the classic effect of EPA and DHA on inflammation, resulting in alterations in the constitution of membrane phospholipids and, consequently, in the synthesis of odd series eicosanoids with reduced proinflammatory potential [8,9,10]. In addition, EPA and DHA have an anti-inflammatory effect, which involves attenuating the activation of the Toll-like receptor 4 (TLR4) signaling pathway [11].
The Brazilian Society of Parenteral and Enteral Nutrition (BRASPEN) recommends omega-3 fatty acids supplementation for patients at risk of malnutrition or who are malnourished and undergoing chemotherapy. It also recommends the use of formulas enriched with omega-3 fatty acids in the surgical preparation of cancer patients, as these have been shown to reduce postoperative complications [6]. However, there is currently no conclusive evidence to support the use of omega-3 for symptom management during breast cancer treatment.
Given the impact of cancer treatment on patients' recovery and survival, a systematic review of the literature was conducted to explore the potential benefits of EPA and DHA supplementation in reducing the side effects of breast cancer treatment in women.
Methods
To ensure the accuracy and transparency of the study, we followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA) [12] and registered a study protocol in the International Prospective Register of Systematic Reviews (PROSPERO), under the number CRD42023403833.
Research question design
To establish the central question of this study, the PICOS anagram (Table 1) was used as a model, composed of the following determinants: population (P), intervention (I), comparison (C), outcome (O), and study type (S).
Search strategy
Searches for Randomized clinical trials (RCTs) were performed in PubMed, Lilacs, Scopus, Embase, Web of Science, Clinical Trials, and Google Scholar in December 2022, without limitations of language or publication year. The structured search strategy was developed using Medical Subject Headings (MeSH) and Boolean operators, as follows: ("Breast Neoplasms") OR ("Breast Cancer") OR ("Breast Carcinoma") AND ("Fatty Acids, Omega-3") OR ("Fish Oils") AND (randomised OR randomized OR randomisation OR randomisation OR placebo* OR (random* AND (allocat* OR assign*) OR (blind* AND (single OR double OR treble OR triple).
Eligibility criteria
The eligibility criteria followed the PICOS strategy (Table 1): (P) adult and/or elderly women undergoing treatment for breast cancer; (I) supplementation with omega-3 fatty acids (EPA/DHA); (C) women under breast cancer treatment taking a placebo; (O) adverse effects of cancer treatment; (S) RCTs.
Based on the results of the pilot search, it was observed that the retrieved studies presented data regarding the effect of supplementation on biomarkers (e.g. inflammatory, lipid biomarkers). Given this, it was added as a secondary outcome and collected if the study presented it.
It was ineligible studies with omega-3 supplementation combined with other nutrients (e.g. vitamin D, protein, minerals); treatment with conjugated drugs; other types of neoplasia; patients with completed or discontinued treatment; animal or in vitro studies; incomplete studies; and unpublished manuscripts such as dissertations and thesis.
Selection of studies and data extraction
The selection stages were independently conducted by two reviewers (N.E.D. and P.N.B.L.). Firstly, we screened articles by titles and abstracts, followed by a full read of all eligible articles in the first stage, and disagreements were resolved by a third reviewer (M.M.R.).
The findings of each study included in this review were summarized in a table of findings, considering the mean age of participants, body weight, BMI, tumor stage, type of treatment, and outcome variables. EPA and DHA doses were presented in grams per day. Only adult participants from twenty years old were considered for analysis.
Assessment of risk of bias
The risk of bias was analyzed independently by two reviewers (N.E.D. and P.N.B.L.) using the "RoB 2.0 Assessment Form" tool developed by Cochrane to assess the risk of bias in randomized studies [13]. This tool includes five main domains: randomization process; deviation in intervention; outcome data loss; outcome measurement; and selective reporting of results. Each domain was evaluated as "low risk," "high risk," or "some concerns." If a study presented no risk in any of these domains, it was considered "low risk." If it presented a risk in at least one domain, it was considered "some concerns." And if it presented risks in several domains, it was considered "high risk."
