Abstract
Background
Previous meta-analyses estimating the prevalence of the post-COVID-19 condition (PCC) were confounded by the lack of negative control groups. This may result in an overestimation of the prevalence of those experiencing PCC, as these symptoms are non-specific and common in the general population. In this study, we aimed to compare the burden of persistent symptoms among COVID-19 survivors relative to COVID-19-negative controls.
Methods
A systematic literature search was conducted using the following databases (PubMed, Web of Science, and Scopus) until July 2023 for comparative studies that examined the prevalence of persistent symptoms in COVID-19 survivors. Given that many of the symptoms among COVID-19 survivors overlap with post-hospitalization syndrome and post-intensive care syndrome, we included studies that compare the prevalence of persistent symptoms in hospitalized COVID-19 patients relative to non-COVID-19 hospitalized patients and in non-hospitalized COVID-19 patients relative to healthy controls that reported outcomes after at least 3 months since infection. The results of the meta-analysis were reported as odds ratios with a 95% confidence interval based on the random effects model.
Results
Twenty articles were included in this study. Our analysis of symptomatology in non-hospitalized COVID-19 patients compared to negative controls revealed that the majority of symptoms examined were not related to COVID-19 infection and appeared equally prevalent in both cohorts. However, non-COVID-19 hospitalized patients had higher odds of occurrence of certain symptoms like anosmia, ageusia, fatigue, dyspnea, and brain fog (P < 0.05). Particularly, anosmia and ageusia showed substantially elevated odds relative to the negative control group at 11.27 and 9.76, respectively, P < 0.05. In contrast, analysis of hospitalized COVID-19 patients compared to those hospitalized for other indications did not demonstrate significantly higher odds for the tested symptoms.
Conclusions
The persistent symptoms in COVID-19 survivors may result from hospitalization for causes unrelated to COVID-19 and are commonly reported among the general population. Although certain symptoms exhibited higher odds in non-hospitalized COVID-19 patients relative to controls, these symptoms are common post-viral illnesses. Therefore, the persistent symptoms after COVID-19 may not be unique to SARS-CoV-2. Future studies including well-matched control groups when investigating persistent symptoms in COVID-19 survivors are warranted to draw a firm conclusion.
Similar content being viewed by others
Introduction
Since the initial outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, there has been a growing trend in the potential long-term implications of Coronavirus disease 2019 (COVID-19), often known as long COVID or post-COVID-19 condition (PCC) [1]. Per the World Health Organization (WHO), the majority of patients who get COVID-19 completely recover. However, contemporary data indicates that about 10–20% of COVID-19-infected patients experience symptoms that can be designated as PCC [2]. Over 200 distinctive signs and symptoms were identified as relevant to PCC [3].
Even though the COVID-19 pandemic has been ongoing for nearly 4 years, there is still no consensus among various recommendations as well as organizations on an accurate depiction of PCC [4,5,6,7]. The WHO’s definition of PCC was developed through a Delphi process, in which PCC comes up in individuals with a history of most likely or verified SARS-CoV-2 infection, usually 3 months upon onset, with symptoms lasting at least 2 months and being unlikely to be attributed to another clinical condition [8]. However, this definition may be skewed because it does not take illness severity [9] into account and is contingent on a history of potential SARS-CoV-2, which is not objective. Furthermore, the elements addressing the timing and duration of symptoms do not meet consensus requirements. Because of this, continual discussion and the incorporation of contemporary proof are required to advance this definition [8].
The previous meta-analyses that aimed to estimate PCC prevalence were confounded by the lack of negative control groups [10,11,12,13]. This results in an overestimation of the prevalence of those experiencing PCC, with preliminary estimates spanning from 45 to 80% [12, 13]. Without negative control groups, it is challenging to properly compare the burden of long COVID and the symptoms profile among individuals who test positive and those who test negative for SARS-CoV-2 because some of the reported long COVID symptoms are non-specific and pervasive in the population [14]. Moreover, these individuals might exhibit the symptoms as a result of pre-existing health disorders or the wider implications of the pandemic, thereby making it more difficult for researchers to draw accurate conclusions without the inclusion of control groups [14].
