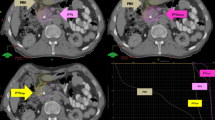
Abstract
Background
Local therapies may benefit patients with oligometastatic cancer. However, there were limited data about pancreatic cancer. Here, we compared the efficacy and safety of stereotactic body radiation therapy (SBRT) to the primary tumor and all oligometastases with SBRT to the primary tumor alone in patients with metastatic pancreatic cancer.
Methods
A retrospective review of patients with synchronous oligometastatic pancreatic cancer (up to 5 lesions) receiving SBRT to all lesions (including all oligometastases and the primary tumor) were performed. Another comparable group of patients with similar baseline characteristics, including metastatic burden, SBRT doses, and chemotherapy regimens, receiving SBRT to the primary tumor alone were identified. The primary endpoint was overall survival (OS). The secondary endpoints were progression frees survival (PFS), polyprogression free survival (PPFS) and adverse events.
Results
There were 59 and 158 patients receiving SBRT to all lesions and to the primary tumor alone. The median OS of patients with SBRT to all lesions and the primary tumor alone was 10.9 months (95% CI 10.2–11.6 months) and 9.3 months (95% CI 8.8–9.8 months) (P < 0.001). The median PFS of two groups was 6.5 months (95% CI 5.6–7.4 months) and 4.1 months (95% CI 3.8–4.4 months) (P < 0.001). The median PPFS of two groups was 9.8 months (95% CI 8.9–10.7 months) and 7.8 months (95% CI 7.2–8.4 months) (P < 0.001). Additionally, 14 (23.7%) and 32 (20.2%) patients in two groups had grade 3 or 4 treatment-related toxicity.
Conclusions
SBRT to all oligometastases and the primary tumor in patients with pancreatic cancer may improve survival, which needs prospective verification.
Similar content being viewed by others
Background
Pancreatic cancer is the fourth and sixth leading cause of cancer-related death in the US and China [1, 2], where the mortality increases slightly. Although surgery is the curative option for pancreatic cancer, only 15–20% patients were eligible for surgical resection at the diagnosis [3,4,5]. About 50% of patients had metastatic pancreatic cancer at the first presentation, and the current standard of care systemic treatment is chemotherapy alone but with dismal outcomes.
Oligometastases, defined as less than five metastases in an organ, has been considered as a clinically significant condition between local and systemic disease, which has been proposed with potential of curability via local therapy [6]. Though resections of liver or lung oligometastases of pancreatic cancer may contributed to improved survival [7], these patients were highly selective and reports were limited to a small number of patients. Therefore, this option for pancreatic cancer still remains controversial, and when it is applied indiscriminately to any patient with metastatic disease, it may not be beneficial. Additionally, emerging evidence has shown that current treatment paradigm demands alterations due to a unique biologically condition of oligometastatic pancreatic cancer [8].
Recently, studies have shown that stereotactic body radiation therapy to all oligometastases could improve survival compared to standard of care [9, 10], especially in prostate and lung cancer [11, 12]. Additionally, several studies have demonstrated that the delivery of radiation to all involved lesions would most likely better promote antigen presentation, improve immune access, and reduce the immunosuppressive barrier effects of bulky lesions not only in one area, but in all areas of disease. All of these effects cannot be optimally achieved using the current, single-site strategy that is designed to promote abscopal effects. Hence, it was advocated that comprehensive irradiation of multiple/all lesions may enhance the likelihood of obtaining meaningful clinical outcomes, especially when the synergy of radiotherapy and immunotherapy does exist [13]. Therefore, we aim to compare outcomes of stereotactic body radiation therapy to all oligometastases and the primary tumor with the primary tumor alone of pancreatic cancer.
