Abstract
Background
COVID-19 is a very harmful pandemic, and its recovery process is highly influenced by nutritional status; however, an appropriate nutritional scale has not yet been proposed for these patients. Therefore, the purpose of this study was to evaluate the effectiveness of the modified Nutrition Risk in the Critically ill (mNUTRIC) score in critically ill patients affected by COVID-19 admitted to the intensive care unit (ICU).
Material and methods
This was a cross-sectional study performed on 204 critically ill patients affected by COVID-19 admitted to the ICU wards. Evaluated indicators include the mNUTRIC Score as well as demographic, and biochemical indicators.
Results
A high percentage of COVID-19 patients (67.2%) had severe disease. Hospital and ICU stay (p > 0.001) and PH (p > 0.001) values were significantly lower in non-survivors than in survivors. mNUTRIC score (p > 0.001), PCO2 (p = 0.003), and CRP levels (p = 0.021) were significantly higher in non-survivors than survivors. mNUTRIC score had a direct correlation with age (p > 0.001), AST (p = 0.000), LDH (p = 0.026), and CRP (p = 0.014) and an inverse correlation with hospital duration (p = 0.031), albumin (p = 0.003) and PH (p < 0.001). Furthermore, there was a non-significant correlation between the mNUTRIC score and mortality chance (OR = 1.085, 95%CI [0.83, 1.42], p = 0.552). While, patients with more severe COVID-19 disease (OR = 8.057, 95%CI [1.33, 48.64], p = 0.023) and higher PCO2 (OR = 1.042, 95%CI [1.01, 1.08], p = 0.023) levels had higher odds of mortality.
Conclusions
Our findings revealed that COVID-19 patients with higher CRP levels and lower PH had higher mortality and poor nutritional condition. Moreover, there was a non-significant association between the mNUTRIC score and mortality chance.
Similar content being viewed by others
Introduction
Today, COVID-19 has received a great deal of attention because of its very high prevalence and mortality [1, 2]. This disease, which is caused by a new virus called Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3], can result in many complications, including pneumonia, coagulopathy, electrolyte imbalance, and can even lead to severe symptoms of acute respiratory distress syndrome (ARDS) and multiple organ failure [4, 5]. Also, this disease, in particular, has many effects on the immune system, including granulocyte and monocyte abnormalities and increased cytokines production [1, 6]. However, there is still no definitive and complete treatment protocol for the current coronavirus (COVID-19) [7]. Therefore, it is necessary to pay more attention to this disease [8].
COVID-19 is directly related to malnutrition, because generally, this disease can cause abnormal weight loss due to indigestion, loss of appetite, nausea, and dysphagia [4, 9,10,11,12]. Also, this disease, which is usually associated with severe infection, increases the patient’s need for energy by increasing the body’s metabolism. Therefore, the patient is eventually exposed to harmful malnutrition [9, 10, 13]. A recent meta-analysis found that the prevalence of malnutrition in COVID-19 patients was significantly high, which increased their risk of mortality because malnutrition could exacerbate their distressing symptoms [14]. Poor nutritional status is important because it affects the balance of immune function and thus increases systemic infection. Malnutrition also increases the length of hospital stay and the likelihood of recurrence [15, 16].
To reduce adverse clinical outcomes, the nutritional risk of critically ill patients should be identified as soon as possible [17]. Most nutrition screening tools are not specific to ICU patients [18], as they are rated in such a way that all critically ill patients are considered at high risk [19, 20]. However, not all critically ill patients have the same nutritional risk, as the study by Alberda et al. showed that increasing calorie or protein intake in these patients generally reduced mortality, but this effect was not the same in critically ill patients with different body mass indexes (BMIs) [17]. In their study, Heyland et al. presented the Nutrition Risk in the Critically ill (NUTRIC) score as a practical tool for critically ill patients, particularly for mechanically ventilated patients. This score assesses age, disease severity, and the number of days hospitalized before admission to the ICU [21]. Therefore, since malnutrition is common in COVID-19 patients and affects their recovery process [14], it is necessary to focus on the nutritional status of these patients with a useful nutrition screening tool. Thus, this study aimed to evaluate the effectiveness of the NUTRIC score as a screening system in critically ill patients affected by COVID-19 admitted to the ICU.
