Abstract
Background
There are no criteria specifically for evaluating the quality of implementation research and recommending implementation strategies likely to have impact to practitioners. We describe the development and application of the Best Practices Tool, a set of criteria to evaluate the evidence supporting HIV-specific implementation strategies.
Methods
We developed the Best Practices Tool from 2022–2023 in three phases. (1) We developed a draft tool and criteria based on a literature review and key informant interviews. We purposively selected and recruited by email interview participants representing a mix of expertise in HIV service delivery, quality improvement, and implementation science. (2) The tool was then informed and revised through two e-Delphi rounds using a survey delivered online through Qualtrics. The first and second round Delphi surveys consisted of 71 and 52 open and close-ended questions, respectively, asking participants to evaluate, confirm, and make suggestions on different aspects of the rubric. After each survey round, data were analyzed and synthesized as appropriate; and the tool and criteria were revised. (3) We then applied the tool to a set of research studies assessing implementation strategies designed to promote the adoption and uptake of evidence-based HIV interventions to assess reliable application of the tool and criteria.
Results
Our initial literature review yielded existing tools for evaluating intervention-level evidence. For a strategy-level tool, additions emerged from interviews, for example, a need to consider the context and specification of strategies. Revisions were made after both Delphi rounds resulting in the confirmation of five evaluation domains – research design, implementation outcomes, limitations and rigor, strategy specification, and equity – and four evidence levels – best, promising, more evidence needed, and harmful. For most domains, criteria were specified at each evidence level. After an initial pilot round to develop an application process and provide training, we achieved 98% reliability when applying the criteria to 18 implementation strategies.
Conclusions
We developed a tool to evaluate the evidence supporting implementation strategies for HIV services. Although specific to HIV in the US, this tool is adaptable for evaluating strategies in other health areas.
Similar content being viewed by others
Introduction
Implementation science is dedicated to improving the uptake and use of evidence-based interventions, practices, and policies to capitalize on scientific knowledge and impact human health. Central to the goals of implementation research is building the evidence for implementation strategies, defined as techniques or change efforts to promote the adoption, implementation, and sustainment of evidence-based interventions (EBIs) [1]. In a recent review, scholars within the field of implementation science recognized that a more robust research agenda related to implementation strategies is needed to yield the promised benefits of improved EBI implementation for practitioners [2]. Within this agenda is a call for more research on the effectiveness of implementation strategies. Expanding on this priority, criteria on which to evaluate evidence quality are needed to assess whether the evidence supporting the effectiveness of any given strategy is sufficient. Without criteria on which to evaluate implementation research focusing on strategies, it is difficult to recommend strategies that are likely to be the most valuable for practitioners or to identify strategies that may hold initial promise but would benefit from more robust research. Evidence criteria are also an foundational element of the creation of a compendium of evidence-based implementation strategies, which is a key dissemination approach for delivering evidence to implementers.
At the intervention level, criteria and rubrics are available to synthesize research outcomes and evaluate research quality behind the evidence supporting an intervention and make recommendations about their use, such as Grading of Recommendations Assessment, Development, and Evaluation (GRADE) or that used by the United States Preventative Services Task Force [3, 4]. These guidelines often consider different domains of research outcomes and quality, like the health outcomes, the research design, and potential for bias in the outcomes because of the research design. Based on these guides, health institutions, like the Preventative Services Task Force, make recommendations about the best interventions across a wide set of health conditions to assist providers and organizations in making clinical and policy-level decisions. To our knowledge, no equivalent set of criteria for implementation strategies are available. As such, it is difficult to discern the quality of evidence supporting an implementation strategy and whether strategies should be recommended to practitioners to support the implementation of EBIs.
Existing criteria, like GRADE, may serve as a valuable starting point for building criteria applicable to the field of implementation research [5]. Effectiveness research and associated evaluation criteria, which heavily emphasizes internal validity, considers the highest quality evidence to be from research designs like double-blind randomized control trials. In implementation research, internal validity tends to be more balanced with external validity so that the results are generalizable to target communities. With external validity in mind, implementation research is typically conducted in practice settings and involves assessment of the organizations and providers who will be impacted by the implementation strategy and subsequently the intervention under consideration. As a result, it is often inappropriate, impractical, and/or undesirable to leverage research designs like randomized controlled trials, because it is not possible to blind practitioners to the strategy and/or intervention or randomize at the unit of analysis [6,7,8]. These realities make direct application of intervention-level criteria inappropriate—necessitating criteria specific to the field [3].
HIV and implementation research in the US
We describe our efforts to develop a set of criteria and evaluation process for implementation strategies to address the HIV epidemic in the United States. Improvements in the US HIV epidemic have been modest over the last two decades, with disparities among communities disproportionally affected by HIV increasing [9]. In an attempt to address HIV incidence, the Centers for Disease Control and Prevention have curated a repository of EBIs to support HIV prevention since the early 2000s and supported dissemination and implementation of a subset of these [10]. Furthermore, major biomedical advancements, such as pre-exposure prophylaxis (PrEP), have proven to be very effective at preventing HIV. Yet many of these interventions have not been widely implemented with equity to yield their intended benefit. Only an estimated 30% of individuals who would benefit from PrEP receive it, with growing disparities by race, gender, income, citizenship status, and intersectional marginalization [11,12,13,14]. Uptake and adherence remain suboptimal along the HIV care continuum (i.e., prevention, testing, diagnosis, linkage-to-care, and treatment), indicating, in part, failed implementation and opportunities to develop evidence-informed implementation strategies [11]. In 2019, the Ending the HIV Epidemic (EHE) Initiative was launched as a coordinated effort among several federal agencies to address HIV-related implementation problems. In alignment with EHE, the National Institutes of Health supported a number of mechanisms and projects to conduct research on implementation strategies [15]. With the growing mass of HIV-related implementation research has come an equally growing knowledge base of implementation strategies targeting multiple aspects of the HIV care continuum, in a wide scope of settings, evaluating various implementation outcomes [16].