Methods
Study design and participants
It was a retrospective study. Eligible patients were aged 18 years or older with a histopathologic diagnosis of pancreatic ductal adenocarcinoma with oligometastases, which was less than five lesions in an organ or a site. Oligometastases were determined by PET-CT via multidisciplinary approach and assessed by two independent radiologists. Additional inclusion criteria were patients without surgical resection, adequate liver, kidney and bone marrow function. These laboratory tests should be completed within a week of treatment initiation. Exclusion criteria were history of radiotherapy or interventional treatment to the primary tumor and metastatic lesions, and immunotherapy or targeted therapy, multiple-site metastases defined as more than five lesions or confirmed in more than one organ or site, periampullary cancer, neuroendocrine tumors, or cholangiocarcinoma. All patients were presented at a multidisciplinary pancreas board meeting where management decisions were discussed. Independent physicians performed prospective follow-up, and assessed the outcomes and safety of stereotactic body radiation therapy (SBRT) and chemotherapy. All patients provided written informed consents before the study. Outcomes of patients receiving SBRT for the all oligometastases and primary tumor were compared with those receiving SBRT for the primary tumor alone. The study was conducted in accordance with Good Clinical Practice guidelines. And it was approved by the institutional review board of our hospital.
Procedures
SBRT was performed via CyberKnife, an image-guided frameless stereotactic robotic radiosurgery system (Accuray Corporation, Sunnyvale CA). Gross tumor volume (GTV) was defined as a radiographically evident gross disease. A 2–5 mm margin expansion on GTV formed planning target volume (PTV). Dose constraints of organs at risk were referred to the American Association of Physicists in Medicine guidelines in TG-101. Target volumes delineations were reviewed and checked by a radiation oncologist and a radiologist for accuracy. 90% of PTV should be covered by the prescription dose. The prescription isodose line was limited to 70–75%, which would restrict the tumor Dmax [14]. Prescription doses were determined according to the sites of lesions.
Chemotherapy was initiated two to three weeks after SBRT, which included gemcitabine- or 5-FU-based regimen. Most of patients received gemcitabine (1000 mg/m2) plus nab-paclitaxel (125 mg/m2) (days 1 and 8, every 14 days for 6–8 cycles) or mFOLFIRINOX (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, irinotecan 150 mg/m2 at day 1, followed by 46 h continuous infusion of 5-fluorouracil 2400 g/m2, every 14 days for 6–8 cycles).
The primary tumor and metastatic lesions were evaluated and measured before and after treatment. Imaging examinations were performed every 2 months. Evaluations were based on RECIST version 1.1 by a blinded independent central imaging vendor. Tumor biomarker (CA19-9) was tested every month. Laboratory evaluations were performed in each cycle of chemotherapy and one week after completion of chemotherapy.
Furthermore, it was clarified that a better response was found in patients with a baseline CA19-9 level of < 200U/mL after neoadjuvant therapy [15]. Therefore, baseline CA19-9 level was stratified with < 200U/mL and ≥ 200 U/mL in our study.
Outcomes
The primary endpoint for the study was overall survival (OS), defined as the time from SBRT to death. Progression was defined according to response evaluation criteria in solid tumor (RECIST) as the appearance of one or more new lesions or a 20% increase in the sum of diameters of target lesions with an absolute increase of at least 0.5 cm. [16]. The secondary endpoints were progression free survival (PFS), calculated by the time from SBRT until documentation of any clinical or radiological disease progression or death. Polyprogression was identified when the number of progressing metastatic tumors exceeded 5 lesions and marks the transition from oligometastatic to polymetastatic status. As a result, polyprogression free survival (PPFS) was the time from SBRT to the identification of the number of lesions of more than 5, or death. Furthermore, treatment-related adverse events were determined by Common Terminology Criteria for Adverse Events (version 4.0) throughout the whole treatment.
Statistical analysis
Demographic, disease and treatment characteristics were summarized with frequency and percentage for categorical variables, and median and interquartile range (IQR) for continuous variables. Fischer’s exact test or χ² test was used for analysis of the categorical binary variables. While normally or non-normally distributed continuous covariates were compared by Student t-test or Mann-Whitney U test. OS and PFS were calculated by Kaplan-Meier methods and compared by Log-rank methods. Factors with a P-value < 0.05 in the univariate regression analysis were entered as candidate variables into multivariate COX regression analysis. Multivariate Cox proportional hazard regression analysis was used for identification of predictors correlating with OS and PFS. Two-sided P-values of less than 0.05 were regarded as statistically significant. Statistical analyses were performed with IBM SPSS version 22.0 (SPSS Inc., Armonk, NY).