Materials and methods
Study design
In this cross-sectional study, the study sample was adult patients with COVID-19 who were admitted to the ICU of Al-Zahra Hospital in Isfahan from July to December 2021.
Inclusion criteria were as follow
1. Age > 18 years.
2. Definitive diagnosis of COVID-19 was based on reverse transcription-polymerase chain reaction (RT-PCR).
3. Critically ill patients admitted to ICU.
4. Intubated and ventilated patients.
5. Admitted to the ICU for more than 48 hours.
Exclusion criteria were as follows
1. Severe malnutrition: Weight loss> 5% in 1 month (> 15% in 3 months) or BMI < 18.5 plus impaired general condition or food intake 0–25% of normal in the past week.
2. Psychiatric disorders that led to severe malnutrition.
3. Patients who were in coma and whose feeding history was unknown.
4. Treated with appetite suppressants.
5. Pregnancy or breastfeeding.
6. Patients who died before spending 24 hours in the ICU.
7. Resistant vomiting (vomiting that does not respond to plasil treatment within 48 hours).
Data collection and variables
In the present study, the modified Nutrition Risk in the Critically ill (mNUTRIC) score was used to assess the nutritional status of critically ill patients with COVID-19 admitted to the ICU. The NUTRIC score is related to the identification of malnourished patients who do not receive enough protein and energy to reduce malnutrition-related mortality by correcting their nutritional status [21]. The NUTRIC score evaluates various parameters, including the number of comorbidities and Interleukin-6 (IL-6) [21]. In this nutrition scale, disease severity is also obtained by Acute Physiology and Chronic Health disease Classification System II (APACHE II) [22] and Sequential Organ Failure Assessment (SOFA) scores [23]. The number of days hospitalized before admission to the ICU and the age of the patients is the other items considered in the NUTRIC score (Table 1) [21].
When IL-6 is available if the score is 0 to 5, the risk of malnutrition is low and if it is 6 to 10, the risk is high and requires aggressive nutrition therapy. Sometimes IL-6 is not available. In the study of Heyland et al., a significant effect of IL-6 on the c-index was not observed, so they stated that if the IL-6 was not available, the mNUTRIC could be used, which deleted IL-6 [21]. Also, Rahman et al. observed that the absence of IL-6 had no significant effect on the NUTRIC score [24]. In this case, if the overall score is 0 to 4, the risk of malnutrition is low and if it is 5 to 9, the risk is high.
For included the patients in the study, on the third day after the patients were admitted to the ICU, if their conditions match the inclusion and exclusion criteria, we entered them into the study after obtaining informed written consent. Then, age, APACHE II, SOFA, number of co-morbidities, and days from hospital to ICU admission were evaluated according to the NUTRIC score. Cases of comorbidity included hypertension, diabetes mellitus, chronic renal failure, neurological disease, coronary artery disease, liver disease, chronic obstructive airway disease, and malignancy. Demographic information was also recorded. BMI was obtained by assessing patients’ weight with a Seca scale with an accuracy of 0.1 kg and height with an inelastic meter with an accuracy of 0.1 cm. However, if it was not possible for the patient to stand on the scales, the patient’s weight and height, which were measured in the hospital in the previous week, were recorded. If this option was also not possible, patients’ height and usual body weight were acquired by asking themselves or their guardians.
Mortality and morbidity were assessed from the beginning of the study until 14 days after discharge from the ICU. Arterial O2 pressure (PaO2) and CO2 pressure (PCO2) were assessed by breathing in normal room air.
Assessment of the severity of the disease
In the present study, PaO2 / FiO2 was measured to classify patients based on the severity of acute respiratory distress syndrome (ARDS). Accordingly, PaO2 / FiO2 > 200 indicates mild, PaO2 / FiO2 101–200 indicates moderate and PaO2 / FiO2 ≤ 100 indicates severe ARDS [25].
