Abstract
Background
The Atherogenic Index of Plasma is a predictive biomarker of atherosclerosis in adults but there is a lack of studies in paediatric population aimed at evaluating the longitudinal changes of the AIP and of the cardiometabolic blood profile related to nutritional interventions. The aim of this study was to compare the effect of individual- versus collective-based nutritional-lifestyle intervention on the Atherogenic Index of Plasma in schoolchildren with obesity.
Methods
One-hundred sixty-four children aged 6–12 years with Body Mass Index z-score > 2 referred to the Paediatric Obesity Clinic, San Paolo Hospital, Milan, Italy, were consecutively enrolled and randomized to undergo to either an individual- (n = 82) or a collective- (n = 82) based intervention promoting a balanced normo-caloric diet and physical activity. In addition, the individual intervention included a tailored personalized nutritional advice and education based on the revised Coventry, Aberdeen, and London-Refined taxonomy. Both at baseline and after 12 months of intervention, dietary habits and anthropometric measures were assessed, a fasting blood sample were taken for biochemistry analysis.
Results
The participation rate at 12 months was 93.3% (n = 153 patients), 76 children in the individual-intervention and 77 children in the collective intervention. At univariate analysis, mean longitudinal change in Atherogenic Index of Plasma was greater in the individual than collective intervention (− 0.12 vs. − 0.05), as well as change in triglyceride-glucose index (− 0.22 vs. − 0.08) and Body Mass Index z-score (− 0.59 vs. − 0.37). At multiple analysis, only change in Body Mass Index z-score remained independently associated with intervention (odds ratio 3.37).
Conclusion
In children with obesity, an individual-based nutritional and lifestyle intervention, including techniques from the CALO-RE taxonomy, could have an additional beneficial effect over a collective-based intervention, although the actual size of the effect remains to be clarified.
Trial Registration Clinical Trials NCT03728621
Similar content being viewed by others
Introduction
Obesity rates have risen rapidly worldwide over the past few decades [1, 2] and obesity is currently one of the most serious global public health burdens [3]. Children with obesity may exhibit metabolic profile derangement [4] and an increased cardiovascular risk [5] lasting possibly even in adulthood [4]. Obesity-related atherogenic dyslipidaemia is an adverse risk factor for cardiovascular disease in children [6] that may be associated with structural and functional vascular changes [7], thus increasing the risk of early detrimental health outcomes [7] and later cardiovascular events [8]. The Atherogenic Index of Plasma (AIP) is a major indicator of the size of pre- and anti-atherogenic lipoprotein particles. It is today considered both a predictive biomarker of atherosclerosis risk in adults [9, 10] and a better indicator than others lipid variables in assessing cardiovascular risk [11, 12], particularly associated with obesity [13].
In both overweight and obese children, nutritional interventions are a crucial step recommended by the international guidelines to profitably manage obesity [4, 14] also because those could result in improvement in the status of obesity [15,16,17] and cardiometabolic blood profile [15, 18]. Despite the potential clinical and social relevance of nutritional interventions, there is paucity of studies in the paediatric population evaluating their effectiveness in outpatients [19, 20]. Moreover, although Hayes et al. [19] suggested that interventions including an individual approach instead of collective nutritional advice may result in better improvement of body weight, there is a lack of randomized trials investigating this hypothesis and any effect on metabolic blood profile.
The primary aim of this study was to evaluate the effect of an individual-based nutritional-lifestyle intervention, compared to a collective one, on the atherogenic plasma index in schoolchildren with obesity. Secondarily, the effect on Body Mass Index (BMI) was investigated.
Material and methods
Settings and participants
This was a longitudinal parallel randomized open trial with an allocation ratio 1:1. Eligibility criteria for participants were as follows. Inclusion criteria: BMI z-score > 2, based on the WHO references [21], age 6 – 12 years, gestational age 37–42 weeks, body weight at birth ≥ 2500 g and < 4000 g, single birth, white parents, family living in Milan or neighbourhood (≤ 30 km). Exclusion criteria: child having syndromic, organic and/or hormonal conditions besides obesity, child on a diet and/or medication that could affect body weight, child needing hospital admission. The data were consecutively collected among children whom the family paediatrician had first addressed for suspected obesity to the Paediatric Obesity Clinic, Department of Paediatrics, San Paolo Hospital, Milan, Italy. At the first ambulatorial general visit, the eligibility criteria were assessed, anamnestic data were recorded, and general routine instructions and recommendations were given to parents and children in accordance with the standard Good Clinical Practice guidelines. The parents were asked if they would be willing to make their child participate in a trial based on specific nutritional and lifestyle interventions, scheduled to start within six months. The parents or legal guardians received a detailed explanation about the aim and design of the study and were requested to sign a consent form. The trial was registered at clinicaltrials.gov (Trial number NCT03728621). Baseline trial data were collected one day before starting intervention, when eligibility criteria were reassessed, and 12 months later, and included anthropometric measurements, dietary habits, physical activity and biochemical evaluations.