In an effort to create, synthesize, and disseminate generalizable knowledge, the Implementation Science Coordination Initiative (ISCI) was funded by the National Institutes of Health to provide technical assistance in implementation research funded by the EHE Initiative, coordinate research efforts, synthesize literature through systematic reviews, develop tools to assist researchers, and disseminate research findings to researchers, policymakers, providers, and more [17, 18]. As part of this effort, we developed a tool to evaluate the quality of evidence of HIV-related implementation strategies to identify best-practice strategies that can promote effective implementation and uptake of EBIs. The long-term goal of this particular project is to accumulate, warehouse, and disseminate a collection of effective strategies that can be used by HIV practitioners nationwide to support the EHE Initiative.
Methods
Overview
We conducted the project in three phases: 1) a literature review in tandem with key informant interviews to generate initial criteria for our tool, 2) a modified Delphi to evaluate and revise our initial tool and criteria; 3) a pilot application of our rubric to a set of implementation research studies. Delphi data were collected from March 2022 to June 2023. Piloting occurred in the fall of 2023. Our data collection protocol was reviewed by the Institutional Review Board at Northwestern University and determined to be non-human subjects research. All data collection instruments have been included as a supplemental file (Supplemental File A), and data are available in a de-identified format from the first author on reasonable request. Methods and results are reported according to STROBE reporting guidelines (Supplemental File B).
Key informant interviews and literature review
We first conducted a review of the scientific and grey literature of existing compilations of criteria for assessing EBIs. Google scholar was used to search for tools or criteria published in academic journals. To identify tools within the grey literature, we focused on federal institutions that frequently provide evidence-recommendations such as the US Preventative Task Force, the Centers for Disease Control and Prevention, and Health Services and Resources Administration. We utilized this literature to identify commonalities across tools, to review current debate on the philosophy of science as it relates specifically to implementation science, and to construct an interview guide for key informant experts with questions to elicit information about key differences between implementation research and existing tools. We also used the literature to identify experts who we then recruited for key informant interviews and our Delphi.
We recruited and interviewed a range of experts, including implementation scientists, HIV providers and implementers, representatives from related fields of public health research (e.g., quality improvement), and public health agency officials. All interviews were scheduled in the Spring of 2022, were approximately 30–45 min long, and were conducted by either VM or az. Briefly, the three main questions were: 1. Do you think existing criteria apply to implementation research studies? 2. What are essential indications of generalizability in implementation research? 3. What are ways to evaluate strategies with multiple components? Each question included follow up probes. Interviews were recorded and transcribed via Zoom. Participants were not given an incentive for participation. Two Ph.D.-level researchers with expertise in qualitative and mixed methods research performed an inductive, thematic process of analysis to explore patterns and categorize responses. Based on their responses, we iteratively developed a preliminary tool and criteria.
Modified Delphi
Identification and recruitment of Delphi participants
We conducted an asynchronous, modified eDelphi with participants of similar expertise as our key informants in two rounds. Participants were recruited using snowball recommendations from those that were interviewed as key informants. Our eligibility criteria included fluent English speakers and those working in either HIV services research or those working in implementation research but in another field that may intersect with HIV, for example, mental health, substance misuse, social services, primary care, or women’s health. If participants were unable to complete the survey, an alternative contact could be recommended. After this first invitation, we sent semiweekly reminder emails for six weeks. A $10 gift card was given to participants for completing the first survey, and a $50 gift card was given to participants for completing the second survey.
Data collection and measures
The surveys were implemented using Qualtrics. The surveys were piloted with members of the ISCI research team to ensure question clarity. Each survey took participants approximately 45–75 min to complete.
First-round Delphi instrument
This survey consisted of 71 items. Participants were first introduced to the purpose of the project at large, which was to create a tool and set of criteria on which to evaluate HIV-related implementation science, and then to the specific goals of the Delphi, which was to generate consensus about which aspects of the tool were most important and least important and whether we had included all the elements that participants felt were necessary. The first portion of the survey gathered demographic and basic information about the participant (e.g., age, race, ethnicity, gender), characteristics of the participant’s work (e.g., I work primarily in… select all areas that apply”), as well as the participant’s experience in implementation research (e.g., How would you describe your knowledge level of implementation science?).