Results
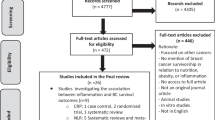
The search identified 1,215 RCTs, resulting in 1,119 after duplicates were removed. After screening the titles and abstracts, 14 studies were selected for full-text reading, of which nine were excluded for not meeting the eligibility criteria. This resulted in five included studies (Fig. 1).
Risk of bias
Overall risk of bias analysis (Fig. 2) showed four studies with low [14,15,16,17] and one study with some concerns [18]. When evaluated each risk domain, 100% had a low risk of bias in the randomization process (D1); deviations from intended interventions (D2), missing outcome data (D3), and measurement of the outcome (D4) [14,15,16,17]. One study presented some concerns in the selection of the reported result (D5) since it did not inform whether the results were analyzed following a previously defined analysis plan [18].
General characteristics of studies and participants
This systematic review included five randomized double-blind placebo-controlled clinical trials that were conducted in four different countries: two in the United States of America [14, 15], Iran [16], Mexico [17], and Indonesia [18] (Tables 2 and 3). These studies provided data from 463 women undergoing treatment for breast cancer using chemotherapy [16,17,18] and hormone therapy with aromatase inhibitors (AI) [14, 15] at various tumor stages.
In all studies, omega-3 fatty acids were orally administered as a gel capsule of fish oil with doses ranging from 1.0 to 4.3 g/day with different concentrations of EPA and DHA. The supplementation period ranged from 51 days to 6 months. Intervention groups supplemented with fish oil were compared to a placebo-controlled group that received supplementation with other forms of lipids, as follows: sunflower oil [16, 17], soybean and corn oil blend [14], a mixture of fats and oils formulated to mirror the proportion of fatty acids typical of the American diet [15]. However, one study did not specify the form of lipid used as a placebo [18].
The results of two studies indicated that omega-3 supplementation led to a significant increase in serum EPA and DHA concentrations [15, 16]. In the first study, participants in the omega-3 group showed a significant increase in red blood cell EPA and DHA concentrations after 12 weeks of supplementation, and this increase continued throughout the 24-week study [15]. In the second study, an increase in the serum phospholipid concentrations of EPA and DHA was observed after approximately 8 weeks of supplementation [16]. Conversely, the placebo groups in both studies did not show any significant changes.
Omega-3 supplementation in the neurological and musculoskeletal symptoms
Reduced total neuropathy score (rTNS) was used to assess the presence and severity of paclitaxel-induced peripheral neuropathy (PIPN) in women. rTNS consists of a subjective assessment of sensory symptoms, sensitivity to pins, deep tendon reflexes, and nerve conduction studies of the sural and peroneal nerves. In one study, omega-3 supplementation for eight weeks reduced the risk of developing peripheral neuropathy by 70% in women receiving paclitaxel chemotherapy compared to the placebo group (Table 3). However, there was no difference in neuropathy severity between supplemented and placebo groups [16].
Two studies [14, 15] investigated the efficacy of PUFA omega-3 supplementation as an adjunct to alleviate musculoskeletal symptoms and joint pain induced by aromatase inhibitors (AI) in postmenopausal women, using specific scales (Table 3). The oral supplementation of 3.3 g/day of omega-3 (2.24 g EPA plus 1.12 g DHA) for 24 weeks resulted in a significant improvement of approximately 50% in AI-associated arthralgia, as assessed by the Brief Pain Inventory (BPI-SF) scale. The BPI-SF scale consists of a 10-point scale, with 0 representing no pain and 10 the worst imaginable pain. The results revealed an average reduction of 1.74 points at 12 weeks and 2.22 points at 24 weeks in the group receiving PUFA omega-3, compared to an average reduction of 1.49 points at 12 weeks and 1.81 points at 24 weeks in the placebo group [14]. However, administering a higher dose of fish oil at 4.3 g/day (2.6 g EPA plus 1.4 g DHA) during the same period did not alleviate the severity of joint pain, as per the BPI-SF scale [15].
Overall survival and quality of life
Four studies assessed the quality of life. In one of them, supplementation with 1 g/day of fish oil (EPA and DHA concentrations not specified) reduced the expression levels of predictive markers of cell proliferation Ki-67 and vascular endothelial growth factor (VEGF), which indicates lower overall survival [18]. Furthermore, overall survival and disease-free survival were higher in the intervention group compared to the control group.