Interventions
The study protocol planned to administer the participants to either an individual- or a collective-based intervention promoting a normo-caloric diet and physical activity. Both in individual and collective intervention, general dietary recommendations were balanced and age- and sex-adjusted in accordance with the national guidelines for treatment of childhood obesity [22] and the Italian Society of Human Nutrition [23], that are Daily Energy Intake (En) 1372–2499 kcal (depending on age and sex); protein: 0.94–0.97 g/kg/die; carbohydrates: 45%-60% En, sugar < 15% En; fats: 20%-35% En (of which saturated fatty acids < 10% En, polyunsaturated fatty acids 5–10% En); fiber: 8.4 g/1000 kcal [22, 23]. Any child was further requested to engage in at least 60 min of moderate to vigorous daily physical activity (MVPA) [24]. The children and their parents were trained in the hospital during an educational two-hours session held by the same expert paediatrician assisted by a nutritionist. The training course was organized to be held in a class of 4 children (collective intervention) or individually (individual intervention), and instructed them and parents about regulation of energy expenditure, body composition, physical activity, consequences of sedentary lifestyle, principles of nutrition, food sources, glycaemic index and glucose metabolism. In the individual intervention, recommendations were refined and personalized on the child's preferences regarding food and lifestyle, and a series of supplementary behaviour modification techniques were recommended in accordance to the Coventry, Aberdeen and London-Refined (CALO-RE) taxonomy (items 1, 2, 5, 6, 8, 16, 21, and 26) [25]. Written guidelines were given to the parents, including general nutritional advice, food choice lists, recommended average servings for principal food categories, according to Italian Dietary Reference Values [23]. General nutritional advices included increasing fruit and vegetables (> 400 g/die, or five portions [26]), legume and fish intakes while decreasing meat consumption, avoiding sugar-sweetened beverages and limiting sweets and introducing more whole grain food, also according to the principles of the Mediterranean diet [27]. An illustrated brochure explaining potential benefits of daily physical activity and a diary for daily recording physical activity of the child in terms of type, frequency, duration and intensity were given to parents. The paediatrician invited parents to contact the hospital staff throughout the intervention period when needed or desired, in any case whenever any adverse event occurred. The educational session was repeated 6 months after starting the intervention. Compliance with nutritional intervention was estimated at 12 months by the rate of daily dietary energy intake within the recommended range, and compliance with physical activity by the rate of MPVA longer than 60 min.
Anthropometry
The body weight and height of any child were measured using a mechanical column scale (SECA 711; SECA GmbH & KG, Hamburg, Germany) with an integrated measuring rod (SECA 220; SECA GmbH & KG). Body Mass Index (BMI) and related z-scores were calculated from the ratio of weight to height squared (kg/m2) using the WHO reference growth charts [21]. Puberty stage was determined.
Dietary habits
Dietary habits of children were assessed by a validated Italian language food frequency questionnaire (FFQ) developed on the original Block FFQ [28], and then updated in 2008 on the basis of the full-length Block 2005 FFQ© (NutritionQuest, Berkeley, CA, USA) and the 2007 new national food composition tables [29]. Parents completed the FFQ during an interview of approximately 50 min, conducted by the same nutritionist. Each meal was analysed to find out which food was eaten and how often. Usual portion sizes were estimated using household measures and the weight (e.g., pasta) or unit (e.g. fruit juice) of the purchase. A 24-h recall was additionally recorded at the end of the interview to standardize the usual serving size. Quantification and analysis of the energy intake and nutrient composition were performed with an ad hoc PC software developed by a consultant.