Laboratory measurements
To evaluate serum albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin (Total and Deconjugate), lactate dehydrogenase (LDH), creatine phosphokinase (CPK), reactive protein C (CRP), and blood PH, 5 ml of blood was obtained after 6 hours of fasting in the morning. To prevent spoilage of the sample, after centrifuging at room temperature for 10 minutes to isolate the serum, the serum was kept at − 80 °C. These blood factors were assessed by commercial kits (Pars Azmun, Karaj, Iran).
Statistical analysis
We used SPSS software version 21 (IBM Corp., Armonk, NY, USA) for data analysis. We reported continuous data as mean and standard deviation (SD) or median (IQR) and categorical data as frequency and percentage and considered P-value < 0.05 as significant. Kolmogorov-Smirnov test, Q-Q plot, and skewness statistics were used to evaluate the normality of the data. Then, if the variables were abnormal, we used the logarithmic transformation approach. We used chi-square or independent t-test to examine the distribution of patients among the mortality status. This distribution was evaluated based on demographic characteristics, anthropometric and biochemical indicators, and the severity of COVID-19 disease. Correlation analysis was used to evaluate the relationship between the studied variables with the NUTRIC Score. Comparison of continuous variables with the NUTRIC Score was performed by analysis of variance (ANOVA) and the relationship between different parameters and death status was analyzed by binary logistic regression analysis.
Results
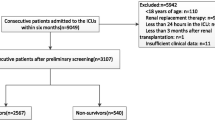
From 208 patients who were admitted into the ICU, 4 patients died within 48 hours of admission (before evaluation of nutritional assessment), thus, were excluded from the analysis. Finally, 204 participants were included in this study. Out of these, 102 patients died and 102 patients survived (Fig. 1). Baseline characteristics of participants are shown in Table 2. The average age of all enrolled patients (n = 204) was 61.98 ± 15.27. The mean age was 60.34 ± 16.28 and 63.62 ± 14.07 in survivors and deceased patients respectively, (p = 0.125). Overall, 121 (59.3%) subjects were female and 83 (40.7%) were male. There was no significant difference between two groups of deceased patients and survivors in terms of sex (p = 0.318). The average length of hospital and ICU stay was 14.99 ± 9.31 and 11.04 ± 7.93 days, respectively in the total sample. The duration of both hospital and ICU stay was longer in survivors (17.76 ± 9.36 and 13.81 ± 7.54) than in deceased patients (12.21 ± 8.43 and 8.28 ± 7.36) (p < 0.001) (Table 2).
Regarding laboratory findings, in all patients, the obtained mean of laboratory and the other parameters were as follow: ALT (58.38 ± 85.20 IU/L), AST (68.81 ± 80.39 IU/L), bilirubin total (0.86 ± 0.85 mg/dl), bilirubin direct (0.312 ± 0.42 mg/dl), LDH (966.32 ± 462.11 U/L), CPK (376.56 ± 622.58 U/L), CRP (75.55 ± 37.23 mg/dl), PaO2 (53.32 ± 27.90 mmHg), albumin (3.38 ± 0.47 g/Dl), international normalized ratio (INR) (1.29 ± 0.44), PH (7.34 ± 0.100), and PCO2 (41.20 ± 11.95 mmHg). A comparison of obtained values in two groups of survivors and deceased individuals revealed that there was a significant difference in CRP (p = 0.021), PH (p < 0.001), and PCO2 (p = 0.003) values (Table 2).
The mean APACHE II and SOFA scores in all participants were 15.22 ± 6.80 and 5.65 ± 2.96; both APACHE II (p = 0.003) and SOFA (p < 0.001) scores were significantly higher in deceased patients in comparison to the survivors. The average of mNUTRIC score in the total sample size was 4.08 ± 1.58. The mean of this score in deceased patients (4.46 ± 1.38) was significantly higher than in survivors (3.71 ± 1.68) (p = 0.001) (Table 2).
