Abstract
Purpose
Crohn’s disease (CD) consists of a state of chronic inflammation that can affect the entire length of the gastrointestinal tract, from the mouth to the anus. Symptoms include diarrhea, abdominal pain, and fever. Corticosteroid therapy has been widely used as the main therapeutic option to induce clinical remission and minimize the deleterious effects of inflammation. However, patients who make continuous use of corticosteroids are likely to develop Cushing’s syndrome. Exclusive enteral nutrition (EEN) is considered the first-line treatment for inducing clinical remission in children and adolescents with active Crohn’s disease. This study aimed to evaluate the use of EEN in inducing remission of CD in children and adolescents and discuss the impact of the EEN on patients’ growth, clinical and endoscopic remission, nutritional aspects, differences between formulas, and the impact on the gut microbiota.
Methods
This is a narrative review. A search for scientific articles was carried out in the PUBMED database, the SciELO electronic library, and the LILACS databases.
Results
Five randomized clinical trials, 7 non-randomized clinical trials, and 5 retrospective studies with document analysis were included. In total, the studies covered 660 children and adolescents, with different degrees of initial CD activity. All 17 articles evaluated had, as a common result, a success of EEN to induce remission of active Crohn’s disease.
Conclusion
EEN is an effective therapy for inducing clinical remission in pediatric patients with active CD, and it is associated with an improvement in the quality of life in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) consists of a state of chronic inflammation that can affect the entire length of the gastrointestinal tract (GIT), from the mouth to the anus, although it is usually focused in the small intestine region, more specifically, in the ileocolonic region [1] The chronicity of inflammation culminates in the manifestation of transmural injuries, leading to the clinical complications inherent to the disease [1, 2]. Symptoms include diarrhea, abdominal pain, rectal blood loss, and fever [3]. The diagnosis is defined by a series of clinical, radiological, endoscopic, and histological examinations. Indices are also used to predict the phenotype of the disease, concerning the location and behavior of the disease [4].
The pathophysiology of CD has a multifactorial character, whose causal relationship is associated with the intestinal microbiota, dietary pattern, and genetic susceptibility [1]. Gut microbiota plays a very important role in the regulation of the immune response by maintaining homeostasis and contributing to the maintenance of the integrity of the intestinal epithelium [5]. The composition of the microbiota is influenced by the food pattern; diets with high lipid intake and low intake of fruits and vegetables are associated with a higher risk of developing CD [6, 7]. Also, people who have a mutation in the nucleotide binding oligomerization domain containing 2 (NOD2) protein gene are more likely to develop CD. NOD2 is a protein present in several cells in the intestinal mucosa, such as dendritic cells, epithelial cells, and Paneth cells. NOD2 can recognize a bacterial receptor, such as the glycan peptide muramyl dipeptide (MDP) and cell wall structures of Gram-positive and negative bacteria. In a healthy situation, when a bacteria comes into contact with a Paneth cell, the MDP is internalized by endo/phagocytosis, which allows the recognition of MDP by NOD2, promoting the activation of nuclear factor kappa B (NF-kB)—is a key transcription factor in the regulation of the innate immune inflammatory response—which induces the production of defensins, an important microbicide. The NOD2 mutation results in a reduction in the expression of defensins, favoring dysbiosis and increasing the number of pathogens in the intestinal lumen [5]. Also, the increase in intestinal permeability facilitates the presentation of antigens to immune system cells, favoring local immune responses [8].
Drug treatment consists of the administration of anti-inflammatory drugs, such as corticosteroids (CS), to combat the acute phase of CD, and immunosuppressive drugs, such as anti-TNF-α antibodies, to prevent relapses [9, 10]. CS therapy has been widely used as the main therapeutic option to induce clinical remission and minimize the deleterious effects of inflammation [11]. However, although the acute effects of this therapy are quite effective, patients who make continuous use of CS are like to develop Cushing’s syndrome, which is characterized by the rise of physical anomalies, such as the “moon face” due to an inadequate accumulation of fat [9]. Prolonged use of this class of drug is also associated with changes in basal metabolism. In the long term, it can impair the maintenance of lean mass, characterizing an even greater problem for the pediatric population [9, 11]. In children and adolescents, there is a decrease in protein turnover that may impact linear growth and maintenance of bone mineral density [11].
Normally, patients with CD are more susceptible to developing secondary malnutrition, because the clinical manifestations directly related to the GIT impact negatively on oral intake and there is a bigger possibility of intestinal malabsorption due to the injured epithelium [1, 3]. Nutritional risk implies an even greater risk for children and adolescents, whose consequences of nutritional deficit are potentially irreversible [1].
Enteral nutrition (EN) consists of the administration of nutrients for patients with nutritional risk, that is when the oral intake is less than 60% of daily nutritional needs and/or when there is a pathophysiological anomaly that makes adequate support impossible. It can be used as a substitute or complementary, depending on the patient’s needs. EN works synergistically with medical treatment, and it is an indispensable tool to prevent or treat nutritional deficiencies because it provides nutritional support adapted to the patient’s metabolic condition [12]. In addition, EN has been increasingly used in CD, not only to restore the nutritional condition but also as a therapeutic alternative with the potential to correct dysbiosis and induce CD clinical remission [1, 9, 10, 13, 14]. In children and adolescents with active CD, exclusive enteral nutrition (EEN) is considered the first line of treatment for inducing clinical remission, because it provides superior results than the CS treatment. For this public, the criterion for prescribing the EN is not restricted to the percentage of oral acceptance. The prescription of the EEN, as a therapy for induction of clinical remission, depends on the disease phenotype and the measurement of disease activity [4]. The Pediatric Crohn’s Disease Activity Index (PCDAI) represents an evaluative instrument—composed of 11 items based on medical aspects—used to measure CD activity. The index was developed specifically for the pediatric population because it includes a topic related to linear growth, an important indicator of healthiness in children. PCDAI results in a score ranging from 0 to 100. Clinical remission is achieved when PCDAI < 10; any score above 10 predicts active CD, only varying in severity [15].