The second portion of the survey evaluated proposed domains for the tool (Overall Evidence of Effectiveness, Study Design Quality, Implementation Outcomes, Equity Impact, Strategy Specification, and Bundled Strategies) and corresponding criteria. Participants were asked to agree or disagree (Yes/No) with the adding/dropping/combining of domains; this was followed by an open-ended question asking why they agreed to said addition/dropping/combining (if applicable). This portion also contained two 5-point Likert-type scales asking participants to rank the domains in order from most important to least important. The third portion of the survey was aimed at gaining the participant’s opinion on the specific criteria (e.g., effect size and effect direction for implementation outcomes) within each domain. For each domain, the participant was asked if there were any criteria that needed to be added/dropped (Yes/No), followed by an open-ended question asking why they would like these items added/dropped (if applicable). The participant was then provided a 5-point Likert scale in which they ranked each item from “Very unimportant” to “Very important”. These questions were repeated for all criteria in all domains.
The final portion of the survey introduced the Levels of Evidence (Best Practice Strategy, Promising Strategy, Emerging Strategy, Undetermined Strategies, and Not Recommended Strategy) and their definitions. The participant was asked if there should be any adding/dropping/combining of the evidence levels (Yes/No), followed by an open-ended question asking why they would like these evidence levels to be added/dropped/combined (if applicable).
Second-round Delphi instrument
This survey consisted of 52 items. All participants from Round 1 were recruited for Round 2. Again, participants were reminded of the overall purpose of the project and the specific goal of the Delphi, which was to confirm changes to the tool made in response to the results of Round 1 and receive feedback. The first portion of the survey gathered the same demographic and basic information as in the first round. The second portion consisted of an overview of the updated tool, including definitions of the domains, criteria, and levels of evidence, and asked for feedback on changes made from the Round 1 results. For example, in the first round of the Delphi survey, participants responded that they would like for greater specificity within the criteria of the Study Design domain. As a response, we split this domain into two domains for Round 2: “Study Design” and “Study Rigor and Limitations.” We presented this change to the participant and asked them to agree or disagree with this change (Yes/No); if “No” was selected, this prompted an open-response question asking for further explanation. Lastly, we asked respondents to apply the criteria and give an evidence-level rating to a set of fictional cases of implementation research studies, allowing respondents to comment on the application and rating process.
Data analysis and management
Quantitative data were managed and analyzed in Excel. Quantitative data were analyzed descriptively, primarily as percent agreement or disagreement for domains, evidence levels, and individual criteria within domains. Qualitative data were analyzed in Dedoose software and Excel, using a rapid direct qualitative content analysis approach [19]. Qualitative data were analyzed by a Ph.D.-level researcher with qualitative research expertise and were intended to confirm or complement quantitative analyses.
Pilot and application to PrEP implementation strategies
To ensure a high-quality process for reviewing literature and consistent application of criteria across the different evidence levels, we piloted and refined the tool with a set of implementation strategies designed to promote the uptake of evidence-based HIV services with members of ISCI, which consists of a mix of faculty, staff, and students holding degrees at the Bachelors, Masters, and PhD levels. VRM led two, hour-long trainings with, four Ph.D.-level members of the ISCI team who were also engaged in systematic reviews of HIV literature on how to apply the criteria. ISCI team members then applied the criteria to an existing set of eight papers reporting on implementation strategies designed to promote PrEP uptake coding a rating for each criteria and domain. Studies were selected by VRM to represent the full range of evidence ratings and different points of the HIV care continuum (i.e., PrEP delivery, HIV testing, and retention in care for HIV treatment). We calculated agreement as a simple percentage of identical ratings between two coders out of the total number of criteria, domain ratings, and overall rating (40 items). In places where there was high disagreement, the tool was revised and refined to provide better guidance and instruction on how to apply specific criteria. In a final application of the tool, two coders, a Master’s and Ph.D. level member of the ISCI team, applied the criteria to an additional set of 18 implementation strategies designed to improve PrEP uptake and use identified through an existing systematic review [20] after a single hour-long training.
Results
We report the primary results from each stage of our process as well as significant changes to the tool made at each stage.
Literature review and key informant interviews
Our initial literature review yielded several existing rubrics, tools, criteria and processes for evaluating evidence supporting a specific intervention identified primarily in the grey literature developed by large institutions responsible for disseminating evidence-based interventions [5, 21]. Many had a similar structure of grouping criteria by domain (e.g., aspects of the research design or strength of the outcomes) and having different evidence ratings or levels (e.g., low, medium, high evidence strength). For example, the Centers for Disease Control and Prevention National Center for Injury Prevention Guide to the Continuum of Evidence of Effectiveness outlines six domains (i.e., effect, internal validity, type of evidence/research design, independent replication, implementation guidance, external and ecological validity) and seven evidence levels (harmful, unsupported, undetermined, emerging, promising direction, supported, and well supported) while the US Preventative Task Force has six domains or “factors” considered when generating an evidence level and five evidence levels or “grades” [21,22,23]. Our literature review also yielded several articles relevant to the generalization of evidence generated from implementation trials. For example, key considerations on whether effects are likely to be transferable from one context to another, balancing internal and external validity, and the need to consider equity in impact [21, 24,25,26].
We conducted a total of 10 interviews representing a mix of expertise in HIV services research, implementation research, and quality improvement research. Informants reflected on different potential domains (e.g., elements of the research design) and listed specific ways that they felt research and evidence quality differed in implementation research from clinical trials. Among factors highlighted were a need to consider the context and specification of strategies, criteria specific to implementation outcomes, and consideration of the equity impact of implementation strategies on the health outcome under consideration. Again, existing implementation science literature helped support and define domains like Proctor's recommendations for strategy specification to ensure that strategies are appropriately described as well as Proctor's implementation outcomes to define and describe implementation outcomes [1, 48].