Results
Patient characteristics
From 2012 to 2022, there were 59 and 158 patients receiving SBRT to both the primary tumor and all oligometastases and to the primary tumor alone. Among patients with SBRT to all lesions, 49 (83.0%), 3 (5.1%), 3 (5.1%) and 4 (6.8%) patients had metastases in the liver, lung, bone and other sites. While regarding patients receiving SBRT to the primary tumor alone, 121 (76.6%), 9 (5.7%), 5 (3.2%) and 23 (14.6%) patients had metastases in the liver, lung, bone and other sites. The median biologically effective dose (BED10, α/β = 10) to the primary tumor of patients with SBRT to all lesions and the primary tumor alone was 59.5 Gy and 58.0 Gy, respectively. And the radiation dose ranged from 30 to 45 Gy/5-8f and 30–43 Gy/5-8 f. For SBRT to liver oligometastases, the radiation dose ranged from 40 to 64 Gy/5-8f, with a median BED10 of 92.625 Gy (interquartile range 85.5–102.6 Gy). There were no statistical differences between two groups regarding baseline characteristics. Details were demonstrated in Table 1.
Survival
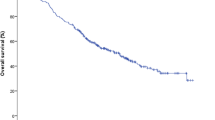
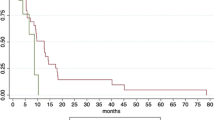
The median OS of patients undergoing SBRT to the primary tumor and all oligometastases and the primary tumor alone was 10.9 months (95% CI 10.2–11.6 months) and 9.3 months (95% CI 8.8–9.8 months) (P < 0.001) (Fig. 1A). One-year OS rate of the two groups was 37.3% (95% CI 31.0%-43.6%) and 2.5% (95% CI 1.3%-3.7%). The median PFS of patients with SBRT to all lesions and the primary tumor alone was 6.5 months (95% CI 5.6–7.4 months) and 4.1 months (95% CI 3.8–4.4 months) (P < 0.001) (Fig. 1B). Six-month PFS rate of the two groups was 52.5% (95% CI 46.0%-59.0%) and 15.2% (95% CI 12.3%-18.1%), respectively. The median PPFS of patients with SBRT to all lesions and the primary tumor alone was 9.8 months (95% CI 8.9–10.7 months) and 7.8 months (95% CI 7.2–8.4 months) (P < 0.001) (Fig. 1C). One-year PPFS rate of the two groups was 25.4% (95% CI 19.7%-31.1%) and 1.3% (95% CI 0.4%-2.2%). After multivariate analysis, BED10 (< 60 Gy as reference; ≥60 Gy HR: 0.52, 95% CI 0.39–0.70, P < 0.001) and irradiated sites (all lesions as reference; primary tumor alone HR: 3.26, 95% CI 2.26–4.70, P < 0.001) correlated with OS (Table 2). Similarly, BED10 (< 60 Gy as reference; ≥60 Gy HR: 0.44, 95% CI 0.32–0.60, P < 0.001) and irradiated sites (all lesions as reference; primary tumor alone HR: 3.84, 95% CI 2.66–5.54, P < 0.001) were also predictive of PFS (Table 3).
Adverse events
Among patients receiving SBRT to all lesions and the primary tumor alone (both plus chemotherapy after SBRT), 14 (23.7%) and 32 (20.2%) patients had grade 3 or 4 treatment-related toxicity. The most common grade 3 or 4 adverse events were neutropenia [12 patients (20.3%) with SBRT to all lesions and 23 (14.6%) with SBRT to the primary tumor alone] and increased ALT or AST [11 (18.6%) with SBRT to all lesions and 32 (20.2%) with SBRT to the primary tumor alone]. Details were shown in Table 4.