On top of that, the recent scientific literature points out that hospitalization could potentially play a crucial role in the development of PCC. The following evidence backs up this claim: First, previous meta-analyses indicated that hospitalized COVID-19 patients had a greater prevalence of PCC than non-hospitalized patients [11, 13, 15]. Second, the risk of developing PCC is significantly higher in hospitalized patients, with a 2.48 odds ratio (OR) compared to non-hospitalized patients [16]. Furthermore, comorbidities and pre-existing medical conditions such as anxiety, depression, asthma, chronic obstructive pulmonary disease (COPD), immunosuppression, and ischemic heart disease had a higher odds ratio of acquiring PCC compared to non-comorbid COVID-19 patients [16].
To circumvent the limitations of past studies and appropriately address the burden of persistent symptoms after COVID-19 relative negative control PCC, we carried out this systematic review and meta-analysis of research studies that involved COVID-19 positive individuals in comparison to COVID-19 negative individuals. Given the potential effect of hospitalization on the prevalence of symptoms after COVID-19, we included studies that compare the prevalence of persistent symptoms following COVID-19 in hospitalized COVID-positive patients relative to those who were hospitalized for reasons other than COVID-19 infection and in non-hospitalized COVID-positive individuals relative to healthy controls. Further, to find out the impact of comorbidities, we did a subgroup analysis of the studies that were comorbidity-matched.
Methods
Search strategy
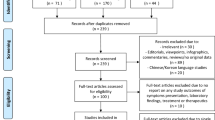
A comprehensive literature search was conducted in the following databases: PubMed, Scopus, and Web of Science, concerning the relevant studies until July 2023. The Preferred Reporting Items for Systematic Reviewers and Meta-analysis (PRISMA) criteria were followed. The reference lists of relevant articles were reviewed for additional studies. The checklist of items to include when reporting a systematic review or meta-analysis and the complete search strategy were presented in Additional file 1: Tables S1 and S2, respectively; see the additional file.
Study selection
Studies were included if they met the following criteria
(a) Articles written in English. (b) Peer-reviewed comparative studies that compare the prevalence of persistent symptoms in COVID-positive and COVID-negative individuals after at least 3 months post-COVID-19 (as defined by WHO). (c) Patients who were hospitalized for COVID-19 compared to patients who were hospitalized due to other infections or causes or (d) non-hospitalized individuals versus healthy controls. (e) Laboratory-confirmed COVID-19; and (f) reporting of follow-up as mean, median, or a set interval after symptom onset, diagnosis, acute illness, or initial computed tomography (CT) chest imaging.
Exclusion criteria for this meta-analysis
The subsequent studies were excluded from the analysis: (a) Studies that reported the prevalence of persistent symptoms in patients hospitalized with COVID-19 versus healthy controls. (b) Studies where the duration of follow-up could not be determined. (c) Studies in which all patients were not assessed for a minimum of 12 weeks. (d) Studies that specifically recruited sub-groups of patients, such as those with diabetes or autoimmune conditions.
Three independent reviewers selected eligible articles based on the aforementioned eligibility criteria. Discrepancies were resolved through consultation with another author.
Data extraction
Three independent reviewers extracted data from the included studies using a standardized data extraction form. Any discrepancies were settled by discussion.
From each included study, the following were extracted: (a) Study details including study design, study aim, study population, country of origin, number of cases (COVID-19 survivors), number of controls (COVID-19 negative individuals), duration, and method of follow-up. (b) Participants’ characteristics, such as age, sex, presence of comorbidities, and hospitalization status. (c) Outcomes associated with post COVID-19 condition in both the case and control groups.
Quality assessment
The assessment of the studies’ quality was carried out using a modified scale based on the Newcastle–Ottawa Scale of case–control studies displayed in Additional file 1: Table S3. The items on the modified scale were: (a) Did the sample size exceed 10,000 or not? (b) How was the ascertainment of COVID-19 infection made; via polymerase chain reaction (PCR) test, serology test, both, or none? c) Was the same method of ascertainment used for cases and controls or not? (d) What was the definition of control; no history of disease or not described? (e) Was the non-response rate the same for both groups? (f) Did the study control for comorbidity, age, and gender or not? g) If control for comorbidity, age, and gender was not achieved, was the study adjusted for confounding factors using multivariate analysis or stratification?