The most used EN to induce clinical remission in the pediatric population is the EEN. This therapy is characterized by the administration of a liquid diet that provides 100% of the patient’s energy requirements, excluding all other dietary sources [16]. There are different enteral formulas available on the market that differ in the composition of proteins, carbohydrates, and lipids. Polymeric formulas have unaltered macronutrients, without any degree of hydrolysis. The semi-elemental formulas have partially hydrolyzed macronutrients and the elementary formulas have fully hydrolyzed macronutrients [12]. The choice of the formula depends on the degree of impairment of the GIT and the patient’s absorptive capacity [2]. The polymeric formula can be taken orally, while the elemental and semi-elemental formulas usually are provided by a nasogastric tube. However, the polymeric formula also can be taken by a nasogastric tube, if the patient condition requires the use of the feeding tube. The duration of EEN therapy normally varies between 6 and 8 weeks. After this period, there is a process of food reintroduction that consists of gradual reinsertion of other dietary sources, until the energy sources are fully supplied by the usual diet [16].
The mechanisms that explain the EEN modulation inflammation are not yet elucidated; however, the hypotheses listed are related to the modulation of the intestinal microbiota and a reduction in the exposure of food antigens in the intestinal lumen [13, 17]. EEN is needed for the success of CD treatment in the pediatric public, as well as plays a therapeutic role. This study aimed to evaluate the use of EEN in inducing remission of CD in children and adolescents and discuss the impact of the EEN on patients’ growth, clinical and endoscopic remission, nutritional aspects, differences between formulas, and the impact on the gut microbiota.
Methods
The methodology adopted for this work was the integrative review, which consists of research in a bibliography with no obligation of replication of the results [18]. This method allows a fluid approach to the topic listed by the researcher, permitting the interpretation of existing scientific literature, without the mandatory systematization of content with pre-defined selection and exclusion criteria, which subsidizes the formation of new problems in scientific research [19].
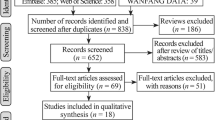
To understand the mechanisms of CD remission induction in children and adolescents in the use of EEN, a search for articles was carried out in the PUBMED database maintained by the National Library of Medicine, in the electronic library in SciELO, and in the Virtual Health Library (VHL) in the databases of Latin American and Caribbean Literature on Health Sciences (LILACS), using the descriptors “Crohn’s disease,” “exclusive enteral nutrition,” “nutrition,” “children,” and “pediatrics.” Corresponding articles that were referenced in specific studies were also consulted. After the reading of the title, the articles found whose theme was related to Crohn’s study in childhood/adolescence and EEN were selected and included in a table containing the year of publication, publication, and reference/link.
The abstract of all selected articles was read and those that presented the following aspects were excluded: enteral nutrition was not exclusive, children and adolescents were not in the active phase of the disease or the public was not exclusively pediatric. All review articles and articles dating from years before 2000 were also excluded. The remaining studies were selected for full reading and included in a table containing the objective and study design, sample and initial PCDAI, mean age at diagnosis, the enteral formula used, route of administration, time of use of the EEN, results, and reference. In Table 1, the final samples consider the children and adolescents who completed the full course of EEN and were analyzed, to minimize any biases related to the overestimation of the sample.
Results
Initially, 38 studies were selected, 35 of which were found in PUBMED, 2 in LILACS, and 1 in SciELO. The studies found in the PUBMED database were all in English, the studies in LILACS were in the Spanish language, and the study found in SciELO was in Portuguese. After reading the abstracts, 21 articles that did not attend to the predetermined inclusion criteria were excluded. Then the 17 remaining articles were the sample for the evaluation in the present study; the articles evaluated were found in the PUBMED database and only 1 in the LILACS database.
The articles included in this review were 5 randomized clinical trials, 7 non-randomized clinical trials, and 5 retrospective studies with document analysis. In total, the studies covered 660 children and adolescents, with different degrees of initial CD activity, ranging mainly from moderate to severe, according to the PCDAI classification. The studies made use of the most diverse formulas available on the market, including polymeric formulas: Modulen IBD, Nutrison Standard, CT3211, AL 110, Nutrison Energy, Nutrini Max, Nutrini Max Energy, Osmolite HiCal, Isosource Energy; the elementary formulas: Elemental O28 and Neocate and the oligomeric formulas: Pregomin and Peptisorb. Some supplements administered exclusively orally were also used, such as the protein supplement Resource Protein and the hyperosmolar supplements: Nutridrink, Nutridrink Yoghurt Style, Nutridrink Juice Style, Ensure Plus, Ensure Plus Fresh, Resource Energy, Resource Fruit, Fresubin Energy Drink, and Fresubin Jucy Drink.
The Modulen IBD polymer formula was the most used in the studies included in this review: 41% of the analyzed studies used this formula during EEN. A single study used a manufactured polymeric formula which was manufactured in a hospital in the UK. Regarding the route of administration of the diet, 9 of the 17 articles preferentially used the oral route. In these cases, the nasoenteral tube was only used when it was a demand of the patient at the beginning or during the treatment. The rest of the studies used preferentially the nasoenteral route or were not specific as to the majority route of administration; only one patient received the diet through a gastrostomy. The studies varied in terms of the duration of EEN, with studies that maintained therapy for only 4 weeks and studies that maintained therapy for 6, 8, 10, 12, and up to 16 weeks. However, most studies carried out the therapeutic intervention for a minimum period of 8 weeks.
Clinical effects
All 17 articles evaluated had as a common result, a success of the EEN to induce remission of active CD. Grover Z. et al. [20] found that EEN was able to induce early mucosal healing and provided sustained remission, reducing relapse rates and hospitalizations in newly diagnosed children. Also, Navas-Lópes V.M et al. [21] evaluated the impact of EEN as primary therapy during the first CD outbreak and observed that 94% of patients who completed treatment achieved clinical remission of the disease, with a significant increase in serum albumin concentration and hematocrit values, concomitantly with a decrease in the values of leukocytes, platelets, erythrocyte sedimentation rate, and serum C-reactive protein concentration. Another parameter evaluated was fecal calprotectin (FC), which acts as an indicative biomarker of neutrophil migration to the intestinal mucosa; this event is closely associated with the occurrence of intestinal inflammation. In this context, the concentration of FC in the feces is considered an important biomarker of disease activity. As a result, the authors found a significant decrease in the concentration of FC, indicating an improvement in the degree of inflammation of the intestinal mucosa. In addition, although no significant difference was found between patients who used EEN early or late, those who were treated with EEN as primary therapy had to use less CS in the long term.