Discussion
Metastases were quite common in pancreatic cancer at the initial diagnosis. In this setting, systemic therapy is given the first priority. However, local treatment may synergize with chemotherapy in the case of oligometastases, which may improve survival of patients with oligometastatic pancreatic cancer. This notion has been proved in lung cancer. In this study, it was demonstrated that SBRT to all oligometastases and the primary tumor contributed to favorable outcomes compared with SBRT to the primary tumor alone.
Since the introduction of the concept of the oligometastatic state and advocation of local therapies due to potential survival benefits [17], no widely accepted definitions of oligometastases have been proposed across cancers. In a recent study on investigation of the biology of polymetastases, it was clarified that ten may be cut-off number of the lesions of polymetastases [18]. We chose up to 5 metastases in our study, and most of the patients had one to three lesions. A retrospective study has compared survival of patients receiving SBRT to all oligometastases and chemotherapy with those receiving chemotherapy alone. The median OS was 42 months and 18 months, which was much longer than ours [19]. The reason contributed to the difference may be ascribed to the included patients. First, in their study, 20 patients received SBRT to all lesions, of whom 14 had history of surgical resections of primary tumors and 3 received surgery after SBRT and chemotherapy. In our study, no patients had surgical resections. Secondly, patients with CA19-9 level of more than 1000U/ml were excluded. However, 21 (35.6%) and 70 (44.3%) patients having SBRT to all lesions and the primary tumor alone had CA19-9 levels of more than 1000U/ml. Therefore, it was indicated that tumor burden of patients in our study was much higher than that in their study, which may result in worse prognosis. Thirdly, patients who had the initial diagnosis of oligometastatic pancreatic cancer and did not have any progressions after 5 months of mutiagent chemotherapy could be included in their study. While no matter whether patients had progressions within 5 months of chemotherapy before SBRT, they were all included in the study. Another study also reported SBRT to oligometastases of 41 stage IV pancreatic cancer patients with up to 5 metastatic lesions, and the median survival was 23 months. Similarly, most of the patients included in the study had a history of surgical resection [20]. Therefore, our study was the first study about the efficacy of SBRT to all oligometastases and the primary tumor for patients without surgery and with a high tumor burden, which showed a larger cohort of patients may benefit from this approach. For radiotherapy to the primary tumor alone, previous studies have shown that the median OS was 6 months and 11.6 months, while the median PFS was 2.4 months and 4 months [21, 22]. The outcomes were similar to those of patients having SBRT to the primary tumor alone in our study.
Regarding therapeutic options, the clinical introduction of online adaptive magnetic resonance (MR) guided systems has allowed dose escalation towards an effective BED, such as SBRT, for pancreatic cancer, theoretically improving clinical efficacy while minimizing the risk of serious toxicity, which may be a promising role in SBRT for pancreatic cancer. According to the previous studies about MR-guided SBRT for pancreatic cancer, the median PFS after treatment ranged from 7.0 to 21.0 months, and 1- and 2-year PFS ranged between 32 and 52% and between 21 and 25% [23,24,25,26,27,28,29,30]. Median OS from treatment ranged from 9.8 to 18.0 months, and 1-year OS ranged between 54% and 80%, and 2-year OS between 28% and 57% [31,32,33]. Hence, these results indicate further investigation of the effectiveness of ablative MR-guided SBRT as a well-tolerated and minimally-invasive therapy for pancreatic cancer in prospective clinical trials.
Disease progression of tumors is classically defined with RECIST criteria. However, it may not be clinically meaningful for patients with all lesions irradiated. In this study, we proposed two categories of progressions: disease progression as the radiological progression of any lesions, polyprogression involving the number of metastases that exceeded 5. We speculated that polyprogression was the biologically, clinically relevant outcome that influenced survival, predictive of poor prognosis. There was a paucity of effects of SBRT to all metastatic lesions on survival. Our study was exploratory due to the small number of patients and should be the evidence for prospective studies. Nevertheless, it was possible that SBRT to all sites of pancreatic cancer would improve survival.