Conversely, two studies [14, 15] investigated the effects of high doses of fish oil with 3.3 g/day (2.24 g EPA plus 1.12 g DHA) and 4.3 g/day (2 0.6 g EPA plus 1.4 g DHA) on women with breast cancer and endocrine symptoms. Validated scales, such as FACT-ES and the pain scale, which assess joint symptoms, functional capacity, health, and disability status (Table 3), were used to evaluate the quality of life. However, the pain scores and quality of life did not demonstrate any significant differences between the intervention group supplemented with PUFA omega-3 and the placebo group.
One study [17] used the Edmonton Scale to assess the reduction in chemotherapy-related toxicity, which also affects the quality of life. They observed improvement only in the symptom of xerostomia after supplementation with 2.4 g/day of fish oil (1.6 g EPA plus 0.8 g DHA), with no differences in the other symptoms analyzed.
Lipid profile and inflammatory markers
Omega-3 supplementation had no impact on serum levels of lipid markers, except in two studies [14, 17]. In the study carried out on women aged about 50 years on aromatase inhibitor treatment, supplementation for 24 weeks of 3.3 g/day of fish oil (2.24 g EPA plus 1.12 g DHA) resulted in a significant drop in plasma triglyceride (TG) concentrations [14]. Women who participated in this study [14] had healthy concentrations of triglycerides before starting treatment [19], and during the study, they reported an increase in fish consumption.
In contrast, patients who received a daily dose of 2.4 g/day of omega-3 fatty acids (1.6 g EPA plus 0.8 g DHA) [17] during the same period and were undergoing multidrug therapy presented a significant increase in TG concentrations at the end of the study, despite the initial levels being within acceptable limits. The average fish consumption was not examined in this study.
Inflammatory biomarkers were measured by two studies [14, 15] that did not find significant differences between the intervention groups supplemented with omega-3 (2.24 g EPA plus 1.12 g DHA and 2.6 g EPA plus 1.4 g DHA, respectively) compared to the control group regarding interleukin (IL)-17, IL-6, C-reactive protein (CRP) and tumor necrosis factor receptor (TNFR)-2. Furthermore, neither study examined the overall composition of diets.
Body composition
Body composition was investigated through body fat mass index (FMI), fat-free mass (FFM), skeletal muscle index (SMI), and body mass index (BMI), assessed by bioelectrical impedance (BIA) in the baseline, and after three and six months of supplementation [17]. Despite patients supplemented with EPA and DHA experiencing an average weight loss of 1.8 kg after six months, no significant differences were found in body weight and BMI over time between the intervention and control groups. The analysis of factors such as physical activity and diet composition that may be related to weight loss was not performed by the authors.
Two other studies [15, 16] provided only baseline mean BMI data from the participants, and it was not possible to assess weight maintenance or gain during cancer treatment. According to mean BMI values at baseline, participants were classified as overweight and obese [15, 16], conforming to the classification of the World Health Organization [20].
Discussion
Approaches and research related to cancer are more focused on curing or delaying the progression of the disease, mitigating other essential aspects, such as the burden of toxic symptoms caused by treatment [21]. This review synthesized the available evidence on omega-3 supplementation during breast cancer treatment in adult women. According to the evaluated results, supplementation with EPA and DHA appears to be a promising strategy to reduce the adverse effects resulting from chemotherapy treatment. The studies analyzed different markers, including PIPN, musculoskeletal symptoms, quality of life, lipid profile, inflammatory biomarkers, and body composition (Table 3).
Among the studies reviewed, the most commonly analyzed benefit of omega-3 supplementation was the improvement in the quality of life. This nutritional intervention promoted a reduction in the disabling toxicities of treatment, such as xerostomia [17] and PIPN [16]. However, the studies [16, 18] did not use validated instruments to assess the quality of life in cancer patients [22,23,24]. This is crucial because the quality of life represents a comprehensive evaluation of the patient's response to the disease and treatment across physical, psychological, and social dimensions [25]. Moreover, while all studies included in this systematic review suggest that the benefits related to quality of life are due to the modulation of the production of pro-inflammatory cytokines such as TNF-α and IL-6, only two of them measured the inflammatory biomarkers [14, 15].