These results are in line with those found by Day As et al. [22] whose study aimed to evaluate the benefits of using EEN to induce CD remission. The aim was to observe possible differences in the therapeutic outcome between newly diagnosed children and children with sustained CD. Both groups improve nutritional aspects, with a significant improvement in the weight Z Score (p = 0.0016) and BMI (p = 0.001), but 80% of newly diagnosed children reached clinical remission with improvement in C-reactive protein, albumin, and platelet values, while only 58% of children with sustained CD achieved remission, improving only the serum albumin concentration and platelet count.
In another study with 77 newly diagnosed children, it was observed that EEN is effective as primary therapy for the clinical remission of the disease, as well as providing improvements in nutritional aspects. [23] Otherwise, in 2009, Buchanan et al. [24] analyzed 110 children with sustained CD and they observed a decrease in erythrocyte sedimentation and serum concentration of C-reactive protein and an improvement in the weight and BMI Z Scores (p < 0.001). These studies have shown a positive correlation between the use of EEN, nutritional support, and reduced inflammation. Thus, in 2004, Bannerjee et al. [25] conducted a study to assess whether the improvement in linear growth provided by EEN was related to the nutritional support or the attenuation of inflammatory response. For this purpose, 12 prepubertal children and adolescents received a polymeric formula for 6 weeks. As a result, they found an improvement in serum IL-6 and an increase in serum IGF-1 concentration, before the change in nutritional biomarkers, which means that the improvement in growth seems to be more associated with attenuation of inflammation than nutritional support itself.
Regarding the therapy used, only 1 study evaluated the difference between the effectiveness of EEN and partial enteral nutritional (PEN). Johnson et al. [26] performed a randomized clinical trial with 50 children to assess which therapy would be most effective. There was no significant difference between the groups regarding nutritional aspects and PCDAI at the end of treatment; however, they observed that the average rate of remission caused by PEN was only 15%, while in the group of children with EEN it was 42%. They also observed that the EEN provided a reduction in episodes of diarrhea.
Exclusive enteral nutrition and corticosteroids
Of the 17 studies included in this study, 4 studies aimed to compare the clinical repercussions of the use of EEN and corticosteroid therapy [27,28,29,30]. Regarding the decrease of Crohn’s disease activity, 3 studies had presented similar results about the efficacy to achieve clinical remission, as both corticosteroid therapy and EEN therapy were successful in inducing remission, with no significant difference in this parameter. However, the group treated with EEN presented a better index of mucosa healing [27, 29, 30]. Otherwise, Connor et al. [28] verified that the group treated with EEN achieved a better rate of remission, because 87% of the patients treated with EEN reached clinical remission, while only 58% of patients that had used the corticotherapy had the same outcome.
Regarding the maintenance of clinical remission, Canani et al. [29] verified that the group of children who used the EEN for inducing clinical remission, as primary therapy, had better results for the maintenance of remission, in the long term, when compared to the group of the children who had used of the corticotherapy as the primary therapy. These results corroborate with the findings by Connor et al. [28], which observed that children who made the use of EEN as the first therapy for inducing clinical remission had a lower probability of using corticosteroids, without increasing the risk of clinical complications, such as surgery or the use of biological agents.
Regarding the nutritional repercussions, Borrelli et al. [27] observed a significant increase in height, weight, and BMI in both groups, although only the polymeric diet group presented superior weight gain. Canani et al. [29] did not verify significant differences in weight gain between the EEN and CS group, although EEN proved to be more effective in improving nutritional status, with an increase in serum iron and albumin concentrations and also the more accentuated linear growth in the group that received EEN. On the other hand, at the end of the 8 weeks of treatment, Pigneur et al. [30] did not find significant differences in the BMI Z-score in both groups.
Another relevant point concerns the side effects. Canani et al. [29] verified that 90% of patients treated with CS had side symptoms typical of corticosteroid therapy, such as moon face, hyperglycemia, muscle weakness, and acne, while only 32% of children in the EEN group had side symptoms such as nausea, diarrhea, and abdominal discomfort. Borrelli et al. [27] obtained a similar result, since the EEN group presented only mild collateral symptoms, such as flatulence and emetic reflex, while children who received CS presented typical symptoms related to drug toxicity, such as Cushingoid appearance and acne.
Formulas differences
Only 3 studies had as main objective to verify if the different compositions or components of the enteral formulas interfere with the therapeutic effect of the EEN [31,32,33]. In 2004, Ludvigsson et al. [33] conducted a multicenter randomized study in which one group received an elemental formula and another group received a polymeric formula over 6 weeks. There was no significant difference in disease remission rates, but the polymeric diet group showed higher rates of weight gain.
Components of the formulas with possible immunomodulatory actions were also evaluated [31, 33]. A study evaluated the use of the polymeric formula CT3211, which presents as the main differential the inclusion of casein, rich in growth factor β, as a protein source. As a result, they found that the children gained weight, had an increase in the standard deviation of BMI, and presented good rates of mucosal healing [32]. The CT3211 formula was also able to induce a reduction in gene expression of IL-1β, IL-8, and interferon-gamma, especially in the terminal ileum and colon [32]. A double-blind randomized trial conducted in 2000, evaluated the efficacy of a polymeric formula rich in glutamine, in which 42% of the total amino acid composition of the formula was glutamine, in comparison with a formula with low content of glutamine (4%). However, the authors did not find significant differences in weight gain and rate of clinical remission between the groups. It should be highlighted that the low-glutamine diet group presented a significantly better result (p = 0.002) in the PCDAI in comparison with the high-glutamine diet group [31].