Biochemistry
Fasting blood samples were taken at 8.00 ± 30 min a.m. and immediately analysed at the hospital laboratory of biochemistry for total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides (TG), apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), glucose and insulin (cobas® 6000 analyzer series, c501 and e601 module, Roche Diagnostics GmbH, Hoffmann-La Roche ltd, Mannheim, Germany). The AIP was calculated as log10 of plasma triglycerides (mg/dL)/HDL-cholesterol (mg/dL) [9]. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as the product of fasting glucose (mmol/L) and fasting insulin (U/mL) divided by 22.5 [30], and insulin resistance was defined as HOMA-IR > 3.16 [31]. The homeostatic model assessment of β-cell function (HOMA-β%) was evaluated as [20 * fasting insulin(U/mL)/fasting glucose (mmol/L)—3.5] [30]. The Quantitative Insulin Sensitivity Check (QUICK) index was calculated as 1/[log10 fasting plasma insulin (U/mL) + log10 glucose (mg/dL)] [32]. The triglyceride-glucose (TyG) index was calculated as ln[triglycerides (mg/dL) /glucose (mg/dL)/2] [33].
Outcomes measures
The primary outcome measure was the change in the AIP after 12 months of starting intervention. The secondary outcome measures were changes in the TyG, HOMA-IR and BMI z-score. No change to trial outcomes was made after the trial started.
Sample size
The sample size was calculated to detect a difference of 0.5 SD or more in the change of the AIP. Assuming a type I error level of 0.05 with a power of 0.80 and a 12 months drop-out rate of 15%, at least 76 children needed to be recruited for each group.
Randomization
Children were randomly allocated to individual or collective intervention by a computer generated, blocked list. A block size of four was used, stratified according to age (± 6 mo.) and sex. The random allocation sequence was implemented sequentially in concealed electronic tables by an informatic blinded to the study. The tables were stored and masked on a single computer. Upon enrolment of a child, the recruiting paediatrician accessed the computer and then the electronic tables via a system-generated numeric code sent to its phone and valid only once for 15 s. By selecting the specific age and sex table, the first sequentially available still masked tile was revealed, and the paediatrician was made aware about the allocation group. The system automatically prevented the paediatrician from selecting any other still masked cells and reading the previously used tiles.
Statistical analysis
Descriptive data are reported as mean, standard deviation (SD) or number of observations (percentage). The 95% confidence interval (CI) was reported as appropriate. Crude significance of within-group and inter-groups differences were tested by the Student’s t test or the Wilcoxon test or the Mann–Whitney U test, as appropriate. A multiple stepwise logistic regression model was fitted (enter at p < 0.15, remove at p > 0.20) to assess if an independent association may exist of change in AIP with the type of intervention. Covariates entered into the model were: age, sex, Tanner stage, baseline dietary energy intake and macronutrients, any examined blood lipid or glucose metabolism variable or index. Two dummy variables were also constructed and included that are compliance at 12 months with dietary energy intake (yes/no) and with MPVA (yes/no). Values of p < 0.05 were considered to indicate statistical significance (two-tailed test). The IBM SPSS Statistics for Windows, Version 25.0, (IBM Corp. Released 2017, Armonk, NY) was used for statistical analysis.
Results
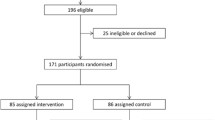
Figure 1 shows the participant flow of the study. Participants were consecutively recruited, from January 01, 2016, to June 30, 2018, and follow-up ended on December 31, 2019. The participation rate at 12 months was 93.3% (n = 153). Preliminary analysis disclosed no difference in any baseline anthropometric, dietary or biochemistry variable between children who completed the trial and those did not (minimum p = 0.173). Participants at 12 months were included in all analysis, and the analysis was by original assigned groups, that is 76 and 77 in the individual or collective intervention group, respectively.
Table 1 shows the baseline characteristics for each intervention group. The daily energy intake was higher than the recommended in 23.7% vs. 27.3% of children in individual vs. collective intervention group, while protein, carbohydrates and fats (as % of energy intake) were outside the recommended values in 42.1% vs. 45.4%, 31.6% vs. 27.3% and 13.2% vs. 14.3%, respectively. No difference occurred between groups (minimum p = 0.559). After 12 months of intervention, energy, macronutrients and fiber intakes, as well MVPA recovered to the recommended values in both groups (minimum p < 0.001) and no difference was observed between groups (minimum p = 0.104). Compliance with the intervention was 90.8% and 88.3% in children who underwent individual or collective intervention respectively (p = 0.617), while compliance with MVPA was 85.5% and 83.1% (p = 0.821).