As shown in Table 2, using the following criteria, PaO2 / FiO2 > 200 indicates mild, PaO2 / FiO2 101–200 indicates moderate, and PaO2 / FiO2 ≤ 100 indicates severe ARDS, in total, 6.4% of individuals had mild, 26.5% had moderate and 67.2% had severe disease. The number of patients with mild and moderate diseases was higher in survivors, while the number of patients with severe diseases was higher in the deceased ones (p = 0.021). Overall, 75 (36.8%) patients had no comorbid disease. Among others, diabetes and hypertension together (12.7%) and diabetes (10.3%) were the most common comorbidities. P-value showed no significant difference between the two groups regarding comorbid diseases (p = 0.470) (Table 2).
We also analyzed the correlation of different parameters with the mNUTRIC score. It was found that there was a significant direct correlation between age (p < 0.001), AST (p < 0.001), LDH (p = 0.026), CRP (p = 0.014), APACHE II (p < 0.001), and SOFA (p = 0.002) with the NUTRIC score. Moreover, the mNUTRIC score was inversely correlated with hospital duration (p = 0.031), albumin (p = 0.003), and PH (p < 0.001). As presented in Table 3, there was no significant correlation between the mNUTRIC score and other indexes.
According to the results of logistic regression analysis, patients with severe disease had higher mortality chance (OR = 8.057, 95%CI [1.33, 48.64], p = 0.023). Increasing PCO2 levels were also associated with higher mortality chance (OR = 1.042, 95%CI [1.01, 1.08], p = 0.023). There was no significant correlation between the mNUTRIC score and the chance of death in participants (OR = 1.085, 95%CI [0.83, 1.42], p = 0.552) (Table 4).
Discussion
There is a high prevalence of malnutrition among hospitalized patients; critically ill patients are also more vulnerable to this condition [26, 27]. Poor nutritional status can affect therapies’ effectiveness, duration, and severity of disease and also increases the mortality chance in COVID-19 patients [28,29,30]. Therefore, a significant focus should be placed on nutritional assessment in these individuals.
The Nutrition Risk Screening-2002 (NRS-2002) and NUTRIC are two main nutritional assessment tools to evaluate nutritional risk in critically ill patients [31]. The NRS-2002 is mainly based on traditional nutrition parameters such as weight loss and changes in food intake [32]. The NUTRIC score is based on the severity of the disease rather than traditional nutrition markers [33]. In a retrospective analysis in 2018, it was shown that when it comes to evaluating malnutrition risk in ICU patients, NUTRIC outperforms NRS-2002 [34]. The NUTRIC score has been shown to be useful in estimating outcomes in critically ill patients in numerous investigations [35,36,37,38]. In our study, we evaluated the effectiveness of the NUTRIC score in critically ill patients affected by COVID-19 admitted to the ICU in the Iranian population. To our knowledge, our study is the first work evaluating the effectiveness of the NUTRIC score in critically ill COVID-19 patients in Iran.
In our results, the mean age of deceased patients was higher than survived individuals, although the difference was not statistically significant between the two groups, many studies have considered older age as an obvious risk factor in COVID-19 that can increase the chance of death in these patients [39,40,41,42]. The duration of both hospital and ICU stays was longer in survived patients than in non-survivors. Higher severity of disease in deceased patients might be the reason of early death that consequently can shorten hospital duration.
In terms of CRP, our findings revealed that the mean CRP level was significantly higher in deceased patients than in survivors. A meta-analysis conducted in 2020 demonstrated that CRP levels were lower in COVID-19 patients with the non-severe disease [43]. There are also a number of studies that suggest that high CRP concentration increases mortality chance in COVID-19 patients [44,45,46,47]. Inflammation has a key role in COVID-19 disease. Virus infection activates immune responses that result in cytokine storm and systemic inflammation, thus, elevated levels of inflammatory markers will be observed in patients [48]. Moreover, bacterial co-infections in COVID-19 patients can increase CRP levels and result in adverse clinical consequences [49].