In addition to the studies in which the main objective was to verify the difference between the formulas in therapy’s efficacy, other studies also addressed this issue. De Bie C. et al. [23] retrospectively evaluated patients who used polymeric and semi-elemental formulas via nasoenteral and patients who used oral hyperosmolar nutritional supplements. The study was not specific about the degree of hydrolysis of the formulas used via oral, only mentioning the hyperosmolarity characteristic of the formulas. Patients who used the hyperosmolar formula were able to make a combination of drinks, depending on preference and taste, which made it impossible to determine how many patients took a particular brand. However, the study covered a wide variety of formulas with this hyperosmolar characteristic. There was no significant difference between the groups regarding the effectiveness of the treatment, although the children who received the hyperosmolar formula had low adherence to treatment compared to the others. Canani et al. [29] did not observe any difference between the polymeric, elemental, and semi-elemental formulas in the clinical outcome. This result is similar to that found by Buchanan et al. [24], in which there was no difference observed between the polymeric and elemental formulas.
Microbiota modulation
Only 3 studies had as main objective to verify the impact of EEN on gut microbiota [34,35,36]. Lionetti et al. [34] carried out a test with 9 children with active CD. The children made use of EEN for 8 weeks. Fecal samples from children on EEN’s therapy were collected 2 to 3 weeks after starting treatment and compared with samples from healthy children. A modification was observed in the pattern of microorganisms residing in the intestinal microbiota, however, which strains were modified were not determined.
Another study carried out in 2015 aimed to understand the effect of the use of the EEN in children with newly diagnosed CD. In this study, 5 children started EEN’s treatment, 2 days after the diagnosis and, in most cases, continued therapy for a period of 8 to 12 weeks. The study evaluated the number of operating taxonomic units before and after therapy. For this purpose, stool samples were collected from patients and the control group, periodically for a period of 26 weeks, after the start of therapy. They observed that there was a positive correlation between the PCDAI and the number of the taxonomic units present in the stool sample, in which there was a gradual increase in bacterial phyla at the beginning of the EEN until the effective clinical remission (PCDAI < 10) [35].
As the study evaluated the post-therapy repercussions, they were also able to verify that the opposite was true and when there was an episode of relapse (PCDAI > 10), there was also an increase in bacterial phyla present in fecal samples. This fact suggests a difference between disease activity and the composition of the intestinal microbiota. However, these changes in the taxonomic unit were not regular, because every child presented a pattern of microorganisms in the baseline and had its specificity previous to the therapy [36]. In 2012, Tjellstrom et al. [36] conducted a study with 18 children, for 6 weeks, to evaluate the changes in intestinal microbiota metabolites, more specifically, in the short-chain fatty acids pattern. For that purpose, stool samples were collected from all patients. These patients presented an increase in butyric acid values, which presents an anti-inflammatory action, suggesting a positive correlation between the use of EEN as the unique food source and the short-chain fatty acids synthesis from intestinal microbiota.
Discussion
The articles included in this review presented similar results regarding the effectiveness of the use of EEN’s therapy to induce clinical remission of CD in pediatric patients. Most studies used PCDAI < 10 as a parameter to define clinical remission, except for one article that did not use PCDAI as a diagnostic parameter [30]. The articles that evaluated the nutritional repercussions of the use of EEN had as a common result the improvement in weight Z-score [22,23,24].
Yu Y et al. [9] infer that the clinical remission promoted by the use of EEN, represented by the PCDAI, may be due to the improvement in nutritional status, since the index covers several aspects of development in children and adolescents, including criteria related to weight gain [15]. However, studies varied in the initial value of PCDAI, which demonstrates the effectiveness of EEN in inducing clinical remission in different degrees of CD [20, 26, 28]. In addition, even if the nutritional support contributes to the definition of clinical remission represented by PCDAI, the attenuation of inflammation promoted by EEN can be demonstrated by the isolated evaluation of biochemical tests, as a decrease in erythrocyte sedimentation rate, serum C-reactive protein concentration, leukocyte, and platelet counts and fecal calprotectin concentration [21, 23, 24].
In this context, Bannerjee et al. [25] observed that EEN induces a reduction in inflammatory markers and IL-6, before changes in nutritional parameters, concomitant with an increase in serum IGF-1 concentration. However, even with the increase in serum IGF-1, no differences were observed in the height for age Z-score. Other nutritional biomarkers related to protein turnover, such as retinol-binding protein and prealbumin, were not evaluated, as they are proteins produced by hepatocytes, which can be suppressed by pro-inflammatory cytokines (IL-1, IL-6, and TNF-alpha) that are present in high levels in CD. The study suggests that EEN provides a direct anti-inflammatory effect that precedes nutritional restitution [25]. To identify whether nutritional restitution is effective, it is necessary to carry out a longitudinal follow-up, mainly to identify significant differences in the height Z-Score, as this indicator is not as sensitive to dietary interventions as the weight Z-Score [25, 37]. In addition, inflammatory markers are more sensitive to the dietary interventions, considering the decrease in food antigens in the intestinal lumen, during EEN’s therapy [37]. Also, the bowel rest may be related to the improvement of the histological and clinical aspects of the disease, since there is a decrease of the intestinal lesions, which contributes to the healing of the inflamed mucosa [37].
Usually, the mucosal healing rate achieved by EEN is similar to anti-TNF treatment and superior to steroids [21]. In the present review, it was possible to verify that EEN is associated with better healing rates of the intestinal epithelium [27, 29]. One of the hypotheses related to the superiority of EEN in promoting mucosal healing is the ability of EEN to correct nutritional deficits, enhancing the process of regeneration of the intestinal epithelium. Based on this premise, Akobeng et al. [31] tested a hypothesis that a formula rich in glutamine, the main energy substrate of the enterocyte, could present superior results in the clinical outcome of patients with CD. They observed that an enriched formula presented inferior results when compared to the usual formula. The hypothesis was tested considering the supply of a possible nutrient with immunomodulatory action, but glutamine is also one of the main energy sources for immune system cells, such as lymphocytes and macrophages. Since the CD is a disease characterized by the dysregulation of the immune response, providing more glutamine to these cells may have contributed to an increase in the local inflammatory response, which denotes the need to consider all possible interactions.