In addition, another important aspect is the lipid profile, which is associated with the inflammatory profile. Despite the literature evidence of the effect of omega-3 supplementation in the plasma TG concentrations reduction [26,27,28], only one study observed reduced in TG concentrations in women with breast cancer [14]. Previous studies demonstrate that the ability of omega-3 fatty acids to reduce TG concentrations is dose-dependent, with reductions of about 5% to 10% for every 1 g of EPA/DHA consumed daily, especially in individuals with higher baseline plasma TG concentrations [29]. However, for a better understanding of the impact of omega-3 fatty acids on the lipid profile and inflammatory biomarkers, it would be interesting to analyze the dietary pattern of the participants in each study, since there may be differences between the groups. This analysis would allow for a better understanding of the effects of omega-3 fatty acids on different dietary patterns, as the ratio of omega-6 to omega-3 polyunsaturated fatty acids in cell membranes reflects dietary intake [30, 31].
Weight and body composition can be an important prognostic factor in individuals with cancer since the systemic inflammation present in this condition result in disease-related malnutrition, leading to body weight loss, changes in body composition, and a decline in physical function [32]. Despite this, only one study included [17] analyzed body composition by electrical bioimpedance (BIA) measurements. Although the BIA is a validated technique for assessing body composition in populations, it should be used with caution in individuals with cancer as these patients may present edema and ascites, causing an overestimation of weight [33, 34]. Other studies [15, 16] evaluated only weight or BMI at the beginning of the interventions, being the mean BMI values corresponded to the overweight and obese ranges. The literature reveals that obesity is strongly associated with the prognosis of breast cancer, especially in the postmenopausal period [35]. Moreover, when associated with lower lean body mass, it is a predictor of negative effects on treatment prognosis [32, 36].
Furthermore, the assessment of individuals could be more comprehensive by taking into account the existence of physical activity practice and its intensity. This is supported by scientific literature, which highlights the positive effects of omega-3 fatty acids supplementation combined with physical activity. This combination has been shown to promote anabolism and reduce muscle catabolism, thereby attenuating the loss of muscle strength, blood biomarkers of inflammation, and muscle damage [31, 37].
Hershman et al. and Lustberg et al. reported adherence and tolerability to a high dose of fish oil of 3.3 g/day and 4.3 g/day, respectively. However, no efficacy was found in preventing or controlling AI-induced musculoskeletal pain in women with breast cancer [14, 15]. It should be noted that the placebo effect found by Hershman et al. may have influenced the results [38]. In studies with continuous subjective outcomes for pain management, the use of a placebo may bring small benefits [39]. This occurs because the presence of anxiety, pain, and autonomic nervous system involvement causes immunobiochemical processes to respond favorably to a placebo [40]. Furthermore, response to PUFA omega-3 supplementation may vary due to factors such as baseline inflammatory status [41], level of physical activity [42], diet composition [11], and genetic variability [43].
Although the safe and optimal dose of PUFA omega-3 (EPA and/or DHA) varies depending on the purpose of use and the individual profile [36], our review suggests that doses starting from 1 g/day of fish oil in combination with chemotherapy have shown promise. Although not all studies achieved the expected benefits, none of the studies showed a worsening of adverse effects in chemotherapy patients with omega-3 supplementation. Moreover, most of the studies analyzed in this review showed a low risk of bias in all evaluated areas, making them more reliable and yielding robust results. These findings corroborate a systematic review that evaluated omega-3 supplementation in patients with different types of cancer undergoing radiotherapy and chemotherapy [44].
Our observations revealed possible limitations in the studies: some studies did not present the sample size calculation in detail [14, 17, 18]; lack of a description of the reasons for dropouts [16]; impairment of blinding justified by the lack of fishy taste in the placebo or by the lack of declaration of the type of placebo used as a control [16, 18]; lack of analysis of the beneficial effects of fatty acids used as a placebo; and absence of isolated analysis of EPA and/or DHA, making it impossible to verify the superiority of EPA over DHA or vice versa. It was also not possible to establish a consensus between studies regarding time versus dose for EPA and/or DHA. Furthermore, new randomized clinical trials that more accurately assess body composition and quality of life in patients with breast cancer at the beginning and end of interventions with PUFA omega-3 would be interesting.