Based on our findings, PH was lower in deceased patients than in survivors and it was lower than the normal range. Low PH is a common problem in various diseases. In COVID-19, adjusting acid-base balance is a therapeutic strategy that can prevent the progression of the disease, as low PH can facilitate viral multiplication and increase inflammatory response [50, 51]. Furthermore, one study found that acidosis was more common among fatal individuals [42]. We found that deceased patients had higher PCO2 than patients who survived but it was still within the normal range.
About the mNUTRIC score, the mean score of the two groups of deceased and survived individuals were both lower than 5. However, the difference was statistically significant between two groups. Survived patients had a lower score than deceased individuals. Previous studies have demonstrated that NUTRIC can be used as a predictor of malnutrition risk and mortality in critically ill COVID-19 patients and a higher NUTRIC score was associated with a higher mortality rate [52,53,54,55].
In this study, we found that there was a positive relationship between NUTRIC score and age, CRP, AST, and LDH. It has previously been stated that poor nutrition status is associated with age and inflammation [56,57,58,59,60]. In addition, it is known that malnutrition can make changes in the levels of liver enzymes and increase AST and ALT because of its impact on liver cells [61,62,63]. High LDH levels have been shown to be a predictor of COVID-19 severity and mortality. Malnutrition can exacerbate the severity of the disease and as a result, LDH levels might increase [64, 65]. Based on our findings, the mNUTRIC score was inversely correlated with hospital duration, albumin, and PH. In a study, it is reported that poor nutritional condition was associated with low albumin levels [66]. Malnutrition can increase the mortality rate in COVID-19 patients [14], which may be one reason for lower hospital duration.
In the current study, a higher mNUTRIC score was associated with higher mortality chance, however, it was not statistically significant. Nevertheless, in some other studies, it is shown that a high NUTRIC score was related to an increased mortality rate in a smaller number of COVID-19 patients [54, 55, 67]. More studies should be done to find results that are statistically meaningful as well. Severe disease and PCO2 were also correlated with the chance of death. In previous studies, it has been shown that other factors like comorbidities, obesity, D-dimer, low SpO2/FiO2 ratio, male gender, and higher age are associated with disease severity and mortality [68,69,70].
Our study has some limitations that should be considered: 1) the sample size was relatively small. 2) There was a lack of more clinical tests. 3) Our design was not a multi-center study. 4) Following up was not possible.
Conclusion
In conclusion, our findings demonstrated that a higher NUTRIC score was non-significantly correlated with higher mortality chance in patients with COVID-19. However, the higher PCO2 and increase in the severity of the disease were also significantly associated with higher odds of mortality. In addition, poor nutritional status and mortality were higher in patients with higher CRP levels and lower PH.
Availability of data and materials
The datasets which was generated and analyzed during the current study and used for the preparation of the manuscript are included in the article submitted for publication.
References
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33.
Farnoosh G, Alishiri G, Zijoud SH, Dorostkar R, Farahani AJ. Understanding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease (COVID-19) based on available evidence-a narrative review. J Milit Med. 2020;22(1):1–11.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13.
Kordzadeh-Kermani E, Khalili H, Karimzadeh I. Pathogenesis, clinical manifestations and complications of coronavirus disease 2019 (COVID-19). Future Microbiol. 2020;15(13):1287–305.
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–8.
Ali MJ, Hanif M, Haider MA, Ahmed MU, Sundas F, Hirani A, et al. Treatment options for COVID-19: a review. Front Med (Lausanne). 2020;7:480.
Ciotti M, Ciccozzi M, Terrinoni A, Jiang W-C, Wang C-B, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57(6):365–88.
Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection: Elsevier; 2020. p. 1631–8.
Miller R, Englund K. Clinical presentation and course of COVID-19. Cleve Clin J Med. 2020;87(7):384–8.
Wischmeyer PE. Nutrition therapy in sepsis. Crit Care Clin. 2018;34(1):107–25.
Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med. 2020;180(7):934–43.
Bedock D, Lassen PB, Mathian A, Moreau P, Couffignal J, Ciangura C, et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN. 2020;40:214–9.