In the case of priming of tumor microenvironment induced by SBRT, radiomics may be a potential option to visualize the changes, predict outcomes and correlate the molecular findings and gene expressions in tumors with image phenotypes. Previous studies have investigated the role of CT- and MR-based radiomics and PET-CT texture for evaluations of outcomes [34,35,36,37,38]. Additionally, two studies have distinguished quasi-mesenchymal subtype from non quasi-mesenchymal subtype of pancreatic cancer and performed survival predictions with machine learning algorithms [39, 40]. While another study has clarified that genetic alterations of KRAS and SMAD4 had significant associations with FDG PET-based radiomic features in pancreatic cancer [41]. An important role to improve prognosis of pancreatic cancer is represented by the innovative approach of personalization through tailored treatment based on stratifications of patients into different groups, and early prediction or simulation of potential treatment response with radiomics, which helps physicians to choose therapeutic patterns accordingly.
Regarding the oligometastases, surgical resection has been performed in highly selected patients in addition to radiotherapy. However, metastasectomy in the management of metastatic pancreas cancer still remains controversial. Previous studies have evaluated the efficacy of surgical resection of patients with both synchronous and metachronous oligometastatic disease. The median OS from the diagnosis and surgery to death was 14.5 months and 12.3 months [42]. The outcomes were similar to those in our study. Nevertheless, only a small number of patients may be eligible for surgery. Some studies have identified several potential criteria of resection, which included major biochemical response (significant decrease of CA19-9) after neoadjuvant therapy, complete or major radiological response with single remaining metastasis or small, few lesions after chemotherapy. Regarding safety of metastasectomy, the reported complication rates of the resection of pancreatic cancer with simultaneous hepatic metastasectomy were 18–68%, with a median complication rate of 45% [42], which was comparable to that of pancreaticoduodenectomy alone [43]. One of the studies reported that 30-day morbidity and mortality of synchronous liver metastases resection was 45.0% and 2.9%, respectively. While after metachronous resection, the 30-day morbidity and mortality was 21.7% and 4.3% [44]. Therefore, in the case of efficacy, outcomes of SBRT to all oligometastases and the primary tumor were comparable with those of surgical resection. While risk of adverse events of SBRT to all lesions were much lower than those of metastasectomy, which may even result in mortality.
Regarding prognosis of the metastatic sites, it had been demonstrated that lung metastases showed favorable outcomes compared with liver metastases [45,46,47]. A median OS of lung-only recurrence was 18.1 to 37.1 months [46, 48,49,50,51], which was longer than that in our study. It had been clarified that patients with lung primary metastasis had lower T stage and less vascular invasion. The possible mechanism of lung metastasis that bypass the portal vein system may be ascribed to lymphatic invasion or lymph node metastases [47]. Other studies have proposed several possible lymphatic spreads from pancreatic cancer: (1) pleural lymphatics along connective tissue septa and into the alveolar spaces and bronchial walls; (2) retrograde lymphatic invasion of the lung from the tracheobronchial or mediastinal glands; and (3) via the systemic circulation with involvement of metastatic lymph nodes in the venous angle [52, 53]. However, this may be one possible pathway for the development of primary lung metastasis, bypassing the liver. Additionally, tumor biology is also vital in the metastasis sites, including organ-specific metastases.
Additionally, though upfront chemotherapy was usually given followed by radiotherapy, SBRT was delivered before administration of chemotherapy in our study. The rationale was due to the shorter duration of SBRT compared with conventional radiotherapy, usually about 5 days, which may not delay the initiation of chemotherapy. Furthermore, some of Chinese patients are not tolerant of mFOLFIRINOX/NALIRIFOX. And for patients with metastatic pancreatic cancer, even though the scores of performance status was 0 to 2 before treatment, most of them were vulnerable to aggressive regimens, which may result in deteriorations of performance status and quality of life. Hence, upfront gemcitabine and nab-paclitaxel was offered to 60% of patients in both two groups. Nevertheless, for the rest patients, mFOLFIRINOX was still given as the first-line therapy.