Finally, it is important to highlight some limitations found in our review study, such as the small number of included studies and the infeasibility of conducting a meta-analysis due to the heterogeneity of the studies about tumor stage, type of treatment, patient characteristics, menopausal status, age, intervention protocols, and analyzed outcomes. Furthermore, we noted the absence of approaches that assessed all expected adverse effects in our research protocol and that met the eligibility criteria. In particular, the lack of studies investigating symptoms such as nausea, vomiting, diarrhea, lack of appetite, and fatigue, as well as radiotherapy treatments. Despite this, the analyzed studies provided outcomes beyond expectations, such as the discussion of inflammatory biomarkers and lipid profiles, allowing us to explore these points. It is also necessary to emphasize that our review stands out for the meticulous approach adopted. The information search was conducted in seven different databases, minimizing the probability of significant omissions. We also highlight the use of an updated tool to measure the risk of bias, which reinforced the robustness of our analysis, providing greater solidity to our conclusions.
Conclusions
The findings of this review study are inconclusive due to the limited number of included studies. Although most studies suggest that supplementation with omega-3 fatty acids (EPA and DHA) during breast cancer treatment in women appears to be a promising strategy to mitigate the adverse effects of chemotherapy and enhance the quality of life, we found great heterogeneity in the analyzed studies. Some limitations include the lack of a more in-depth analysis of body composition, the absence of an isolated analysis of EPA and/or DHA, and the need to consider other factors such as diet, level of physical activity, baseline inflammatory profile, analysis by subgroups, considering the effects of chemotherapy and tumor staging. More research is needed to confirm the benefits and determine an optimal omega-3 dosage for patients being treated for breast cancer.
Data availability
All the data elaborated are contained within the article.
References
International Agency for Research on Cancer.Estimated number of new cases in 2020, World, females, all ages. In: World Health Organization. 2023. http://gco.iarc.fr./. Accessed 29 Mar 2023
Instituto Nacional de Câncer (INCA). Tratamento. In: Ministério da Saúde. 2022. https://www.gov.br/inca/pt-br/assuntos/gestor-e-profissional-de-saude/controle-do-cancer-de-mama/acoes/tratamento. Accessed 2 Mar 2023
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81. https://doi.org/10.1093/carcin/bgp127.
Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer. 2011;11:384. https://doi.org/10.1186/1471-2407-11-384.
Mooney K, Berry DL, Whisenant M, Sjoberg D. Improving cancer care through the patient experience: how to use patient-reported outcomes in clinical practice. Am Soc Clin Oncol Educ Book. 2017;37:695–704. https://doi.org/10.1200/EDBK_175418.
Horie LM, Barrére APN, Castro MG, de Alencastro MG, Alves JTM, Bello PPD. Diretriz BRASPEN de terapia nutricional no paciente com câncer. BRASPEN J. 2019;34(1):02–32.
Nabavi SF, Bilotto S, Russo GL, Orhan IE, Habtemariam S, Daglia M, et al. Omega-3 polyunsaturated fatty acids and cancer: lessons learned from clinical trials. Cancer Metastasis Rev. 2015;34(3):359–80. https://doi.org/10.1007/s10555-015-9572-2.
Sawyer MB, Field CJ. Possible Mechanisms of ω-3 PUFA Anti-tumour Action. In: Calviello G, Serini S, editors. Dietary Omega-3 Polyunsaturated Fatty Acids and Cancer. 1st ed. Netherlands: Springer; 2010. p. 3–38.
Guilherme Campos F, Waitzberg DL, Flávia Logulo A, Susana Torrinhas R, Gemio JW. Imunonutrição em colite experimental: efeitos benéficos dos ácidos graxos ômega-3. Arq Gastroenterol. 2002;39(1):48–54. https://doi.org/10.1590/s0004-28032002000100009.
Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2(3):355–74. https://doi.org/10.3390/nu2030355.
Rogero MM, Calder PC. Obesity, inflammation, toll-like receptor4 and fatty acids. Nutrients. 2018;10(4):432. https://doi.org/10.3390/nu10040432.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement:na updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester (UK) p: Wiley; 2019. p. 694.