Blood lipid and glucose metabolism profiles for each group at baseline and after 12 months of intervention are shown in Tables 2 and 3 respectively. No difference between groups was observed for any lipid variable except total and LDL cholesterol. Triglycerides decreased in both groups, but a reduction of the AIP was found in the individual group only (Table 2). No difference between groups was observed for any variable of the glucose metabolism but a significant reduction of the TyG index was found in the individual intervention group only (Table 3). No difference between groups was found for the rate of insulin resistance neither at baseline (48.7% vs. 37.7%, p = 0.169) nor at 12 months (39.5% vs. 32.5%, p = 0.347). Longitudinal change in rate was − 9.2% (95% CI − 17.5 to − 0.91%, p = 0.065, McNemar test) and − 5.2% (95% CI − 11.3 to 0.93%, p = 0.219, McNemar test) in individual and collective group respectively, and it did not differ between the groups (p = 0.336).
The longitudinal change of lipid and glucose metabolic variables for each group is shown in Table 4. Mean absolute change was higher in individual compared to collective intervention for the AIP (difference − 0.07, 95% CI, − 0.14 to − 0.02), TyG index (difference − 0.14, 95% CI − 0.26 to − 0.02) and triglycerides (difference -0.14, mg/dL 95% CI − 0.28 to − 0.006).
Through the 12 months of intervention, mean BMI z-score decreased both in the individual and collective intervention group (p < 0001). The mean longitudinal change was higher in individual intervention group (p < 0.0001) (Table 5), with an absolute mean difference between groups of 0.22 (95% CI 0.09 to 0.35). BMI z-score decreased to below 2 in 7 (9.2%, binomial 95% CI 3.8 to 18.1%) and 6 (7.8%, binomial 95% CI 2.9 to 16.3%) of children in individual and collective intervention groups, respectively.
At multiple stepwise analysis, only change in BMI z-score remained independently associated with intervention (individual vs. collective intervention, odds ratio 3.37, 95% CI 1.29 to 8.77, p < 0.001), that means a 5% (95% CI 2 to 8%) higher probability of a 1% BMI z-score reduction in individual vs. collective intervention. Statistical significance of change in AIP and TyG was p = 0.146 and p = 0.069, respectively.
No adverse events were reported in any participant during the study period.
Discussion
This intervention study aimed to assess if promoting a normo-caloric and balanced diet together with physical activity in children with obesity may affect differently on the Atherogenic Index of Plasma in those undergoing an individual- or collective-based intervention. The study included a consecutive series of children recruited in a centre for childhood obesity activated in 2009 and accredited as an EASO Paediatric Collaborating Centre for Obesity Management (https://easo.org/coms-2/). The study had adequate power and the retention rate was satisfactorily high after 12 months (> 90%).
Intervention was primarily based on well-established and standardized nutritional recommendations [22,23,24]. Furthermore, in the individual-based intervention behavioural changes techniques from CALO-RE taxonomy were applied [25]. Overall compliance with intervention was acceptable, with 90% of children who assumed at 12 months within the recommended ranges of energy intake.
Only few studies evaluated the AIP in the general paediatric population [34, 35] and to our knowledge no trial was conducted in children with obesity. Nogay et al. [34] estimated in the subgroup of children with BMI ≥ 95th percentile a mean AIP of 0.01 and 0.05 in those aged 6–11 and 12–18 years, respectively. Higher mean values were found in this trial. Indeed, a direct comparison with results by Nogay et al. [34] could be misleading, as in this trial children exhibited at baseline a mean BMI z-score higher than 3, corresponding to a value over the 99th percentile [36]. Further, it is unclear if in Nogay et al. [34] the 95th percentile was referenced to the general population or estimated in the examined sample. Anyway, the findings would suggest a possible better effectiveness on the AIP of the individual intervention (mean reduction of 41.4% after 12 months) over the collective intervention (mean reduction of 18.5%), although the comparative size of the effect could be overestimated in this trial, as at baseline children in the individual intervention group showed higher total cholesterol and LDL cholesterol values than children in the collective intervention group. The potential clinical meaning of these findings would be possibly interesting, as the AIP reflects the relationship between protective and atherogenic lipoprotein and is associated with the size of pre- and anti-atherogenic lipoprotein particles [9, 11]. Dobiàsova and Frohlich [10] pooled together 35 cohorts for a total of 1433 subjects (children and adults) and found an association between AIP and cholesterol esterification rate in apoB-lipoprotein-depleted plasma (FERHDL), an indicator inversely related to the size of HDL particles [10]. In addition, these authors found a maximum AIP value of 0.12 in adults without high risk of coronary artery disease while the weighted pooled mean of AIP in subjects with high risk of or angiographically proven coronary artery disease was 0.27 and 0.30, respectively [10]. In children aged 5–14 years, all without metabolic diseases and obesity, the authors estimated a mean of AIP ranging from -0.09 (girls 5–9 years) to 0.02 (girls 10–14 years) [10]. In this study, the relatively high overall mean of AIP observed at baseline (0.28) supports that attention should be paid in the management of obesity in the paediatric population, and that interventions should be hopefully long lasting targeted and strictly monitored.