Based on these collective results, conceptually, we modeled our initial tool by grouping criteria according to domain and having a series of evidence levels similar to many tools and criteria that we reviewed. We also worked to integrate current thinking and perspectives on implementation science, evidence, and generalizability into our tool from both the literature and key informant interviews. Briefly, we structured our initial tool along six domains: overall effectiveness, study design quality, implementation outcomes, equity impact, strategy specification, and a bundled strategies domain. Each domain included a set of criteria. For example, criteria for the implementation outcomes domain included operationalization of implementation outcomes; validity and reliability of measure used; significance and direction of effect for quantitative outcomes; and reported effects as beneficial, neutral, or harmful. We also developed and defined five evidence levels with associated recommendations: best practice strategy, promising strategy, emerging strategy, undetermined strategy, non-recommended strategy. As an example, promising strategies were described as demonstrating mostly positive outcomes that may need more rigorous examination to ensure they are having the intended effect or are generalizable to a wider context. Practitioners would be recommended to take caution when using a promising strategy in practice and ensure it is having a similar outcome as demonstrated in the original research.
Modified Delphi
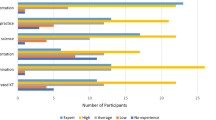
For the Delphi Round 1, we recruited from a pool of 68 experts. Two individuals responded stating their inability to participate, with one participant suggesting a replacement. Forty-one participants completed the survey, and two participants partially completed the survey for a total of 43 participants (63% response rate). For the Delphi Round 2, we recruited among the responders from Round 1 with no refusals to participate and no partial responses. Thirty participants in total completed the Round 2 survey (70% response rate). Respondent characteristics are provided in Table 1 for both Delphi Rounds. Briefly, one half of Respondents in both rounds self-identified as women (55.8%; 50% in rounds 1 and 2 respectively), with the majority white (83.7%; 80%) and not Hispanic or Latino (86%; 100%). Most respondents worked in academic settings (81.4%; 80%), with most working in HIV in round 1 but not round 2 (83.7%; 36.7% respectively). The highest number respondents had 11–20 years of experience in their area of expertise (44.2%; 43.3% respectively), and three quarters reported experience with leading implementation research projects (76.7%; 73.3%). Both complete and partially complete responses were included in subsequent analyses.
Delphi round 1
Table 2 presents the quantitative outcomes regarding whether the participant believed that domains should be added, dropped, or combined. More than half (58%) of participants thought no new domains should be added, while 44% of participants thought domains should be dropped or combined. When examining the evidence levels, 79% of individuals felt that no additional evidence levels were needed, while 47% thought one or more of the evidence levels could be dropped or combined.
Table 3 summarizes open-ended responses with example quotes for domains and evidence levels that were commented on most often. When reviewing the qualitative responses of those who indicated a domain should be added, most respondents suggested adding specific criteria or wanted greater clarity in how the domains and criteria within domains were defined. For example, regarding the equity domain, individuals desired greater clarity, operationalization, and description of how equity is being considered and evaluated. Of these, four sought greater clarity of equity-related outcomes, and six recommended inclusion of equity metrics or different ways of operationalizing equity. Three participants felt equity should be examined in combination with implementation outcomes. Three suggested greater consideration of community partnership development and inclusion of the target population in the development of the strategy or design of a study. Finally, participants recommended combining promising, emerging, and/or undetermined as levels of evidence and better specifying and operationalizing the levels.
Briefly, we revised the structure of our tool along five domains: study design, implementation outcomes, study rigor and limitations, strategy specification, and equity impact. These domains each included a revised set of criteria. For example, based on the recommended additions to the study design and rigor domain, we split this domain into two domains: 1) study design; and 2) study rigor and limitations. We considered several of the comments on dropping equity but ultimately opted to keep this domain, relax the criteria, and heavily refine the description. Other cross-cutting changes included combining the criteria for bundled strategies and strategy specification. We combined two of the evidence levels (emerging and undetermined) and revised the definitions to include: best practice, promising practice, needs more evidence, and harmful.
Delphi round 2
For the second round of the Delphi, we asked respondents to confirm major changes to the tool based on the first round of the Delphi (Table 2), and have respondents evaluate our proposed process for applying the criteria. Most respondents agreed with changes to the domains and evidence levels although there remained some commentary on the equity domain. When examining the open-ended responses among those disagreeing with the changes to the equity domain, we grouped responses into individuals that did not agree with the domain (i.e., a hard no to the revisions) and others who still had additional suggestions for the domain but approved of the domain overall (i.e., a soft no with suggested revisions; Table 3). Based on these responses, we finalized the domains and made several additional adjustments to the definitions of equity including defining which target populations can be considered in determining whether the strategy has a positive equity impact or not. Finally, we revised our process for applying the rubric based on the recommendation to apply the criteria across each domain in addition to giving an overall rating. While this did increase time in the review process, this change allowed us to still provide information on how strategies rate across all domains, enabling researchers and practitioners to compare how strategies rate on different domains or select a strategy that is strong in a specific domain, like equity supporting for example.