Hershman DL, Unger JM, Crew KD, Awad D, Dakhil SR, Gralow J, Greenlee H, Lew DL, Minasian LM, Till C, Wade JL 3rd, Meyskens FL, Moinpour CM. Randomized multicenter placebo-controlled trial of Omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J Clin Oncol. 2015;33(17):1910–7. https://doi.org/10.1200/JCO.2014.59.5595.
Lustberg MB, Orchard TS, Reinbolt R, Andridge R, Pan X, Belury M, Cole R, Logan A, Layman R, Ramaswamy B, Wesolowski R, Berger M, Patterson E, Loprinzi C, Shapiro CL, Yee L. Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Res Treat. 2018;167(3):709–18. https://doi.org/10.1007/s10549-017-4559-z.
Ghoreishi Z, Esfahani A, Djazayeri A, Djalali M, Golestan B, Ayromlou H, Hashemzade S, Asghari Jafarabadi M, Montazeri V, Keshavarz SA, Darabi M. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo-controlled trial. BMC Cancer. 2012;12:355. https://doi.org/10.1186/1471-2407-12-355.
De la Rosa OF, Meneses García A, Ruiz Calzada H, Astudillo de la Vega H, Bargalló Rocha E, Lara-Medina F, Alvarado Miranda A, Matus-Santos J, Flores-Díaz D, Oñate-Acuña LF, Gutiérrez-Salmeán G, Ruiz García E, Ibarra A. Effects of omega-3 fatty acids supplementation on neoadjuvant chemotherapy-induced toxicity in patients with locally advanced breast cancer: A randomized, controlled, double-blinded clinical trial. Nutr Hosp. 2019;36(4):769–76. https://doi.org/10.20960/nh.2338.
Darwito D, Dharmana E, Riwanto I, Budijitno S, Suwardjo S, Purnomo J, Widodo I, Ghozali A, Aryandono T, Anwar SL. Effects of Omega-3 supplementation on Ki-67 and VEGF expression levels and clinical outcomes of locally advanced Breast Cancer patients treated with Neoadjuvant CAF chemotherapy: A randomized controlled trial report. Asian Pac J Cancer Prev. 2019;20(3):911–6. https://doi.org/10.31557/APJCP.2019.20.3.911.
Nordestgaard BG, Langsted A, Mora S, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. 2016;37(25):1944–58. https://doi.org/10.1093/eurhearti/ehw152.
WHO Consultation on Obesity (1999: Geneva S, Organization WHO 2000) Obesity: preventing and managing the global epidemic: report of a WHO consultation. World Health Organization. https://apps.who.int/iris/handle/10665/42330
Cleeland CS, Allen JD, Roberts SA, et al. Reducing the toxicity of cancer therapy: Recognizing needs, taking action. Nat Rev Clin Oncol. 2012;9(8):471–8. https://doi.org/10.1038/nrclinonc.2012.99.
Kanatas A, Velikova G, Roe B, Horgan K, Ghazali N, Shaw RJ, Rogers SN. Patient-reported outcomes in breast oncology: a review of validated outcome instruments. Tumori Journal. 2012;98(6):678–88. https://doi.org/10.1177/030089161209800602.
Anota A, Sprangers, MAG. EORTC QLQ-C30. In: Maggino, F. (eds) Encyclopedia of Quality of Life and Well-Being Research. Springer, Cham. 2021. https://doi.org/10.1007/978-3-319-69909-7_901-2.
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–86. https://doi.org/10.1200/JCO.1997.15.3.974.
Deng M, Lan Y, Luo S. Quality of life estimate in stomach, colon, and rectal cancer patients in a hospital in China. Tumour Biol. 2013;34(5):2809–15. https://doi.org/10.1007/s13277-013-0839-3.
Dogay Us G, Mushtaq S. N-3 fatty acid supplementation mediates lipid profile, including small dense LDL, when combined with statins: a randomized double-blind placebo-controlled trial. Lipids Health Dis. 2022;21(1):84. https://doi.org/10.1186/s12944-022-01686-y.