A decrease in triglyceride-glucose index was observed with better longitudinal change in the individual. Reduction of insulin, HOMA-IR and HOMA-β%, and increase of QUICK index occurred in the individual intervention group only. Overall, the results suggest a possible supplementary beneficial effect of the individual intervention over the collective intervention on TyG and insulin, suggesting an improvement of insulin sensitivity [35]. Other studies investigated TyG in children and adolescents [37,38,39], although they were not designed to evaluate the role of nutritional interventions in the obese paediatric population. Mohd et al. [38] reported in 225 obese adolescents with normal glucose tolerance, prediabetes, and type 2 diabetes, an association of the TyG index with in vivo insulin sensitivity, evaluated by the hyperinsulinemic–euglycemic clamp. Rodrıguez-Moran et al. [39] noted that the TyG index was highly sensitive compared to the euglycemic-hyperinsulinemic clamp and that it had a high diagnostic concordance with the HOMA-IR in a cohort of 2779 Mexican schoolchildren, as also observed by Kang et al. [37]. Moreover, it should be also considered that while HOMA-IR may mainly reflect hepatic insulin resistance, the TyG index may mainly reflect muscle insulin resistance [40]. Also, an increase in plasma triglycerides may interfere with normal glucose metabolism of the muscle, thus reducing insulin sensitivity [41].
Lastly, BMI z-score decreased in about 95% of children with a mean decrease of 0.63 (19.6%) and 0.41 (13.1%) in individual- and collective-based intervention, respectively. These findings agree with previous studies [35, 42,43,44,45], reporting after 5–12 months of nutritional only or lifestyle interventions in overweight/obese children a mean decrease of BMI z-score ranging from about 5% [43, 44] to 20% [43]. Interestingly, it should be noted that the effectiveness of an intervention on the cardio-vascular risk has been recognized when the BMI z score reduction is at least 0.5 [46, 47].
This study has some limitations that need to be highlighted. The main limitation arises from the unfortunate circumstance that unexpectedly, at baseline, children in the individual intervention group had significantly higher total cholesterol and LDL cholesterol values than children in the intervention group. This makes the quality of the trial sub-optimal, as the difference in these basic parameters of atherogenesis warns that drawing conclusions about the effect size of the individual intervention, could be partially misleading when not discussed carefully. On October 2020, we conducted a review of all the trial procedures. It was noted that the family paediatricians involved in the recruitment possibly did not homogeneously referred the children for the hospital visit. When they were re-contacted anonymously some of them admitted sometimes not to refer the child to hospital, as they deemed unnecessary at that time, contravening the criteria and recommendations they had received during a classroom lesson held one month before the start of the trial. This unpredictable and deplorable attitude may have introduced a hidden bias into the randomization procedure. Anyhow, this limitation deserves to be shortly discussed. Confidently, one could firstly remark that the AIP index is defined by TG and HDL and in turn the observed beneficial effect on this index in individual vs. collective intervention, might be, at least in part, overestimated in this trial, possibly due to baseline worse cholesterol profile exhibited by children in individual intervention group. Accordingly, it could be not unexpected that children in the individual intervention showed more beneficial effects. However, one should also note that in this study baseline AIP index (the primary outcome measure we used) did not differ at baseline between the two intervention groups (mean 0.29 in individual vs. 0.27 in collective intervention). Moreover, even hypothetically assuming the same mean baseline value of 0.27 the mean reduction in the individual group would be 0.10 (observed value 0.12) vs. the value of 0.05 observed in the collective group, and the difference would still be significant. This would suggest that the greater improvement in the AIP index in the individual intervention group may not only resulted from a worse baseline blood lipid profile, but, at least in part, from the type of intervention itself. Another question, related to the baseline differences in cholesterols, may arise concerning at the baseline carbohydrate intake of about 30 g higher in the individual intervention group, and if it may be a possible confounder. However, one should note that although studies suggest that while higher total carbohydrate intake, as percentage of