Pilot application to PrEP implementation strategies
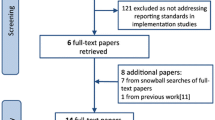
To ensure a consistent, high-quality process for applying criteria to research studies examining implementation strategies, we initially piloted the rubric with eight existing studies on implementation strategies to promote the uptake of evidence-based HIV services including PrEP, HIV testing, and retention in care [27,28,29,30,31,32,33,34]. At the conclusion, we were able to achieve 90% reliable application of the criteria, resulting in dropping some criteria and clarifying other criteria and their application. Two members of the ISCI team then applied the rubric to a set of 18 implementation strategies identified through an ongoing systematic review designed to promote uptake of PrEP in a second pilot application, achieving 98% reliability and taking approximately 15–30 min per article.
Among the 18 strategy studies, summarized in Table 4, one was assigned an overall rating as Best Practice and the remaining were assigned as Needs More Evidence. The primary domains where strategies failed to exceed the Needs More Evidence criteria were in Research Design as well as Study Rigor and Limitations. This was largely because these studies only utilized post-implementation assessment, were intended as pilot or feasibility studies, or were conducted only at a single site. Given the early state of the implementation research related to PrEP implementation in the US, we felt that this mix of ratings was relatively appropriate. While the domains that have parallels in other rating systems resulted in relatively low ratings among our studies, we observed a good mix of ratings on domains unique to our tool and implementation research (i.e., strategy specification and equity) at the Best, Promising, and Needs More Evidence levels, suggesting these domains are sufficiently discerning among the existing set of studies.
A summary of major changes to the rubric and criteria are summarized in Table 5. The final domains and evidence-levels are provided in Table 6, and a summary of the criteria by domain at each evidence level is provided in Table 7. The final tool with domains, criteria, evidence levels, and application instructions are available as a supplement (Supplemental file C).
Discussion
To our knowledge, this is the first set of criteria to evaluate evidence for implementation strategies and serve as a basis for recommendations to practitioners. Our Best Practice tool was initially informed by existing criteria and interviews, refined by a Delphi, and then piloted with implementation strategies. This process yielded a rating scale (i.e., best, promising, needs more evidence, and harmful) and domains (e.g., study design, implementation outcomes, rigor and limitations), which are common to other tools and rubrics. Yet, implementation research’s system-level focus required tailoring to our rubric for some domains, like study design and outcomes, and the development of entirely new domains, specifically strategy specification and equity. To help define the criteria for the domains, we used results from key informant interviews and existing implementation science literature to help ensure appropriateness for the field [1, 6, 48]. As a specific example of tailoring, we have outlined criteria for the research design domain that considers the realities of where implementation research is conducted and does not require blinding or randomization for strategies to be considered the highest rating. While these helped provide structure and specific criteria at each of the evidence levels, in conducting the pilot we noted missing information which sometimes made it difficult to evaluate the research. We recommend using Standards for Reporting Implementation Studies (StaRI) guidelines as well as Proctor’s recommendations for strategy specification when reporting implementation research to help ensure the needed details to evaluate the research are reported and available for potential practitioners to understand what resources and efforts are needed for implementation strategies [1, 49].
In addition to being a new resource for implementation science, to our knowledge this is also the first evidence rating criteria that considers the potential to improve equity in a health issue. Because implementation science directly impacts communities with the potential to improve or exacerbate inequities, HIV included, experts reiterated that equity was a critical domain to include. However, our work among participants, who primarily identified as white and non-Latin, demonstrates a lack of consensus in the implementation science field about what equity in implementation science means. We also encourage continued discussion within the implementation science community that includes diverse perspectives to help foster consensus and bring additional attention to this problem.
For the Best Practices Tool, the criteria within the Equity domain emphasizes community engagement in the research process, a research focus on populations experiencing inequities, as well as equity in outcomes for the Best Practice evidence level rating. as a means to These criteria encourage attention to and improvement in HIV-related inequities as many in the field have advocated [50,51,52] with additional, more relaxed criteria for lower evidence ratings. However, we recognize that no single implementation strategy (or intervention) is going to adequately address the deeply rooted structural determinants, like racism and homophobia, which keep inequities entrenched. Implementers who are interested in utilizing strategies may wish to consider additional factors that are relevant to their specific contexts, like whether communities they serve are reflected in the strategies they are considering or whether the strategy responds to the determinants driving inequity in their context. However, it is our hope that by including equity improvement as criteria to be considered the highest quality research, we can bring additional attention to and encourage equity in HIV outcomes in the US.
Our tool and criteria are designed to discern among studies for which there is best evidence specific to HIV implementation strategies in the US, which is a rapidly growing field, rather than having an absolute threshold of effectiveness that studies must meet. There are other heath areas, such as cancer and global HIV implementation research, for which there are more studies leveraging more rigorous research designs to evaluate implementation strategies [53, 54]. If applied in these areas, it may be more appropriate to have more stringent criteria to adequately discern among studies for which there is relatively good evidence compared to those which would need additional study. We encourage others who may consider using this tool in their area of implementation science to consider adapting the specific criteria within each of the domains and at each of the evidence-levels to ensure that it appropriately discerns among available studies before routine application. Continuing with the example of more rigorous research designs, it may be appropriate to require better replication of results or more diverse settings than we have incorporated into our specific criteria. However, we would suggest that the overall structure of the tool, specifically the domains and recommendation levels could remain the same regardless of the health field. Conversely, we received many suggestions for more stringent criteria that participants felt like should be included that we were not able to include because it would have resulted in few-to-no strategies identified as best practice. US focused HIV implementation science is still in its adolescence, with many pilots and full-fledged trials underway but not yet published. It is our hope that in the future, we will be able to include more stringent criteria within the rubric so that the needed evidence quality improves over time within HIV implementation research.