The results of the study should be interpreted with cautions given the limitations. The small number of patients included in the study and retrospective design limit the robustness of results. However, patients in our study had synchronous metastases without surgical resection of the primary tumor and a higher tumor burden compared with those in aforementioned studies, which implied that patients in our study were less selective. Also, there was no statistical difference between characteristics of two groups. However, potential bias still could not be eliminated without prospective selection. Additionally, the statistical methods in the study had the inherent statistical limitations. The Kaplan-Meier method provides unadjusted survival probabilities. The results of the study did not provide definitive evidence, suggesting larger cohort of confirmative studies should be needed. However, the study may be an initial step to shed light on the benefits of SBRT to all lesions for an appropriate cohort of patients with oligometastatic pancreatic cancer, which may be a distinct disease condition with clinically favorable outcomes.
Our study showed that patients with oligometastatic pancreatic cancer may benefit from SBRT to all lesions with improved survival and slowing polyprogression. Nevertheless, the perception should be verified prospectively in the robust design trials.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- SBRT:
-
Stereotactic body radiation therapy
- GTV:
-
Gross tumor volume
- PTV:
-
Palnning target volume
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- PPFS:
-
Polyprogression free survival
- IQR:
-
Interquartile range
- BED10, α/β = 10:
-
Biologically effective dose
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, et al. National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health. 2023;8:e943–55.
Shaib Y, Davila J, Naumann C, et al. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. population-based study. Am J Gastroenterol. 2007;102:1377–82.
Mayo SC, Nathan H, Cameron JL, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012;118:2674–81.
Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–62.
Weichselbaum RP, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–82.
Hashimoto D, Satoi S, Fujii T, Sho M, He J, Hackert T, et al. Is surgical resection justified for pancreatic ductal adenocarcinoma with distant abdominal organ metastasis? A position paper by experts in pancreatic surgery at the Joint Meeting of the International Association of Pancreatology (IAP) & the Japan Pancreas Society (JPS) 2022 in Kyoto. Pancreatology. 2023;23:682–8.
Damanakis AI, Ostertag L, Waldschmidt D, Kütting F, Quaas A, Plum P, et al. Proposal for a definition of oligometastatic disease in pancreatic cancer. BMC Cancer. 2019;19:1261.
Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–8.
Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38:2830–8.
Francolini G, Gaetano Allegra A, Detti B, Di Cataldo V, Caini S, Bruni A, et al. Stereotactic body radiation therapy and abiraterone acetate for patients affected by oligometastatic castrate-resistant prostate cancer: a randomized phase II trial (ARTO). J Clin Oncol. 2023;41:5561–8.
Tsai CJ, Yang JT, Shaverdian N, Patel J, Shepherd AF, Eng J, et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (consolidative use of Radiotherapy to Block [CURB] oligoprogression): an open-label, randomised, controlled, phase 2 study. Lancet. 2023. https://doi.org/10.1016/S0140-6736(23)01857-3.
Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol. 2019;16:123–35.
Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–101.
Cloyd JM, Wang H, Egger ME, et al. Association of clinical factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg. 2017;152:1048–56.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:2282–2247.
Hellman S, Weichselbaum RR, Oligometastases. J Clin Oncol. 1995;13:8–10.
Maddipati R, Norgard RJ, Baslan T, et al. MYC levels regulate metastatic heterogeneity in pancreatic adenocarcinoma. Cancer Discov. 2022;12:542–61.
Elamir AM, Karalis JD, Sanford NN, Polanco PM, Folkert MR, Porembka MR, et al. Ablative radiation therapy in oligometastatic pancreatic cancer to delay polyprogression, limit chemotherapy, and improve outcomes. Int J Radiat Oncol Biol Phys. 2022;114:792–802.
Scorsetti M, Comito T, Franceschini D, Franzese C, Prete MG, D’Alessio A, et al. Is there an oligometastatic state in pancreatic cancer? Practical clinical considerations raise the question. Br J Radiol. 2020;93:20190627.