calories from carbohydrate, can relate to blood cholesterol profile (e.g., [48,49,50]), also when %carbohydrate ranges around 50%, orientation of the association (negative/positive) is not currently conclusively clear. For example, Ma et al. [51] found at a cross-sectional analysis that percentage of calories from carbohydrates, as well as total intake of carbohydrates (on units of 20 g), were inversely associated with total cholesterol, LDL cholesterol and HDL cholesterol at unadjusted analysis. After adjusting for confounders (including BMI, percentage fats intake, season of year at lipids assessment and others) none of these associations remained significant (except HDL cholesterol/%carbohydrates, p = 0.03). On longitudinal adjusted analysis, they found a significant decrease in HDL cholesterol and increase in total cholesterol/ HDL cholesterol ratio related to %carbohydrate. On a whole, they concluded that "further studies in the form of clinical trials are required to investigate these associations and to disentangle the relationship between dietary carbohydrate intake and serum lipids". While these results were reported in adult population Nicklas et al. [52], assessing schoolchildren aged 7–11 years (a range of age comparable to children in our trial), observed that the effect of diet on serum lipids in children is similar to the one observed in adults. Not only, they reported also that total fat and saturated fat were positively associated with total cholesterol and HDL cholesterol levels, saturated fat was positively associated with total cholesterol, while carbohydrate was inversely associated with both total cholesterol and HDL cholesterol. Although it cannot be proved that the above considerations may be translated to children with obesity, in absence of specific trials they strongly warn that anyway a complex interrelationship might exist of carbohydrate intake with serum lipids, that needs to be purposely investigated in childhood obesity. Lastly, another limitation was that this study based the dietary assessment on the Food Frequency Questionnaire technique. However, one should note that it was used merely as a tool to estimate compliance with nutritional recommendations. Future research should hopefully include more accurate procedures, such as the 7-day food diary.
Conclusion
On the whole, within the limitations of this study, one may conclude that in children with obesity, an individual-based nutritional and lifestyle intervention, including techniques from the CALO-RE taxonomy, could have an additional beneficial effect over a collective-based intervention, although the actual size of the effect remains to be clarified.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due the raw information gathered in this research were kept strictly confidential as stated in respondents’ consent agreement but are available from the corresponding author on reasonable request.
Abbreviations
- AIP:
-
Atherogenic Index of Plasma
- WHO:
-
World Health Organisation
- CALOR-RE taxonomy:
-
Coventry, Aberdeen and London- Refined taxonomy
- BMI:
-
Body Mass Index
- MVPA:
-
Moderate to vigorous daily physical activity
- FFQ:
-
Food Frequency Questionnaire
- HDL:
-
High-density lipoprotein
- LDL:
-
Low- density lipoprotein
- TG:
-
Triglycerides
- ApoA1:
-
Apolipoprotein A1
- ApoB:
-
Apolipoprotein B
- HOMA-IR:
-
Homeostatic models assessment of insulin resistance
- HOMA-β%:
-
Homeostatic model assessment of β-cell function
- QUICK:
-
Quantitative sensitivity check
- TyG:
-
Triglyceride-glucose Index
- FERHDL :
-
Fractional esterification rate of cholesterol in high density lipoprotein
- EASO:
-
European Association of the Study of Obesity
References
World Health Organisation (WHO). Report of the Commission on Ending Childhood Obesity. Commission on Ending Childhood Obesity (ECHO): Geneva, Switzerland, 2016. https://www.who.int/end-childhood-obesity/en/. Accessed 19 Nov 2020.
Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, Lu Y, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96.
World Health Organization. Childhood overweight and obesity. http://www.who.int/dietphysicalactivity/childhood/en/. Accessed 19 Nov 2020.
Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(3):709–57.
May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129(6):1035–41.
Zimmet P, Alberti KGM, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents: an IDF consensus report. Pediatr Diabetes. 2007;8(5):299–306.
D’Adamo E, Guardamagna O, Chiarelli F, Bartuli A, Liccardo D, Ferrari F, et al. Atherogenic dyslipidemia and cardiovascular risk factors in obese children. Int J Endocrinol. vol. 2015. doi.org/https://doi.org/10.1155/2015/912047
Cook S, Kavey REW. Dyslipidemia and pediatric obesity. Pediatr Clin North Am. 2011;58(6):1363–73.