Abate SM, Chekole YA, Estifanos MB, Abate KH, Kabthymer RH. Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: a systematic review and meta-analysis. Clin Nutr ESPEN. 2021;43:174–83.
Ehwerhemuepha L, Bendig D, Steele C, Rakovski C, Feaster W. The effect of malnutrition on the risk of unplanned 7-day readmission in pediatrics. Hosp Pediatr. 2018;8(4):207–13.
Correia MIT, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22(3):235–9.
Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35(10):1728–37.
Anthony PS. Nutrition screening tools for hospitalized patients. Nutr Clin Pract. 2008;23(4):373–82.
Detsky A, McLaughlin J, Baker J, Johnston N, Whittaker S, Mendelson R, et al. What is subjective global assessment of nutritional status? 1987. Classical article. Nutr Hosp. 2008;23(4):400–7.
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Group AahEW. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–36.
Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15(6):1–11.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29.
Moreno R, Vincent J-L, Matos R, Mendonca A, Cantraine F, Thijs L, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Intensive Care Med. 1999;25(7):686–96.
Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland DK. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr. 2016;35(1):158–62.
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. Jama. 2012;307(23):2526–33.
Edington J, Boorman J, Durrant ER, Perkins A, Giffin CV, James R, et al. Prevalence of malnutrition on admission to four hospitals in England. The malnutrition prevalence group. Clin Nutr (Edinburgh, Scotland). 2000;19(3):191–5.
White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement: academy of nutrition and dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr. 2012;36(3):275–83.
Fedele D, De Francesco A, Riso S, Collo A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: an overview. Nutrition (Burbank, Los Angeles County, Calif). 2021;81:111016.
Holdoway A. Nutritional management of patients during and after COVID-19 illness. Br J Commun Nurs. 2020;25(Sup8):S6–s10.
Vong T, Yanek LR, Wang L, Yu H, Fan C, Zhou E, et al. Malnutrition increases hospital length of stay and mortality among adult inpatients with COVID-19. Nutrients. 2022;14(6):1310.
Lew CCH, Yandell R, Fraser RJL, Chua AP, Chong MFF, Miller M. Association between malnutrition and clinical outcomes in the intensive care unit: a systematic review [formula: see text]. JPEN J Parenter Enteral Nutr. 2017;41(5):744–58.
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr (Edinburgh, Scotland). 2003;22(3):321–36.
Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care (London, England). 2011;15(6):R268.
Canales C, Elsayes A, Yeh DD, Belcher D, Nakayama A, McCarthy CM, et al. Nutrition risk in critically ill versus the nutritional risk screening 2002: are they comparable for assessing risk of malnutrition in critically ill patients? JPEN J Parenter Enteral Nutr. 2019;43(1):81–7.
Zhang P, He Z, Yu G, Peng D, Feng Y, Ling J, et al. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin Nutr (Edinburgh, Scotland). 2021;40(2):534–41.
Ata Ur-Rehman HM, Ishtiaq W, Yousaf M, Bano S, Mujahid AM, Akhtar A. Modified nutrition risk in critically ill (mNUTRIC) score to assess nutritional risk in mechanically ventilated patients: a prospective observational study from the Pakistani population. Cureus. 2018;10(12):e3786.
de Vries MC, Koekkoek WK, Opdam MH, van Blokland D, van Zanten AR. Nutritional assessment of critically ill patients: validation of the modified NUTRIC score. Eur J Clin Nutr. 2018;72(3):428–35.
Kalaiselvan MS, Renuka MK, Arunkumar AS. Use of nutrition risk in critically ill (NUTRIC) score to assess nutritional risk in mechanically ventilated patients: a prospective observational study. Indian J Crit Care Med. 2017;21(5):253–6.
Kang S-J, Jung SI. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020;52(2):154.
Leung C. Risk factors for predicting mortality in elderly patients with COVID-19: a review of clinical data in China. Mech Ageing Dev. 2020;188:111255.
Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;23(5):1416–24.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–74.