Another possible immunomodulator is casein, a protein derived from milk, rich in TGF-β transforming beta transformer, which function is associated with immunoglobulins, cell differentiation, and the growth of several cells. This protein may play a central role in anti-inflammatory processes related to autoimmune diseases and tolerance mechanisms [38]. Fell et al. [32] observed that the CT3211 formula, whose only protein source is casein, was able to promote a downregulation in the gene expression of IL-1β, IL-8, and interferon γ. However, no significant differences were observed in clinical outcomes from the use of other polymeric formulas such as Nutrison Standard™, whose protein source also includes whey protein, or even hydrolyzed formulas, so it is not possible to infer the superiority of casein as a protein source [26, 33].
Another mechanism related to the superior healing rates is associated with a lower exposure of food antigens in the intestinal lumen during the EEN’s therapy period, which reduces the exacerbated immune response, responsible for the histological changes inherent to CD [37]. In this context, EEN presents better results in terms of CD remission rate when compared to PEN [26]. The first studies reported on the EEN used an elemental diet in line with the theory that free amino acids had lower antigenic potential concerning whole proteins [37]. However, among the selected studies in this review, none of them showed superiority in the use of the elemental formula to induce clinical remission, when compared to the polymeric or semi-elemental formulas, which demonstrates that the effectiveness of the therapy seems to be more associated with a decrease in exposure to food residues present in a standard diet in comparison to the degree of hydrolysis of the nutrients.
Modulation of the gut microbiota may also be associated with the success of enteral therapy [35]. The metabolites produced by bacteria, particularly associated with SCFA, may be responsible for the effectiveness of the modulation since EEN provides an increase in the concentration of butyric acid, which has an anti-inflammatory character [36]. Butyric acid is one of the fermentation products of GIT’s bacteria and one of the main energy substrates of colonocytes. The role of butyric acid in the inflammatory response is associated with the inhibition of the synthesis of TNF-alpha, IL-6, and IL-12 cytokines, which have a pro-inflammatory action, and stimulation of the synthesis of IL-10, an anti-inflammatory cytokine. Butyric acid can reduce the release of IL-2 and IFN-γ, playing a crucial role in inflammatory modulation in autoimmune conditions [39] which may also contribute to the effectiveness of EEN as therapy in CD. However, it is not possible to predict the exact factor by which the EEN provides this modulation, as any change in the food supply can interfere with the microbiota, its substrates, and the pH, which interferes with the fermentation rates [36].
It is still unclear which microorganisms would be directly involved in intestinal dysbiosis associated with CD. Patients with CD may have great variability of microorganisms in the microbiota [34], which supports the theory that several strains may be related to the pathogenesis of the disease [35]. Some studies suggest that the increase in Bifidobacterium is associated with a positive clinical outcome [35, 40]. Otherwise, Pigneur et al. [30] observed enrichment of Bifidobacterium after steroid therapy, which suggests that this modulation may be possible by another pathway, not exclusively with EEN. Also, in 2019, Yilmaz B et al. verified in their study about the microbial network in CD that the Bacteroidetes and Ruminococcus strains are more associated with CD, while Faecalibacterium would be more associated with ulcerative colitis [41].
The composition of the microbiota is influenced by numerous factors, such as dietary patterns, medication use, and lifestyle habits. This fact can explain the large divergence in the results of the studies regarding the microbiota of patients with CD [42]. In this context, changing dietary patterns is a possible modulation tool, which may minimize the exposure to endotoxins and the risk of abnormal immune responses [42]. During the EEN, the dietary intervention occurs for a limited period, and even then, it already allows a change in the profile of bacteria, which demonstrates the effectiveness of modulating the microbiota by the diet. A long-term intervention can be beneficial considering the maintenance of this primary modulation since EEN is also associated with a positive clinical outcome acting as maintenance therapy [43].
Regarding the route of administration of the diet, most studies used preferentially the oral one. Buchanan et al. [24] verified that children with nasoenteral feeding were more successful in the treatment in comparison to the children who received the diet orally. This may be related to the palatability of the oral formula, which impacts adherence to treatment and, consequently, the expected clinical outcome. The improvement of the palatability is a determining factor to enhance acceptance of the oral formula [32] since the oral polymeric formulas are usually more accepted than the elemental formulas [22].
About the low acceptance of the diet, none of the articles evaluated considered the acceptance of a normal oral diet prior to treatment. The criterion for indicating EEN was, mostly, the degree of disease activity; only patients who presented some functional impediment to the indication of therapy, such as patients who presented fistulas or total intestinal obstruction, were not indicated for the EEN protocols [33]. In fact, one of the possible principles of therapy is the promotion of intestinal rest, in order to minimize the antigenic factors present in the normal diet, so the exclusion of other dietary components is essential for treatment success [37]. However, the studies evaluated encompass a large number of children and adolescents, who differ in the degree of severity of the diseases [21, 26, 27, 33]. Therefore, it is possible that a child is in the active phase of the disease, to a moderate degree, for example, and still manages to tolerate a normal oral diet, being able to complete their daily energy needs via oral feeding. As the criterion for inclusion of EEN does not consider previous oral acceptance, the abrupt suspension of normal oral feeding can negatively impact treatment adherence. Therefore, evaluating previous oral acceptance and performing the insertion of EEN gradually, as well as food reinsertion [16], can be an alternative to improve acceptance of the diet, and make the period of treatment less painful for these children and adolescents due to the food restriction.
In this context, the studies differed between the time of the use of the EEN, including studies that lasted for a time of 4 weeks [31] and studies in which the EEN was maintained for up to 12/16 weeks [28, 35]. It is possible to infer that children who stayed longer with EEN suffered a bigger impact on quality of life, considering the absence of normal oral feeding. Overall, no differences were observed regarding the duration of EEN and the effectiveness of the therapy, since the patients who remained on therapy for only 4 weeks achieved clinical remission, as well as patients who remained on therapy for 16 weeks [28, 31].