Dobiášová M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: theoretical and practical implications. Clin Chem. 2004;50(7):1113–5.
Dobiášová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FERHDL). Clin Biochem. 2001;34(7):583–8.
Bhardwaj S, Bhattacharjee J, Bhatnagar MK, Tyagi S, Delhi N. Atherogenic index of plasma, castelli risk index and atherogenic coefficient—new parameters in assessing cardiovascular risk. IJPBS. 2013;3(3):359–64.
Guo Q, Zhou S, Feng X, Yang J, Qiao J, Zhao Y, et al. The sensibility of the new blood lipid indicator-atherogenic index of plasma (AIP) in menopausal women with coronary artery disease. Lipids Health Dis. 2020;19(1):27–34.
Zhu X, Yu L, Zhou H, Ma Q, Zhou X, Lei T, et al. Atherogenic index of plasma is a novel and better biomarker associated with obesity: a population-based cross-sectional study in China. Lipids Health Dis. 2018;17(1):37–42.
Mead E, Brown T, Rees K, Azevedo LB, Whittaker V, Jones D, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst Rev. 2017;6(6):CD012651.
Ho M, Garnett SP, Baur LA, Burrows T, Stewart L, Neve M, et al. Impact of dietary and exercise interventions onweight change andmetabolic outcomes in obese children and adolescents a systematic review and meta-analysis of randomized trials. JAMA Pediatr. 2013;167(8):759–68.
Flodmark CE, Lissau I, Moreno LA, Pietrobelli A, Widhalm K. New insights into the field of children and adolescents’ obesity: the European perspective. Int J Obes Relat Metab Disord. 2004;28(10):1189–96.
Nowicka P, Höglund P, Pietrobelli A, Lissau I, Flodmark CE. Family Weight School treatment: 1-year results in obese adolescents. Int J Pediatr Obes. 2008;3(3):141–7.
Ho M, Garnett SP, Baur L, Burrows T, Stewart L, Neve M, et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics. 2012;130(6):e1647–71.
Hayes JF, Altman M, Coppock JH, Wilfley DE, Goldschmidt AB. Recent updates on the efficacy of group-based treatments for pediatric obesity. Curr Cardiovasc Risk Rep. 2015;9(4):16–33.
Wilfley DE, Hayes JF, Balantekin KN, Van Buren DJ, Epstein LH. Behavioral interventions for obesity in children and adults: evidence base, novel approaches, and translation into practice. Am Psychol. 2018;73(8):981–93.
World Health Organization (WHO). Child Growth Standards: WHO Anthro and macros. (version 3.2.2, January 2011). https://www.who.int/childgrowth/software/en/. Accessed 19 Nov 2020.
Valerio G, Maffeis C, Saggese G, Ambruzzi MA, Balsamo A, Bellone S, et al. Diagnosis, treatment and prevention of pediatric obesity: consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital J Pediatr. 2018;44(1):88–108.
Società Italiana di Nutrizione Umana. LARN Livelli di Assunzione di Riferimento di Nutrienti ed energia per la popolazione italiana. IV revisio. 2014. https://sinu.it/tabelle-larn-2014/. Accessed 19 Nov 2020.
World Health Organization (WHO). Recommended population levels of physical activity for health. In Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. https://www.who.int/dietphysicalactivity/factsheet_recommendations/en/. Accessed 19 Nov 2020.
Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011;26(11):1479–98.
Amine EK, Baba NH, Belhadj M, Deurenberg-Yap M, Djazayery A, Forrestre T, et al. Diet, nutrition and the prevention of chronic diseases. Report of a joint WHO/FAO expert consultation (WHO Technical Report Series 916). World Health Organization. https://www.who.int/nutrition/publications/obesity/WHO_TRS_916/en/. Accessed 19 Nov 2020.
Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274–84.
Bellù R, Ortisi MT, Riva E, Banderali G, Cucco I, Giovannini M. Validity assessment of a food frequency questionnaire for school-age children in Northern Italy. Nutrition Res. 1995;15(8):1121–8.
Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione. Tabelle di composizione degli alimenti. 2018 https://www.alimentinutrizione.it/sezioni/tabelle-nutrizionali/. Accessed 19 Nov 2020.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):e500–3.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative Insulin Sensitivity Check Index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Nogay NH. Assessment of the correlation between the atherogenic index of plasma and cardiometabolic risk factors in children and adolescents: Might it be superior to the TG/HDL-C ratio? J Pediatr Endocrinol Metab. 2017;30(9):947–55.