Chilimuri S, Sun H, Alemam A, Mantri N, Shehi E, Tejada J, et al. Predictors of mortality in adults admitted with COVID-19: retrospective cohort Study from new York City. West J Emerg Med. 2020;21(4):779–84.
Milenkovic M, Hadzibegovic A, Kovac M, Jovanovic B, Stanisavljevic J, Djikic M, et al. D-dimer, CRP, PCT, and IL-6 levels at admission to ICU can predict in-hospital mortality in patients with COVID-19 pneumonia. Oxidative Med Cell Longev. 2022;2022.
Smilowitz NR, Kunichoff D, Garshick M, Shah B, Pillinger M, Hochman JS, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42(23):2270–9.
Ullah W, Thalambedu N, Haq S, Saeed R, Khanal S, Tariq S, et al. Predictability of CRP and D-dimer levels for in-hospital outcomes and mortality of COVID-19. J Commun Hosp Intern Med Perspect. 2020;10(5):402–8.
Bivona G, Agnello L, Ciaccio M. Biomarkers for prognosis and treatment response in COVID-19 patients. Ann Lab Med. 2021;41(6):540–8.
Ming DK, Myall AC, Hernandez B, Weiße AY, Peach RL, Barahona M, et al. Informing antimicrobial management in the context of COVID-19: understanding the longitudinal dynamics of C-reactive protein and procalcitonin. BMC Infect Dis. 2021;21(1):1–7.
Kellum JA, Song M, Li J. Science review: extracellular acidosis and the immune response: clinical and physiologic implications. Crit Care (London, England). 2004;8(5):331–6.
Shevel E. Conditions favoring increased COVID-19 morbidity and mortality: their common denominator and its early treatment. Mo Med. 2021;118(2):113–5.
Bodolea C, Nemes A, Avram L, Craciun R, Coman M, Ene-Cocis M, et al. Nutritional risk assessment scores effectively predict mortality in critically ill patients with severe COVID-19. Nutrients. 2022;14(10):2105.
Frias-Toral E, Garcia E, Morocho C, Peralta F, Ramirez Y, Fuentes D. Association of the nutric score with 30 and 60-day mortality in sars Covid 19 critically ill patients in an intensive care unit, in Guayaquil - Ecuador. Clin Nutr ESPEN. 2020;40:622. https://doi.org/10.1016/j.clnesp.2020.09.648 Epub 2020 Nov 9.
Kumar N, Kumar A, Kumar A, Pattanayak A, Singh K, Singh PK. NUTRIC score as a predictor of outcome in COVID-19 ARDS patients: a retrospective observational study. Indian J Anaesth. 2021;65(9):669–75.
Osuna-Padilla IA, Moguel NCR, Vargas AA, Llamazares SR. High nutritional risk using NUTRIC-score is associated with worse outcomes in COVID-19 critically ill patients. Nutr Hosp. 2021;38(3):540–4.
Fatyga P, Pac A, Fedyk-Łukasik M, Grodzicki T, Skalska A. The relationship between malnutrition risk and inflammatory biomarkers in outpatient geriatric population. Eur Geriatr Med. 2020;11(3):383–91.
Gioulbasanis I, Patrikidou A, Kitikidou K, Papadimitriou K, Vlachostergios PJ, Tsatsanis C, et al. Baseline plasma levels of interleukin-8 in stage IV non-small-cell lung cancer patients: relationship with nutritional status and prognosis. Nutr Cancer. 2012;64(1):41–7.
Hickson M. Malnutrition and ageing. Postgrad Med J. 2006;82(963):2–8.
Honda H, Qureshi AR, Heimbürger O, Barany P, Wang K, Pecoits-Filho R, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47(1):139–48.
Mahieux F, Couderc R, Fénelon G, Maachi M. Relationships between weight loss and circulating cytokines in patients with Alzheimer’s disease. Psychologie & neuropsychiatrie du vieillissement. 2006;4(4):281–6.
Chowdhury MSI, Rahman AZ, Haque M, Nahar N, Taher A. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in different grades of protein energy malnutrition. J Bangladesh Soc Physiol. 2007;2:17–9.