Lionnet P [34] conducted a study for 8 weeks and observed that in the 4th week, all children had reached clinical remission, which is in agreement with the findings of JF Luvidgsson [33] who verified a gradual improvement in the degree of activity of the Chron’s disease between 4 and 6 weeks, but observed no difference in the first 2 weeks. These findings may indicate that the use of EEN for only 4 weeks may be enough to achieve the expected clinical outcome. Corroborating these findings, Day AS et al. [22] observed continuous improvements in PCDAI during weeks 4 and 8, although the mean change in PCDAI from baseline at 4 to 8 weeks was not significant.
There is no consensus about the perfect time to maintain EEN, although there is a certain standardization in maintaining therapy for a minimum period of 8 weeks, as adopted by the vast majority of articles evaluated in the present study. The 8-week period seems to be a good time to elicit clinical improvement, related to inflammation minimization and nutritional parameters [22]. However, even if the therapy impacts the lives of these children and adolescents during EEN, the clinical remission of the disease, per se, provides an improvement in quality of life in long term, since there are data that suggest that EEN is able to prevent relapses and minimize the risk of hospitalizations within 1 year [20, 34].
Another relevant point concerns the specificities of the evaluated public. EEN appears to be quite effective in inducing clinical remission in the general pediatric population, and it may be even more useful when used as a first-line intervention in newly diagnosed children and adolescents [22, 23]. It is not clear why EEN is more effective as a primary treatment, but some data suggest that this therapy, when adopted as a primary treatment, is superior to CS, in the remission rate after 12 months [29]. Also, it is worth mentioning that even if there is a superiority in inducing clinical remission in newly diagnosed children, this does not cancel the effectiveness of the therapy in children with an already well-defined diagnosis [22]. The EEN’s use proved to be more effective in providing improvements in global nutritional status than corticosteroid therapy [27, 29]. The side effects promoted by EEN can also be considered less debilitating than those caused by CS since the corticotherapy is largely associated with the development of facial anomalies resulting from Cushing’s syndrome and a delay in linear growth [9]. In general, CD patients are more likely to have a deficit in their ability to socialize due to low self-esteem and barriers to understanding their sexuality. In this sense, both the clinical manifestations of the disease itself and the secondary repercussions resulting from the treatment can negatively affect the quality of life of children and adolescents, representing serious psychosocial problems [1]. The absence of serious collateral symptoms that interfere with the appearance, concomitant with the promotion of linear growth and sexual maturation, turn EEN into a more interesting therapeutic alternative for this public, both in terms of social and immunological repercussions. The improvement in life’s quality and, consequently, the decrease in physiological stressors can lead to a decrease in the pro-inflammatory cytokine, which may be related to an improvement in disease activity and mucosal healing [44].
The present work has limitations due to the inherent characteristics of the research design itself. The narrative review has a broader approach to data selection, with no well-established selection and exclusion protocols. The choice of articles is at the sole discretion of the author, who can suffer from subjective influences, which contributes to the emergence of possible biases [45]. The selected studies presented a wide variety of years of publication, study design, and sample size. In the present review, only 5 randomized clinical studies were included, which have greater scientific support regarding the level of evidence [46]. Many studies covered a smaller sample of 50, which demonstrates the need for more representative samples. Also, the studies differed in terms of the protocol for including patients for evaluation, as well as in the parameters analyzed.
Conclusion
EEN is an effective therapy for inducing clinical remission in pediatric patients with active CD and it is associated with an improvement in the quality of life in this population. The present work allowed us to explore the possible mechanisms behind the effectiveness of the EEN, regarding the possible metabolic interactions. There is still a lot of uncertainty about how EEN provides clinical remission; hypotheses have been pointed out about the modulation of the microbiota, the correction of nutritional deficits, and the minimization of food antigens with proinflammatory potential. Therefore, there is a need to carry out more clinical trials, which allow a better understanding of the metabolic interactions that result in the remission of inflammation, so that it is possible to use this knowledge for therapeutic purposes. In summary, EEN is presented as an effective therapy, with sufficient evidence to support its use in the pediatric population. However, clinical studies with larger samples are needed to better understand the mechanisms behind its effectiveness.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- CD:
-
Crohn’s disease
- EEN:
-
Exclusive enteral nutrition
- GIT:
-
Gastrointestinal tract
- PCDAI:
-
Pediatrics Crohn’s Disease Activity Index
- CS:
-
Corticosteroids
- EN:
-
Enteral nutrition
- PEN:
-
Partial enteral nutritional
References
Santos GM, Silva LR, Santana GO. Repercussões nutricionais em crianças e adolescentes na presença de doenças inflamatórias intestinais. Revista Paulista de Pediatria. 2014;32(4):403–11. https://doi.org/10.1590/S0103-05822014000400018.
Forbes A, Escher J, Hébuterne X, Klek SM, Krznaric Z, Schneider S, et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36:321–47. https://doi.org/10.1016/j.clnu.2016.12.027.
Santos LAA, Dorna MS, Vulcano DSB, Augusti L, Franzoni LC, Gondo FF, Romeiro FG, Sassaki LY. Terapia nutricional nas doenças inflamatórias intestinais: artigo de revisão. Nutrire Rev Soc Bras Aliment Nutr. 2015;383–396 https://doi.org/10.4322/2316-7874.56714
Lafferty L, Tuohy M, Carey A, Sugrue S, Hurley M, Hussey S. Outcomes of exclusive enteral nutrition in paediatric Crohn’s disease. Eur J Clin Nutr. 2016;71(2):185–91. https://doi.org/10.1038/ejcn.2016.210.
Al Nabhani Z, Dietrich G, Hugot Jp, Barreau F. Nod2: the intestinal gate keeper. PLoS Pathog. 2017;13(3):e1006177. https://doi.org/10.1371/journal.ppat.1006177.g001
Sidiq T, Yoshihama S, Downs I, Kobayashi KS. Nod2: a critical regulator of ileal microbiota and Crohn’s disease. Front Immunol. 2016;7:367. https://doi.org/10.3389/fimmu.2016.00367.