Verduci E, Lassandro C, Giacchero R, Miniello VL, Banderali G, Radaelli G. Change in metabolic profile after 1-year nutritional-behavioral intervention in obese children. Nutrients. 2015;7(12):10089–99.
De Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta Pædiatrica, 2006; Suppl 450:76–85.
Kang B, Yang Y, Lee EY, Yang HK, Kim HS, Lim SY, et al. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int J Obes (Lond). 2017;41(5):789–92.
Noor Shafina MN, SoJung L, Fida B, Hala T, Silva A. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic–euglycemic clamp. Pediatr Diabetes. 2016;17(6):458–65.
Rodríguez-Morán M, Simental-Mendía LE, Guerrero-Romero F. The triglyceride and glucose index is useful for recognising insulin resistance in children. Acta Paediatr. 2017;106(6):979–83.
Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, et al. Markers of insulin resistance and carotid atherosclerosis: a comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–72.
Kelley DE, Goodpaster BH. Skeletal muscle triglyceride: an aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24(5):933–41.
Pozzato C, Verduci E, Scaglioni S, Radaelli G, Salvioni M, Rovere A, et al. Liver fat change in obese children after a 1-year nutrition-behavior intervention. J Pediatr Gastroenterol Nutr. 2010;51(3):331–5.
Kolsgaard MLP, Joner G, Brunborg C, Anderssen SA, Tonstad S, Andersen LF. Reduction in BMI z-score and improvement in cardiometabolic risk factors in obese children and adolescents. The Oslo Adiposity Intervention Study - a hospital/public health nurse combined treatment. BMC Pediatr. 2011;11(1):47–54.
Harder-Lauridsen NM, Birk NM, Ried-Larsen M, Juul A, Andersen LB, Pedersen BK, et al. A randomized controlled trial on a multicomponent intervention for overweight school-aged children – Copenhagen, Denmark. BMC Pediatr. 2014;14(1):273–86.
Blüher S, Petroff D, Wagner A, Warich K, Gausche R, Klemm T, et al. The one-year exercise and lifestyle intervention program KLAKS: Effects on anthropometric parameters, cardiometabolic risk factors and glycemic control in childhood obesity. Metabolism. 2014;63(3):422–30.
Ford AL, Hunt LP, Cooper A, Shield JPH. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Arch Dis Child. 2010;95(4):256–61.
Reinehr T, Lass N, Toschke C, Rothermel J, Lanzinger S, Holl RW. Which amount of BMI-SDS reduction is necessary to improve cardiovascular risk factors in overweight children? J Clin Endocrinol Metab. 2016;101(8):3171–9.
Ford ES, Liu S. Glycemic index and serum high-density lipoprotein cholesterol concentration among US adults. Arch Intern Med. 2001;161(4):572–6.
Frost G, Leeds AA, Doré CJ, Madeiros S, Brading S, Dornhorst A. Glycaemic index as a determinant of serum HDL-cholesterol concentration. Lancet. 1999;353(9158):1045–8.
Liu S, Manson JAE. Dietary carbohydrates, physical inactivity, obesity, and the “metabolic syndrome” as predictors of coronary heart disease. Curr Opin Lipidol. 2001;12(4):395–404.
Ma Y, Chiriboga DE, Olendzki BC, Li W, Leung K, Hafner AR, et al. Association between carbohydrate intake and serum lipids. J Am Coll Nutr. 2006;25(2):155–63.
Nicklas TA, Dwyer J, Feldman HA, Luepker RV, Kelder SH, Nader PR. Serum cholesterol levels in children are associated with dietary fat and fatty acid intake. J Am Diet Assoc. 2002;102(4):511–7.
Acknowledgements
We thank children families and all the people involved in this work.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
E.V. and G.R. conceived and designed the experiments; G.B. and G.Z. coordinated the clinical examinations and data collection; G.R. analysed the data; all authors contributed to writing the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Hospital Ethics Committee approved the study protocol and gave ethical clearance.
Consent for publication
All the authors give their consent to for publication.
Competing interests
The authors declare they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Verduci, E., Banderali, G., Di Profio, E. et al. Effect of individual- versus collective-based nutritional-lifestyle intervention on the atherogenic index of plasma in children with obesity: a randomized trial. Nutr Metab (Lond) 18, 11 (2021). https://doi.org/10.1186/s12986-020-00537-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-020-00537-w