There are some notable limitations to the processes used to develop the Best Practice Tool and the criteria themselves. We used a modified eDelphi approach to develop the rubric and criteria with some loss to follow up from the first to the second round of the Delphi, particularly among HIV service providers which may mean the results are not sufficiently representative of this context. However, we did retain many individuals working in settings where HIV services intersect, like substance misuse, mental health, and social services. Our use of a modified Delphi method did not result in consensus, but instead resulted in an approximation of consensus. In addition, we were not able to elicit the opinions about the appropriateness of the tool from the perspective of front-line HIV service implementers on balance with those of the research community. We hope to address this in future iterations of this work.
We envision several future directions for this tool with implications for both researchers and practitioners that will advance the goals of ISCI and support the EHE Initiative. Systematic reviews of HIV-related implementation strategies are currently underway through ISCI [55]. The next phase will entail applying these criteria to implementation strategies identified through these reviews and developing a compendium of strategies. We recognize that a rating and recommendation is not sufficient to support uptake, and we also have a complementary dissemination effort underway to provide the needed information and materials for wide adoption and scale up which will be available on the ISCI website [18]. Our criteria and rating system will also yield benefits for researchers conducting HIV implementation research. Through our efforts, we will also identify strategies that hold promise but would benefit from additional research and additional evidence supporting their effectiveness. Researchers can also use these criteria in designing studies of new strategies so that they can score better on these criteria.
Conclusion
For practitioners to fully benefit from research developing and testing implementation strategies targeting HIV services, clear evaluation criteria and recommendations are needed to assess which strategies are the most likely to have benefit and impact. We developed domains and criteria appropriate to evaluate evidence quality in HIV-related implementation strategies. This rubric includes recommendations for practitioners about strategies for which there is best evidence and recommendations for research about strategies for which more evidence is needed. Establishing criteria to evaluate implementation strategies advances implementation science by filling a much-needed gap in HIV implementation research which can be extended to other areas of implementation science.
Availability of data and materials
The Delphi dataset generated during the current study available from the corresponding author on reasonable request.
References
Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8(1):139.
Powell BJ, Fernandez ME, Williams NJ, Aarons GA, Beidas RS, Lewis CC, et al. Enhancing the impact of implementation strategies in healthcare: a research agenda. Front Public Health. 2019;7:3.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Sawaya GF, Guirguis-Blake J, LeFevre M, Harris R, Petitti D. for the U.S. Preventive services task force. Update on the methods of the U.S. Preventive services task force: estimating certainty and magnitude of net benefit. Ann Intern Med. 2007;147(12):871.
Terracciano L, Brozek J, Compalati E, Schünemann H. GRADE system: new paradigm. Current opinion in allergy and clinical immunology. 2010;10(4):377–83.
Kilbourne A, Chinman M, Rogal S, Almirall D. Adaptive designs in implementation science and practice: their promise and the need for greater understanding and improved communication. Annu Rev Public Health. 2024;45(1):69.
Lamont T, Barber N, de Pury J, Fulop N, Garfield-Birkbeck S, Lilford R, et al. New approaches to evaluating complex health and care systems. BMJ. 2016;352:i154.
Schliep ME, Alonzo CN, Morris MA. Beyond RCTs: Innovations in research design and methods to advance implementation science. Evid-Based Commun Assess Interv. 2017;11(3–4):82–98.
CDC. The State of the HIV Epidemic in the U.S. | Fact Sheets | Newsroom. 2022. https://www.cdc.gov/nchhstp/newsroom/fact-sheets/hiv/state-of-the-hiv-epidemic-factsheet.html. Accessed 10 Oct 2022.
CDC. Compendium | Intervention Research | Research | HIV. 2022. https://www.cdc.gov/hiv/research/interventionresearch/compendium/index.html. Accessed 10 Oct 2023.
CDC. Volume 28 Number 4| HIV Surveillance | Reports | Resource Library | HIV/AIDS. 2023. https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-4/index.html. Accessed 30 Nov 2023.
Zamantakis A, Li DH, Benbow N, Smith JD, Mustanski B. Determinants of Pre-exposure Prophylaxis (PrEP) implementation in transgender populations: a qualitative scoping review. AIDS Behav. 2023;27(5):1600–18.
Brooks RA, Landrian A, Lazalde G, Galvan FH, Liu H, Chen YT. Predictors of awareness, accessibility and acceptability of Pre-exposure Prophylaxis (PrEP) among English- and Spanish-speaking Latino men who have sex with Men in Los Angeles California. J Immigr Minor Health. 2020;22(4):708–16.
Namara D, Xie H, Miller D, Veloso D, McFarland W. Awareness and uptake of pre-exposure prophylaxis for HIV among low-income, HIV-negative heterosexuals in San Francisco. Int J STD AIDS. 2021;32(8):704–9.