Two independent reviewers assessed the quality of the studies, and discrepancies were resolved through consultation with a third author.
Data synthesis
Results were reported as odds ratios with a 95% confidence interval (CI) based on the random effects model. We used the I2 statistic to measure statistical heterogeneity between the results of the studies, with values less than 50% indicating low heterogeneity and values greater than or equal to 50% indicating high heterogeneity. Since different studies reported varying symptom patterns and numbers, meta-analysis was only performed for symptoms with data from at least three studies. Subgroup analyses were conducted based on age categories and the studies that were matched in terms of comorbidities. In addition, sensitivity analyses were conducted using a one-leave-out approach to identify any outlier studies that may have significantly affected the overall results. Testing for publication bias by funnel plots and Egger's tests was considered for analysis in more than 10 studies [17]. All statistical analysis was performed using Comprehensive Meta-Analysis version 3.0 (Biostat, Englewood, NJ, USA).
Results
Study selection and characteristics of included studies
In total, 2320 titles were identified through database searches, of which 20 were included in this review [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] (Fig. 1). The list of excluded studies with reasons for exclusion was presented in Additional file 1: Table S4. As displayed in Table 1, the current systematic review comprised 20 studies representing a variety of countries. Each of the subsequent countries had one research conducted: Sweden [36], Switzerland [29], Germany [18], Italy [19], Slovakia [25], Norwegian [9], France [33], Netherlands [34], Denmark [27], and Spain [30]. Two studies were done in Norway [22, 31], while another 2 studies were reported in Israeli [21, 26], four studies were performed in the UK [23, 24, 28, 32] and one study in the USA [20]. One study involved participants from many nations including Argentina, Canada, Costa Rica, Italy, Paraguay, Singapore, Spain, and the United States [35].
Stratification of the included studies based on the hospitalization status of COVID-19 patients and negative control
The twenty included studies resulted in 21 estimates, with Funk et al. aiming to estimate PCC for non-hospitalized children relative to the negative control and also for hospitalized children relative to non-COVID-19 hospitalized children [35].
Sixteen of the twenty-one studies assessed PCC in non-hospitalized COVID-19 patients in comparison to negative controls [9, 18, 19, 22,23,24,25,26, 28, 29, 31,32,33,34,35,36]. The remaining five studies addressed PCC in COVID-19 patients who were hospitalized [20, 21, 27, 30, 35]. Three studies compared hospitalized COVID-19 patients to those hospitalized for other indications without specifying the cause of hospitalization [20, 30, 35]. Another study evaluated hospitalized COVID-19 patients in comparison to non-COVID-19 hospitalized patients due to pneumonia [21]. One study investigated hospitalized COVID-19 patients relative to those hospitalized with acute myocardial infarction [27].
Age category
When categorizing studies by age, four studies were performed on children [28, 29, 32, 35], two studies involved adolescents and young adults [9, 18], 12 studies included adults [19,20,21, 23,24,25, 27, 30, 31, 33, 34, 36] and only 2 studies included a wide age range [22, 26].
Follow-up since COVID-19 onset
The included studies in this research encompassed a diverse range of follow-up durations aimed at monitoring participants’ conditions after diagnosis. Some studies allowed for extended, short-term observation [23, 36]. Most of the studies focused on a 6-month follow-up, providing insights into the mid-term progression of the studied conditions [9, 24, 27,28,29]. However, other studies conducted comprehensive 7-month, 9-month, and 10-month follow-up periods, [18, 21, 25]. Effectively, more studies focused on the long-term progression of the condition [22, 30, 33]. Some studies implemented a flexible approach toward follow-up and had a follow-up period of 6–12 months, 3–8 months, and 3–5 months [20, 26, 31]. It’s worth noting that some studies had the shortest median follow-up period of 3 months [32, 34, 35].
Quality assessment of the included studies
The detailed score for each included study was presented in Additional file 1: Table S5. Fifteen of the twenty studies have a score of more than six out of 10 and are considered of fair quality. While five studies had a score of five or less and may have a high risk of bias either because of a small sample size, different rates of non-response, subjective assessment of outcomes, or non-adjustment for confounding factors [18, 19, 21, 29, 33].