Webking S, Sandoval ML, Chuong MD, Ucar A, Aparo S, De Zarraga F, et al. Ablative 5-fraction stereotactic MRI-guided adaptive radiotherapy for oligometastatic pancreatic adenocarcinoma. Cancer Control. 2023. https://doi.org/10.1177/10732748231219069.
Wu L, Lu Y, Chen L, Yue X, Cen C, Zheng C, et al. The effects of radiotherapy on pancreatic ductal adenocarcinoma in patients with liver metastases. Curr Oncol. 2022;29:7912–24.
Bruynzeel AME, Lagerwaard FJ. The role of biological dose-escalation for pancreatic cancer. Clin Transl Radiat Oncol. 2019;18:128–30.
Tyagi N, Liang J, Burleson S, et al. Feasibility of ablative stereotactic body radiation therapy of pancreas cancer patients on a 1.5 Tesla magnetic resonance linac system using abdominal compression. Phys Imaging Radiat Oncol. 2021;19:53–9.
Bryant JM, Palm RF, Herrera R, et al. Multi-institutional outcomes of patients aged 75 years and older with pancreatic ductal adenocarcinoma treated with 5-fraction ablative stereotactic magnetic resonance image-guided adaptive radiation therapy(A-SMART). Cancer Control. 2023;30:10732748221150228.
Tringale KR, Tyagi N, Reyngold M, et al. Stereotactic ablative radiation for pancreatic cancer on a 1.5 Telsa magnetic resonance linac system. Phys Imaging Radiat Oncol. 2022;24:88–94.
Kim H, Olsen JR, Green OL, et al. MR-guided radiation therapy with concurrent gemcitabine/nab-paclitaxel chemotherapy in inoperable pancreatic cancer: a TITE-CRM phase I trial. Int J Radiat Oncol Biol Phys. 2023;115:214–23.
Chuong MD, Herrera R, Kaiser A, et al. Induction chemotherapy and ablative stereotactic magnetic resonance image-guided adaptive radiation therapy for inoperable pancreas cancer. Front Oncol. 2022;12:888462.
Chuong MD, Bryant JM, Herrera R, et al. Dose-escalated magnetic resonance image-guided abdominopelvic reirradiation with continuous intrafraction visualization, soft tissue tracking, and automatic beam gating. Adv Radiat Oncol. 2021;7:100840.
Chuong MD, Bryant J, Mittauer KE, et al. Ablative 5-fraction stereotactic magnetic resonance guided radiation therapy with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer. Pract Radiat Oncol. 2021;11:134–47.
Bordeau K, Michalet M, Keskes A, et al. Stereotactic MR-guided adaptive radiotherapy for pancreatic tumors: updated results of the Montpellier prospective registry study. Cancers (Basel). 2022;15:7.
Michalet M, Bordeau K, Cantaloube M, et al. Stereotactic MR-guided radiotherapy for pancreatic tumors: dosimetric benefit of adaptation and first clinical results in a prospective registry study. Front Oncol. 2022;12:842402.
Rudra S, Jiang N, Rosenberg SA, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8:2123–32.
Cozzi L, Comito T, Fogliata A, et al. Computed tomography based radiomic signature as predictive of survival and local control after stereotactic body radiation therapy in pancreatic carcinoma. PLoS ONE. 2019;14:e0210758.
Yue Y, Osipov A, Fraass B, et al. Identifying prognostic intratumor heterogeneity using pre- and post-radiotherapy 18F-FDG PET images for pancreatic cancer patients. J Gastrointest Oncol. 2017;8:127–38.
Simpson G, Spieler B, Dogan N, et al. Predictive value of 0.35 T magnetic resonance imaging radiomic features in stereotactic ablative body radiotherapy of pancreatic cancer: a pilot study. Med Phys. 2020;47:3682–90.
Parr E, Du Q, Zhang C, et al. Radiomics-based outcome prediction for pancreatic cancer following stereotactic body radiotherapy. Cancers. 2020;12:1051.