Kastelein JJP, Maki KC, Susekov A, et al. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: The EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8(1):94–106. https://doi.org/10.1016/j.jacl.2013.10.003.
Zibaeenezhad MJ, Ghavipisheh M, Attar A, Aslani A. Comparison of the effect of omega-3 supplements and fresh fish on lipid profile: a randomized, open-labeled trial. Nutr Diabetes. 2017;7(12):1. https://doi.org/10.1038/s41387-017-0007-8.
Jacobson TA. Role of n−3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. Am J Clin Nutr. 2008;87(6):1981S-1990S. https://doi.org/10.1093/ajcn/87.6.1981S.
de Izar MCO, Lottenberg AM, Giraldez VZR, et al. Posicionamento sobre o Consumo de Gorduras e Saúde. Arq Bras Cardiol. 2021;116(1):160–212. https://doi.org/10.36660/abc.20201340.
Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60(9):502–7. https://doi.org/10.1016/j.biopha.2006.07.080.
Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, de van der Schueren MA, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64. https://doi.org/10.1016/j.clnu.2016.09.004.
Selberg O, Sel S. The adjunctive value of routine biochemistry in nutritional assessment of hospitalized patients. Clin Nutr. 2001;20(6):477–85. https://doi.org/10.1054/clnu.2001.0427.
Schoeman J. Nutritional assessment and intervention in a pediatric oncology unit. Indian J Cance. 2015;52(2):186–90. https://doi.org/10.4103/0019-509X.175832.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. International agency for research on cancer handbook working group. Body fatness and cancer-viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–8. https://doi.org/10.1056/NEJMsr1606602.
Troesch B, Eggersdorfer M, Laviano A, Rolland Y, Smith AD, Warnke I, Weimann A, Calder PC. Expert opinion on benefits of long-chain Omega-3 fatty acids (DHA and EPA) in aging and clinical nutrition. Nutrients. 2020;12(9):2555. https://doi.org/10.3390/nu12092555.
Corder KE, Newsham KR, Mcdaniel JL, et al. Effects of short-term docosahexaenoic acid supplementation on markers of inflammation after eccentric strength exercise in women. J Sports Sci Med. 2016;15(1):176–83.
Wampold BE, Minami T, Tierney SC, Baskin TW, Bhati KS. The placebo is powerful: estimating placebo effects in medicine and psychotherapy from randomized clinical trials. J Clin Psychol. 2005;61(7):835–54. https://doi.org/10.1002/jclp.20129.
Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? N Engl J Med. 2001;344(21):1594–602. https://doi.org/10.1056/NEJM200105243442106.
Papakostas YG, Daras MD. Placebos, placebo effect, and the response to the healing situation: the evolution of a concept. Epilepsia. 2001;42(12):1614–25. https://doi.org/10.1046/j.1528-1157.2001.41601.x.
Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, Mischoulon D. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016;21(1):71–9. https://doi.org/10.1038/mp.2015.22.
Gammone MA, Riccioni G, Parrinello G, D’Orazio N. Omega-3 polyunsaturated fatty acids: benefits and endpoints in sport. Nutrients. 2018;11(1):46. https://doi.org/10.3390/nu11010046.
Roke K, Mutch DM. The role of FADS1/2 polymorphisms on cardiometabolic markers and fatty acid profiles in young adults consuming fish oil supplements. Nutrients. 2014;6(6):2290–304. https://doi.org/10.3390/nu6062290.
De AguiarPastore Silva J, de Souza E, Fabre M, Waitzberg DL. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: A systematic review. Clin Nutr. 2015;34(3):359–66. https://doi.org/10.1016/j.clnu.2014.11.005.
Author information
Authors and Affiliations
Contributions
Conceptualization and investigation, N.E.D. and P.N.B.-L.; writing – original draft preparation, N.E.D.; writing – review and editing, N.E.D., P.N.B.-L., M.M.R. and M.L.P.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Delmicon, N.E., Brandão-Lima, P.N., Rogero, M.M. et al. Omega-3 fatty acids as adjuvant therapy in the adverse effects of antineoplastic treatment for breast cancer: a systematic review. Nutrire 48, 45 (2023). https://doi.org/10.1186/s41110-023-00231-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-023-00231-w