Post-COVID consequences in non-hospitalized COVID-19 patients compared to negative controls
Non-hospitalized patients with COVID-19 were found to have a stronger association with certain symptoms compared to negative controls (Table 2). These symptoms included anosmia, ageusia, dyspnea, fatigue, and brain fog or confusion, with pooled odds ratios greater than 1, as shown in Figs. 2, 3, 4, 5 and 6. Anosmia had the highest odds ratio of 11.27, followed by ageusia with 9.76. Some manifestations, such as chest pain, dizziness, skin conditions, myalgia/arthralgia, and ear problems, showed slightly elevated odds ratios of 1.9, 1.37, 1.42, 1.25, and 1.35, respectively, P < 0.05. After matching for comorbidities, the pooled odds ratios for certain symptoms changed. Anosmia, dyspnea, fatigue, and brain fog continued to exhibit significantly higher odds ratios in COVID-19 patients even after matching for comorbidities (4.91, 2.29, 2.2, and 2.91, P < 0.05), respectively. However, chest pain, skin conditions, myalgia/arthralgia, and ear problems no longer showed a significant association.
Post-COVID consequences in non-hospitalized children with COVID-19 compared to negative controls
Anosmia, fatigue, brain fog or confusion, and dizziness were found to be associated with non-hospitalized children with COVID-19 compared to negative controls (P < 0.05) as shown in Table 3. Especially anosmia had a very high odds ratio of 10.45. However, the pooled odds ratios for specific symptoms varied when comorbidities were matched. Brain fog and dizziness were no longer significant (P > 0.05), while there were not enough studies on fatigue to be tested.
Post-COVID consequences in non-hospitalized adults with COVID-19 compared to negative controls
Abdominal pain was significantly greater in the negative control group (OR 0.83) (P < 0.05). In contrast, symptoms significantly associated with non-hospitalized COVID-19 compared to controls included fatigue, dyspnea, brain fog, anosmia, chest pain, sleep disturbances, and tachycardia (OR 1.68, 1.99, 2.29, 4.95, 1.44, 1.14, and 1.34 P < 0.05, respectively). However, subgroup analyses matching for comorbidities revealed that associations with sleep disturbances, chest pain, tachycardia, and abdominal pain were no longer statistically associated in non-hospitalized COVID-19 patients, as presented in Table 4.
Post-COVID consequences in hospitalized COVID-19 patients compared to non-COVID-19 hospitalized patients
Of the five symptoms tested, only headache and sleep disorders (OR 0.86 and 0.89, respectively, P < 0.05) showed significantly lower odds of occurrence in hospitalized COVID-19 patients compared to patients hospitalized for other reasons, as shown in Table 5. The other three symptoms, brain fog, anxiety, and fatigue, did not have significantly higher odds in COVID-19 patients, although brain fog and anxiety had slightly elevated odds ratios of 1.19 and 1.04, respectively. In contrast, the odds ratios for fatigue were slightly lower at 0.94.
Included studies comparing myocardial parameters (structure, function, tissue characteristics, and perfusion) between patients with mild COVID-19 and COVID-negative healthy controls
As shown in Table 6, two studies aimed to assess myocardial structure, function, and tissue characterization in those with mild COVID-19 syndrome and COVID-negative healthy controls after more than 12 weeks and 6 months since infection.
Gorecka et al. [23] found that most patients with a long COVID-19 syndrome and no previous cardiovascular disease did not show any signs of abnormalities in their myocardial energetics, structure, function, blood flow, or tissue characteristics. Likewise, Joy et al. [24] demonstrated that there was no significant difference in cardiovascular abnormalities between seropositive individuals and those who were seronegative, even among otherwise healthy participants.
Post-COVID consequences in COVID-positive patients relative to COVID-negative controls across all included studies overall and stratified by patients’ age category
Overall, compared to negative control, anosmia, dyspnea, chest pain, brain fog, dizziness, tachycardia/palpitation, ageusia, fatigue, and myalgia/arthralgia were significantly higher in COVID-positive patients (pooled OR 6.9, 2.1, 1.72, 1.77, 1.35, 1.34, 6.57, 1.53, and 1.22, respectively). Nevertheless, when age-based subgroup analysis was done, there was no statistically significant difference in chest pain in both the adult and children’s categories (Additional file 1: Table S6).