Cusumano D, Boldrini L, Yadav P, et al. Delta radiomics analysis for local control prediction in pancreatic cancer patients treated using magnetic resonance guided radiotherapy. Diagnostics. 2021;11:72.
Kaissis GA, Ziegelmayer S, Lohöfer FK, et al. Image-based molecular phenotyping of pancreatic ductal adenocarcinoma. J Clin Med. 2020;9:724.
Kaissis G, Ziegelmayer S, Lohöfer F, et al. A machine learning model for the prediction of survival and tumor subtype in pancreatic ductal adenocarcinoma from preoperative diffusion-weighted imaging. Eur Radiol Exp. 2019;3:41.
Lim CH, Cho YS, Choi JY, et al. Imaging phenotype using 18F-fluorodeoxyglucose positron emission tomography–based radiomics and genetic alterations of pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging. 2020;47:2113–22.
Macfie R, Berger Y, Sarpel U, Hiotis S, Golas B, Labow D, et al. Surgical management of pancreatic cancer liver oligometastases. Crit Rev Oncol Hematol. 2022;173:103654.
Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530–6.
Hackert T, Niesen W, Hinz U, Tjaden C, Strobel O, Ulrich A, et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol. 2017;43:358–63.
Suenaga M, Fujii T, Kanda M, et al. Pattern of first recurrent lesions in pancreatic cancer: hepatic relapse is associated with dismal prognosis and portal vein invasion. Hepatogastroenterology. 2014;61:1756–61.
Morimoto D, Yamada S, Sonohara F, et al. Characteristics of lung metastasis as an initial recurrence pattern after curative resection of pancreatic cancer. Pancreas. 2020;49:699–705.
Zheng B, Ohuchida K, Yan Z, et al. Primary recurrence in the lung is related to favorable prognosis in patients with pancreatic cancer and postoperative recurrence. World J Surg. 2017;41:2858–66.
Homma Y, Endo I, Matsuyama R, et al. Outcomes of lung metastasis from pancreatic cancer: a nationwide multicenter analysis. J Hepatobiliary Pancreat Sci. 2022;29:552–61.
Groot VP, Blair AB, Gemenetzis G, et al. Isolated lung recurrence after resection of pancreatic cancer: the effect of patient factors and treatment modalities on survival. HPB (Oxford). 2019;21:998–1008.
Decoster C, Gilabert M, Autret A, et al. Heterogeneity of metastatic pancreatic adenocarcinoma: lung metastasis show better prognosis than liver metastasis: a case control study. Oncotarget. 2016;7:45649–55.
Otsuka H, Uemura K, Kondo N, et al. Clinical characteristics of initial recurrence in lung after surgical resection for pancreatic ductal adenocarcinoma. Pancreatology. 2020;20:1472–8.
Kamisawa T, Isawa T, Koike M, et al. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas. 1995;11:345–9.
Lisa JR, Trinidad S, Rosenblatt MB. Pulmonary manifestations of carcinoma of the pancreas. Cancer. 1964;17:395–401.
Acknowledgements
We appreciated Dr. Jiuhong Chen for her constructive advice in patients’ follow-up.
Funding
This study was funded by Ministry of Science and Technology of China (2022YFC2503700, 2022YFC2503701), National Natural Science Foundation of China (82272742), Shanghai Health Commission Leading Talent Program (2022LJ019) and Changhai Hospital affiliated to Naval Medical University (2019YPT004).
Author information
Authors and Affiliations
Contributions
ZHJ designed the study. ZXF, JLG and FZR were responsible for the treatment. ZXF, JLG, ZXZ and LWY performed the follow-up. FZR and YYS were responsible for data curation. ZXF, YYS and CYS analyzed the data. ZXF and JLG wrote the manuscript. ZHJ revised the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the institutial review board of Changhai Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, L., Ye, Y., Feng, Z. et al. Stereotactic body radiation therapy for the primary tumor and oligometastases versus the primary tumor alone in patients with metastatic pancreatic cancer. Radiat Oncol 19, 111 (2024). https://doi.org/10.1186/s13014-024-02493-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-024-02493-8