Hou JK, Abraham B, El-serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–73. https://doi.org/10.1038/ajg.2011.44.
Turner J. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. https://doi.org/10.1038/nri2653.
Yu Y, Chen KC, Chen J. Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn’s disease: a meta-analysis. World J Pediatr Fev. 2019;15(1):26–36. https://doi.org/10.1007/s12519-018-0204-0.
Verburgt CM, Ghiboub M, Benninga MA, de Jonge WJ, Van Limbergen JE, Nutritional therapy strategies in pediatric Crohn’s disease. Nutrients. 2021;13:212 https://doi.org/10.3390/nu13010212
Steiner S, Noe J, Denne S. Corticosteroids increase protein breakdown and loss in newly diagnosed pediatric Crohn disease. Pediatr Res. 2011;70:484–8. https://doi.org/10.1203/PDR.0b013e31822f5886.
Cardoso MGC, Prates SMS, Anastácia LR. Fórmulas para nutrição enteral padrão e modificada disponíveis no Brasil: Levantamento e classificação. Braspen J. 2018;33(4):402–17.
Levine A, Wine E, Assa A, Sigall Boneh R, Shaoul R, Kori M, Cohen S, et al. Crohn ’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157(2):440-450.e8. https://doi.org/10.1053/j.gastro.2019.04.021.
Gavin J, Ashton JJ, Heather N, Marino LV, Beattie RM. Nutritional support in paediatric Crohn’s disease: outcome at 12 months. Acta Pediatr. 2018;107:156–62. https://doi.org/10.1111/apa.14075.
Turner D, Griffiths AM, Walters TD, Seah T, Markowitz J, Pfefferkorn M, Keljo D, Otley A, Leleiko NS, Mack D, Hyams J, Levine A. Appraisal of the pediatric Crohn’s disease activity index on four prospectively collected datasets: recommended cutoff values and clinimetric properties. Am J Gastroenterol. 2010;105(9):2085–92. https://doi.org/10.1038/ajg.2010.143.
Verburgt CM, Ghiboub M, Benninga MA, de Jonge WJ, Van Limbergen JE. Nutritional therapy strategies in pediatric Crohn’s disease. Nutrients. 2021;13:212. https://doi.org/10.3390/nu13010212
Souza GN, Draghi PF, Yonamine GH. Terapia nutricional oral e enteral nas doenças inflamatórias intestinais em crianças e adolescentes: uma revisão na literatura. Revista Paulista de Pediatria, v. 38, 2020. São Paulo. https://doi.org/10.1590/1984-0462/2020/38/2019032
Rother ET. Revisão sistemática X revisão narrativa. Acta Paulista de Enfermagem. 2007;20(2). https://doi.org/10.1590/S0103-21002007000200001
Elias CSR, Silva LA, Martins MTSL, Ramos NAPR, Souza, MGG, Hipolito R. Quando chega o fim? Uma revisão narrativa sobre terminalidade do período escolar para alunos deficientes mentais. SMAD: Revista Electrónica em Salud Mental, Alcohol y Drogas, 2012; 1(8):48–53.
Grover Z, Muir R, Lewindon P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J Gastroenterol. 2013;49(4):638–45. https://doi.org/10.1007/s00535-013-0815-0.
Navas-López VM, Blasco-Alonso J, Lacasa Maseri S, Girón Fernández-Crehuet F, Serrano Nieto MJ, Vicioso Recio MI, Sierra Salinas C. La nutrición enteral exclusiva continua siendo el tratamiento de primera línea en la enfermedad de Crohn pediátrica en la era de los biológicos An Pediatr (Barc). 2015;83(1):47–54. https://doi.org/10.1016/j.anpedi.2014.02.027. Spanish.
Day AS, Whitten KE, Lemberg DA, Clarkson C. Vitug-Sales M, Jackson R, Bohane TD. Exclusive enteral feeding as primary therapy for Crohn’s disease in Australian children and adolescents: a feasible and effective approach. J Gastroenterol Hepatol. 2006;21(10):1609–1614. https://doi.org/10.1111/j.1440-1746.2006.04294.x
De Bie C, Kindermann A, Escher J. Use of exclusive enteral nutrition in paediatric Crohn’s disease in The Netherlands. J Crohn’s Colitis. 2013;7(4):263–70. https://doi.org/10.1016/j.crohns.2012.07.001.
Buchanan E, Gaunt WW, Cardigan T, Garrick V, McGrogan P, Russell RK. The use of exclusive enteral nutrition for induction of remission in children with Crohn’s disease demonstrates that disease phenotype does not influence clinical remission. Aliment Pharmacol Ther. 2009;30(5):501–7. https://doi.org/10.1111/j.1365-2036.2009.04067.x.
Bannerjee K, Camacho-Hübner C, Babinska K, Dryhurst KM, Edwards R, Savage MO, Sanderson IR, Croft NM. Anti-inflammatory and growth-stimulating effects precede nutritional restitution during enteral feeding in Crohn disease. J Pediatr Gastroenterol Nutr. 2004;38(3):270–5. https://doi.org/10.1097/00005176-200403000-00007.
Johnson T, Macdonald S, Hill SM, Thomas A, Murphy MS. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut. 2006;55(3):356–61. https://doi.org/10.1136/gut.2004.062554.
Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, Russo PM, Cucchiara S. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol. 2006;4(6):744–53. https://doi.org/10.1016/j.cgh.2006.03.010.
Connors J, Basseri S, Grant A, Giffin N, Mahdi G, Noble A, Rashid M, Otley A, Van Limbergen J. Exclusive enteral nutrition therapy in paediatric Crohn’s disease results in long-term avoidance of corticosteroids: results of a propensity-score matched cohort analysis. J Crohns Colitis. 2017;11(9):1063–70. https://doi.org/10.1093/ecco-jcc/jjx060.
Berni Canani R, Terrin G, Borrelli O, Romano MT, Manguso F, Coruzzo A, D’Armiento F, Romeo EF, Cucchiara S. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig Liver Dis. 2006;38(6):381–7. https://doi.org/10.1016/j.dld.2005.10.005.