Sensitivity analysis and publication bias testing
We conducted a sensitivity analysis using the leave-one-out approach for symptoms or conditions that showed statistically significant higher odds in COVID patients relative to COVID-negative controls in both the matched comorbidity and overall analysis. Dyspnea, fatigue, brain fog, and anosmia all showed statistical significance in non-hospitalized COVID-19 relative to negative control. The analysis indicated that the pooled odds ratios of these tested symptoms were reliable and were not dependent on any study, as shown in Figs. 7, 8, 9, 10 and 11.
Publication bias testing was conducted for symptoms that displayed statistical significance and were present in at least 10 studies. The results of the funnel plots and Egger's tests did not provide any indications of publication bias for the three evaluated symptoms, namely dyspnea, fatigue, and brain fog, with corresponding p-values of 0.28, 0.58, and 0.09, respectively (Additional file 1: Figs. S1–S3).
Discussion
The persistent symptoms after COVID-19 are non-specific and frequently reported among the general population. In addition, these symptoms may result from hospitalization for causes unrelated to COVID-19. In light of these considerations, we aimed to compare the burden of persistent symptoms after COVID-19 among individuals who tested positive and those who tested negative for SARS-CoV-2.
Our study highlighted the following main findings: (1) Analysis of symptoms in non-hospitalized COVID-19 patients compared to negative controls revealed that the majority of the tested symptoms were unrelated to COVID-19, and appeared to be equally prevalent in both groups. However, some symptoms, such as anosmia, ageusia, dyspnea, fatigue, and brain fog, seemed to be associated with COVID-19 in non-hospitalized patients. Particularly, anosmia and ageusia exhibited high odds ratios of 11.27 and 9.76, respectively. (2) The matched comorbidity subgroup analysis revealed that the presence of pre-existing medical conditions may influence the odds of experiencing specific symptoms and conditions in both children and adults. This was more evident in children than adults. However, it is important to note that the limited number of studies included in the analysis warrants caution in interpreting the findings. (3) The limited evidence so far indicates that there is no significant difference in cardiovascular abnormalities between individuals who had mild COVID-19 relative to seronegative healthy participants. But larger studies are still warranted to draw firmer conclusions. (4) Analysis of hospitalized COVID-19 patients compared to hospitalized patients for other indications did not show significantly higher odds of tested symptoms. Unexpectedly, headache and sleep disorders had higher odds of occurring in non-COVID hospitalized patients.
Similarly, a meta-analysis comparing the odds of post-COVID-19 symptoms in children relative to negative controls found that out of the 13 tested symptoms, persistent dyspnea, loss of smell or taste (anosmia/ageusia), and fever had odds ratios of 2.69, 10.68, and 2.23 respectively, indicating that these symptoms were significantly associated with COVID-19 infection [37]. However, the findings of this study may be limited by comparing COVID-infected children of varying severity to negative controls and the smaller number of the included studies (3 studies).
According to our analysis of symptoms in non-hospitalized COVID-19 patients relative to negative controls, anosmia exhibited a significantly high odds ratio of 11.27 in non-hospitalized COVID-19 patients when compared to the negative control group, which comprised individuals without any infection. Although anosmia was prevalent as a persistent symptom after COVID, it is challenging to determine whether it occurred more frequently than other upper respiratory tract infections (URTI) of similar severity. This is supported by the high frequency of post-viral olfactory disorders, which range from 11 to 40% [38]. Also, the prevalence of anosmia in the mixed hospitalized and non-hospitalized population of COVID survivors is 14.12% [13]. Furthermore, our meta-analysis of proportions showed that the prevalence of anosmia in both hospitalized and non-hospitalized COVID survivors is 14.3%. In addition, a recent study found that patients recovering from COVID-19 infections exhibited a lower incidence of complete anosmia compared to those recovering from non-COVID-19 infections [39]. Therefore, the uniqueness of anosmia to SARS‑CoV‑2 may be questioned.