Karajiban M, Montazerifar F, Hosseini R, Suni F, Dashipour AR, Fadaaeimokhtarkanlo M. The relationship between malnutrition and liver enzymes inHospitalized children in Zahedan: a case-control study. Zahedan. J Res Med Sci. 2021;23(1).
Karmacharya K, Islam M. Role of serum alanine aminotransferase aspartate aminotransferase and alkaline phosphatase in early detection of protein energy malnutrition. J Nepal Paediatr Soc. 2007;27(2):68–72.
Skevaki C, Fragkou PC, Cheng C, Xie M, Renz H. Laboratory characteristics of patients infected with the novel SARS-CoV-2 virus. J Infect. 2020;81(2):205–12.
Wei W, Wu X, Jin C, Mu T, Gu G, Min M, et al. Predictive significance of the prognostic nutritional index (PNI) in patients with severe COVID-19. J Immunol Res. 2021;2021:9917302.
Eckart A, Struja T, Kutz A, Baumgartner A, Baumgartner T, Zurfluh S, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. 2020;133(6):713–22 e7.
Leoni MLG, Moschini E, Beretta M, Zanello M, Nolli M. The modified NUTRIC score (mNUTRIC) is associated with increased 28-day mortality in critically ill COVID-19 patients: internal validation of a prediction model. Clin Nutr ESPEN. 2022;48:202–9.
Akgül F, Sevim B, Arslan Y, Şencan M, Atabey P, Aktaş A. Predictors of severity and mortality in COVID-19: a retrospective study from batman. Turkey Infect Dis Clin Microbiol. 2022;4(1):18–30.
Alharthy A, Aletreby W, Faqihi F, Balhamar A, Alaklobi F, Alanezi K, et al. Clinical characteristics and predictors of 28-Day mortality in 352 critically ill patients with COVID-19: a retrospective study. J Epidemiol Glob Health. 2021;11(1):98–104.
Ziuzia-Januszewska L, Januszewski M, Sosnowska-Nowak J, Janiszewski M, Dobrzyński P, Jakimiuk AA, et al. COVID-19 severity and mortality in two pandemic waves in Poland and predictors of poor outcomes of SARS-CoV-2 infection in hospitalized young adults. Viruses. 2022;14(8):1700.
Acknowledgements
We would like to thank all patients, research assistants, and clinicians for their involvement in the study.
Funding
This study is approved and supported by Isfahan University of Medical Sciences with grant number 299055. The institution role is only in funding this study and not in design, analyzing, and interpretation of outcomes, collecting data, and in writing the final manuscript.
Author information
Authors and Affiliations
Contributions
B. Alikiaii, ST. Hashemi, Z. Heidari, B. Nazemroaya, M. Golparvar, S. Daneshmanesh, SH. Fattahpour, M. Shojaei, and M. Bagherniya equally contributed to the conception and design of the research. B. Alikiaii, ST. Hashemi, Z. Kiani, B. Nazemroaya, M. Golparvar, S. Daneshmanesh, SH. Fattahpour, S. Amini, and M. Shojaei contributed to the acquisition of the data. Z. Heidari, Z. Kiani, S. Amini, M. Shojaei, and M. Bagherniya contributed to the analysis and interpretation of the data. B. Alikiaii, ST. Hashemi, Z. Kiani, Z. Heidari, S. Amini, and M. Shojaei drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethic committee of Isfahan University of Medical Sciences approved this study with the ethical code IR.MUI.MED.REC.1399.721. Written informed consent was received from all subjects or their care givers before beginning the study. All methods were carried out in accordance with relevant guidelines and regulations or Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alikiaii, B., Hashemi, S.T., Kiani, Z. et al. Evaluation of the effectiveness of the modified nutrition risk in the critically ill (mNUTRIC) score in critically ill patients affected by COVID-19 admitted to the intensive care unit (ICU). BMC Nutr 8, 158 (2022). https://doi.org/10.1186/s40795-022-00659-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-022-00659-9