Pigneur B, Lepage P, Mondot S, Schmitz J, Goulet O, Doré J, Ruemmele FM. Mucosal healing and bacterial composition in response to enteral nutrition vs steroid-based induction therapy—a randomised prospective clinical trial in children with Crohn’s disease. J Crohns Colitis. 2019;13(7):846–55. https://doi.org/10.1093/ecco-jcc/jjy207.
Akobeng AK, Miller V, Stanton J, Elbadri AM, Thomas AG. Double-blind randomized controlled trial of glutamine-enriched polymeric diet in the treatment of active Crohn’s disease. J Pediatr Gastroenterol Nutr. 2000;30(1):78–84. https://doi.org/10.1097/00005176-200001000-00022.
Fell JM, Paintin M, Arnaud-Battandier F, Beattie RM, Hollis A, Kitching P, Donnet-Hughes A, MacDonald TT, Walker-Smith JA. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2000;14(3):281–9. https://doi.org/10.1046/j.1365-2036.2000.00707.x.
Ludvigsson JF, Krantz M. Bodin L, Stenhammar L, Lindquist B Elemental versus polymeric enteral nutrition in paediatric Crohn’s disease: a multicentre randomized controlled trial. Acta Paediatr. 2004;93(3):327–35. https://doi.org/10.1111/j.1651-2227.2004.tb02956.x.
Lionetti P, Callegari ML, Ferrari S, Cavicchi MC, Pozzi E, de Martino M, Morelli L. Enteral nutrition and microflora in pediatric Crohn’s disease. J Parenter Enteral Nutr. 2005;29(4 supl):S173–S178. https://doi.org/10.1177/01486071050290s4s173.
Kaakoush NO, Day AS, Leach ST, Lemberg DA, Nielsen S, Mitchell HM. Effect of exclusive enteral nutrition on the microbiota of children with newly diagnosed Crohn’s disease. Clin Transl Gastroenterol. 2015;6(1):e71–e71. https://doi.org/10.1038/ctg.2014.21.
Tjellstrom B, Hogberg L, Stenhammar L, Magnusson KE, Midtvedt T, Norin E, Sundqvist T. Effect of exclusive enteral nutrition on gut microflora function in children with Crohn’s disease. Scand J Gastroenterol. 2012;47(12):1454–9. https://doi.org/10.3109/00365521.2012.703234.
Issa M, Binion DG. Bowel Rest and Nutrition Therapy in the Management of Active Crohn’s Disease. Nutr Clin Pract. 2008;23(3):299–308. https://doi.org/10.1177/0884533608318675.
Agin M, Yucel A, Gumus M, Yuksekkaya HA, Tumgor G. The Effect of Enteral Nutrition Support Rich in TGF-β in the Treatment of Inflammatory Bowel Disease in Childhood. Medicina (Kaunas). 2019;55(10):620. https://doi.org/10.3390/medicina55100620.
Vinolo MAR. Efeito dos ácidos graxos de cadeia curta sobre neutrófilos. 2010. Tese (Doutorado em Fisiologia Humana) - Instituto de Ciências Biomédicas, University of São Paulo, São Paulo. 2010. https://doi.org/10.11606/T.42.2010.tde-10012011-152253
Neurath MF. Host-microbiota interactions in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(2):76–7. https://doi.org/10.1038/s41575-019-0248-1.
Yilmaz B, Juillerat P, Øyås O, Ramon C, Bravo FD, Franc Y, Fournier N, Michetti P, Mueller C, Geuking M, Pittet VEH, Maillard MH, Rogler G; Swiss IBD Cohort Investigators, Wiest R, Stelling J, Macpherson AJ. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat Med. 2019;25(2):323–336. https://doi.org/10.1038/s41591-018-0308-z.
Sanz Y, Romaní-Perez M, Benítez-Páez A, Portune KJ, Brigidi P, Rampelli S, Dinan T, Stanton C, Delzenne N, Blachier F, Neyrinck AM, Beaumont M, Olivares M, Holzer P, Günther K, Wolters M, Ahrens W, Claus SP, Campoy C, Murphy R, Sadler C, Fernández L, Kamp JV. Towards microbiome-informed dietary recommendations for promoting metabolic and mental health: Opinion papers of the MyNewGut project. Clin Nutr. 2018;37(6 Pt A):2191–2197. https://doi.org/10.1016/j.clnu.2018.07.007.
Duncan H, Buchanan E, Cardigan T, Garrick V, Curtis L, McGrogan P, Barclay A, Russell RK. A retrospective study showing maintenance treatment options for paediatric CD in the first year following diagnosis after induction of remission with EEN: supplemental enteral nutrition is better than nothing! BMC Gastroenterol. 2014;14:50. https://doi.org/10.1186/1471-230x-14-50.
Afzal NA, Van Der Zaag-Loonen HJ, Arnaud-Battandier FS. Davies S. Murch B, Derkx R. Heuschkel, Fell JMN. Improvement in quality of life of children with acute Crohn’s disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment Pharmacol Ther. 2004;20(2):167–72. https://doi.org/10.1111/j.1365-2036.2004.02002.x.
Cordeiro AM, Oliveira GM, Renteria JM, Guimarães CA. Revisão sistemática: uma revisão narrativa. Rev Col Bras Cir. 2007;34(6):428–31. https://doi.org/10.1590/S0100-69912007000600012.
Galvão CM. Níveis de evidência. Acta Paulista de Enfermagem. 2006;19(2):5. https://doi.org/10.1590/S0103-21002006000200001.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
M.M.R. was responsible for the study concept. H.C.V. performed the search of the studies and the initial manuscript preparation. H.C.V. and M.M.R. were responsible for the study analyses, design, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vidal, H.C., Rogero, M.M. The use of exclusive enteral nutritional therapy in children and adolescents with active Crohn’s disease: an integrative review. Nutrire 47, 25 (2022). https://doi.org/10.1186/s41110-022-00177-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-022-00177-5