Glenshaw MT, Gaist P, Wilson A, Cregg RC, Holtz TH, Goodenow MM. Role of NIH in the ending the HIV epidemic in the US initiative: research improving practice. JAIDS J Acquir Immune Defic Syndr. 2022;90(S1):S9.
Queiroz A, Mongrella M, Keiser B, Li DH, Benbow N, Mustanski B. Profile of the portfolio of NIH-Funded HIV implementation research projects to inform ending the HIV epidemic strategies. JAIDS J Acquir Immune Defic Syndr. 2022;90(S1):S23.
Mustanski B, Smith JD, Keiser B, Li DH, Benbow N. Supporting the growth of domestic HIV implementation research in the United States through coordination, consultation, and collaboration: how we got here and where we are headed. JAIDS J Acquir Immune Defic Syndr. 2022;90(S1):S1.
HIV Implementation Science Coordination Initiative. https://hivimpsci.northwestern.edu/. Accessed 21 Oct 2023.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Merle JL, Benbow N, Li DH, Zapata JP, Queiroz A, Zamantakis A, McKay V, Keiser B, Villamar JA, Mustanski B, Smith JD. Improving Delivery and Use of HIV Pre-Exposure Prophylaxis in the US: A Systematic Review of Implementation Strategies and Adjunctive Interventions. AIDS Behav. 2024;28(7):2321–39.
Frieden TR, Degutis LC, Mercy JA, Puddy RW, Wilkins N. Understanding Evidence. https://www.cdc.gov/violenceprevention/pdf/understanding_evidence-a.pdf. Accessed 30 Nov 2023.
United States Preventive Services Taskforce. Methods and Processes. https://www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/procedure-manual/procedure-manual-section-6-methods-arriving-recommendatio. Accessed 24 April 2024.
United States Preventive Services Taskforce. Grade Definitions. https://www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/grade-definitions. Accessed 24 April 2024.
Schloemer T, Schröder-Bäck P. Criteria for evaluating transferability of health interventions: a systematic review and thematic synthesis. Implement Sci. 2018;13(1):88.
Geng EH, Peiris D, Kruk ME. Implementation science: Relevance in the real world without sacrificing rigor. Plos Med. 2017;14(4):e1002288.
Lifsey S, Cash A, Anthony J, Mathis S, Silva S. Building the evidence base for population-level interventions: barriers and opportunities. Health Educ Behav Off Publ Soc Public Health Educ. 2015;42(1 Suppl):133S-140S.
Burns PA, Omondi AA, Monger M, Ward L, Washington R, Sims Gomillia CE, et al. Meet me where i am: an evaluation of an HIV patient navigation intervention to increase uptake of PrEP among black men who have sex with men in the deep south. J Racial Ethn Health Disparities. 2022;9(1):103–16.
Clement ME, Johnston BE, Eagle C, Taylor D, Rosengren AL, Goldstein BA, et al. Advancing the HIV pre-exposure prophylaxis continuum: a collaboration between a public health department and a federally qualified health center in the Southern United States. AIDS Patient Care STDs. 2019;33(8):366–71.
Brant AR, Dhillon P, Hull S, Coleman M, Ye PP, Lotke PS, et al. Integrating HIV pre-exposure prophylaxis into family planning care: a RE-AIM framework evaluation. AIDS Patient Care STDs. 2020;34(6):259–66.
Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. Plos One. 2014;9(10):e108742.
Cunningham WE, Ford CL, Kinsler JJ, Seiden D, Andrews L, Nakazono T, et al. Effects of a laboratory health information exchange intervention on antiretroviral therapy use, viral suppression and racial/ethnic disparities. J Acquir Immune Defic Syndr 1999. 2017;75(3):290–8.
Havens JP, Scarsi KK, Sayles H, Klepser DG, Swindells S, Bares SH. Acceptability and feasibility of a pharmacist-led human immunodeficiency virus pre-exposure prophylaxis program in the Midwestern United States. Open Forum Infect Dis. 2019;6(10):ofz365.
Horack CL, Newton SL, Vos M, Wolfe BA, Whitaker A. Pre-exposure prophylaxis in a reproductive health setting: a quality improvement project. Health Promot Pract. 2020;21(5):687–9.
Ezeanolue EE, Obiefune MC, Ezeanolue CO, Ehiri JE, Osuji A, Ogidi AG, et al. Effect of a congregation-based intervention on uptake of HIV testing and linkage to care in pregnant women in Nigeria (Baby Shower): a cluster randomised trial. Lancet Glob Health. 2015;3(11):e692-700.
Buchbinder SP, Havlir DV. Getting to zero San Francisco: a collective impact approach. J Acquir Immune Defic Syndr. 2019;82 Suppl 3(Suppl 3):S176-82.
Bunting SR, Saqueton R, Batteson TJ. A guide for designing student-led, interprofessional community education initiatives about HIV risk and pre-exposure prophylaxis. MedEdPORTAL J Teach Learn Resour. 2019;18(15):10818.
Bunting SR, Saqueton R, Batteson TJ. Using a student-led, community-specific training module to increase PrEP uptake amongst at-risk populations: results from an exploratory pilot implementation. AIDS Care. 2020;32(5):546–50.
Coleman M, Hodges A, Henn S, Lambert CC. Integrated pharmacy and PrEP navigation services to support PrEP uptake: a quality improvement project. J Assoc Nurses AIDS Care JANAC. 2020;31(6):685–92.
Gregg E, Linn C, Nace E, Gelberg L, Cowan B, Fulcher JA. Implementation of HIV preexposure prophylaxis in a homeless primary care setting at the Veterans affairs. J Prim Care Community Health. 2020;11:2150132720908370.
Hoth AB, Shafer C, Dillon DB, Mayer R, Walton G, Ohl ME. Iowa TelePrEP: a public-health-partnered telehealth model for human immunodeficiency virus preexposure prophylaxis delivery in a Rural State. Sex Transm Dis. 2019;46(8):507–12.
Khosropour CM, Backus KV, Means AR, Beauchamps L, Johnson K, Golden MR, et al. A pharmacist-led, same-day, HIV pre-exposure prophylaxis initiation program to increase PrEP uptake and decrease time to PrEP initiation. AIDS Patient Care STDs. 2020;34(1):1–6.
Lopez MI, Cocohoba J, Cohen SE, Trainor N, Levy MM, Dong BJ. Implementation of pre-exposure prophylaxis at a community pharmacy through a collaborative practice agreement with San Francisco Department of Public Health. J Am Pharm Assoc JAPhA. 2020;60(1):138–44.
Pathela P, Jamison K, Blank S, Daskalakis D, Hedberg T, Borges C. The HIV Pre-exposure Prophylaxis (PrEP) Cascade at NYC sexual health clinics: navigation is the key to uptake. J Acquir Immune Defic Syndr 1999. 2020;83(4):357–64.
Roth AM, Tran NK, Felsher M, Gadegbeku AB, Piecara B, Fox R, et al. Integrating HIV preexposure prophylaxis with community-based syringe services for women who inject drugs: results from the project SHE demonstration study. J Acquir Immune Defic Syndr. 2021;86(3):e61-70.
Saberi P, Berrean B, Thomas S, Gandhi M, Scott H. A simple Pre-Exposure Prophylaxis (PrEP) optimization intervention for health care providers prescribing PrEP: pilot study. JMIR Form Res. 2018;2(1):e2.
Tung EL, Thomas A, Eichner A, Shalit P. Implementation of a community pharmacy-based pre-exposure prophylaxis service: a novel model for pre-exposure prophylaxis care. Sex Health. 2018;15(6):556–61.
Wood BR, Mann MS, Martinez-Paz N, Unruh KT, Annese M, Spach DH, et al. Project ECHO: telementoring to educate and support prescribing of HIV pre-exposure prophylaxis by community medical providers. Sex Health. 2018;15(6):601–5.
Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76.
Pinnock H, Barwick M, Carpenter CR, Eldridge S, Grandes G, Griffiths CJ, Rycroft-Malone J, Meissner P, Murray E, Patel A, Sheikh A. Standards for reporting implementation studies (StaRI) statement. bmj. 2017; 356.
Brownson RC, Kumanyika SK, Kreuter MW, Haire-Joshu D. Implementation science should give higher priority to health equity. Implement Sci. 2021;16(1):28.
Shelton RC, Adsul P, Oh A, Moise N, Griffith DM. Application of an antiracism lens in the field of implementation science (IS): Recommendations for reframing implementation research with a focus on justice and racial equity. Implement Res Pract. 2021;1(2):26334895211049480.
Baumann AA, Shelton RC, Kumanyika S, Haire‐Joshu D. Advancing healthcare equity through dissemination and implementation science. Health services research. 2023;58:327–44. Accessed 19 Feb 2024.
Neta G, Sanchez MA, Chambers DA, Phillips SM, Leyva B, Cynkin L, et al. Implementation science in cancer prevention and control: a decade of grant funding by the National Cancer Institute and future directions. Implement Sci. 2015;10(1):4.
Hwang S, Birken SA, Melvin CL, Rohweder CL, Smith JD. Designs and methods for implementation research: advancing the mission of the CTSA program. J Clin Transl Sci. 2020;4(3):159–67.
Merle JL, Li D, Keiser B, Zamantakis A, Queiroz A, Gallo CG, et al. Categorising implementation determinants and strategies within the US HIV implementation literature: a systematic review protocol. BMJ Open. 2023;13(3):e070216.
Acknowledgements
We would like to acknowledge members of the ISCI leadership team and Melissa Mongrella who developed the survey instruments within REDCap.
Funding
This work was supported by an Ending the HIV Epidemic supplement to the Third Coast Center for AIDS Research, an NIH funded center (P30 AI117943). Author az’s time was supported by a training grant from the NIMH (T32MH30325). Author JLM’s time was supported by a post-doctoral training grant from the National Library of Medicine (2 T15 LM 007124–26).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization of this project and manuscript. VM and AMP were responsible for drafts on Delphi sections of this manuscript. Az was responsible for qualitative portions of the manuscript. BM, NB, DL, JM, and JS supported research conceptualization and research design for the project. LH, MS, and MP were responsible for drafting the introduction and discussion portions of the manuscript. All authors reviewed, revised, and provided feedback on later drafts of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocols and data collection were determined to be non-human subjects research by Northwestern University’s Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
McKay, V.R., Zamantakis, A., Pachicano, A.M. et al. Establishing evidence criteria for implementation strategies in the US: a Delphi study for HIV services. Implementation Sci 19, 50 (2024). https://doi.org/10.1186/s13012-024-01379-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13012-024-01379-3