Similarly, non-hospitalized COVID-19 patients were more likely to experience ageusia than negative controls, which also comprised individuals without any infection, with a pooled odds ratio of 6.57. According to a recent study, patients recovering from COVID-19 infections displayed ageusia less frequently than those recovering from non-COVID-19 infections [39]. Anosmia and ageusia are frequently reported after recovering from COVID-19; however, further research is necessary to determine conclusively if these chemosensory symptoms occur more often compared to other post-viral illnesses.
Non-hospitalized COVID-19 patients were also more likely to experience brain fog than negative controls in both adults and children. It is not yet clear whether to directly blame SARS-CoV2 itself for these symptoms or whether they are a result of particular idiopathic stressors. Moreover, there is no quantifiable standardized definition of brain fog, and the methodologies and outcome measures differ [40].
Fatigue is a frequently reported symptom of COVID-19 [41] and is considered the most frequent symptom among COVID survivors [13]. It is not unexpected that some individuals may experience fatigue after COVID-19 recovery, as post-infectious fatigue is a widely recognized phenomenon that has been observed in both viral and non-viral infections and is well-documented in the literature [42]. Conferring to our study, non-hospitalized COVID-19 patients have significantly higher odds of fatigue than negative controls, which persisted even after matching for comorbidity (with pooled odds of 2.2). However, it is important to note that fatigue is not limited to SARS-CoV-2 and has been observed in other infectious diseases, as mentioned earlier.
Similarly, dyspnea is strongly associated with non-hospitalized COVID-19 patients, even after matching for comorbidities. Although the specific mechanism responsible for dyspnea in mild COVID-19 survivors has yet to be determined, studies suggest that it is typically caused by hyperventilation rather than organ damage [43,44,45,46,47]. This hyperventilation may arise from an abnormality in ventilatory control or a failure of inhibitory systems (such as endorphins) following pulmonary infections [47].
From another perspective, persistent symptoms after COVID-19 may result from hospitalization for causes unrelated to COVID-19, which is referred to as post-hospital syndrome (PHS) or Post-intensive care syndrome (PICS). Throughout hospital stays, inpatients are subjected to considerable levels of stress, which might raise their risk for a wide range of adverse health events referred to as PHS [48]. Allostatic overload is thought to be the cause of PHS [48]. Additionally, the PICS has been identified as a distinct condition that specifically arises from ICU stays. PICS refers to a collection of physical, cognitive, and emotional symptoms that can occur in individuals who have survived a critical illness and received treatment in the ICU [49]. Either PHS or PICS could potentially explain why COVID-19 patients who have been hospitalized, do not exhibit a notably higher odds of tested symptoms compared to patients hospitalized for other causes. Interestingly, headaches and sleep disorders (OR 0.86 and 0.89, respectively, P < 0.05) showed significantly lower odds of occurrence in hospitalized COVID-19 patients compared to patients hospitalized for other reasons. However, the upper limits of the 95% CI nearly reach the null effect (one), as shown in Table 5. This borderline significance likely stems from the limited number of studies included (only three). With more studies, we could reach more definitive conclusions regarding this association. Similarly, Quinn et al. showed that the burden of post-acute physical and mental health disorders among patients who survived hospitalization for COVID-19 was comparable to that of other acute infectious diseases, suggesting that rather than being direct effects of the SARS-CoV-2 infection, many of the post-acute consequences of COVID-19 may be attributable to the severity of the infection illness requiring hospitalization [50]. Another study revealed that neurological risk was high in COVID-19 survivors, but not more than that observed after other infections of similar severity [51]. This highlights the importance of including well-matched control groups when investigating PCC.
Limitations
This study has several limitations that should be considered when interpreting the results. These include the potential for heterogeneity due to differences in study design, population characteristics, and outcome definitions, as well as the risk of recall bias. Furthermore, some symptoms with borderline odds ratios warrant further investigation due to their limited research and occasionally smaller sample sizes, hindering their definitive association with PCC.
Future prospective studies aimed at achieving a more precise understanding and drawing explicit solid conclusions about the post-acute consequences of COVID-19 should consider several key factors. First, using a well-standardized clinical definition of outcomes instead of relying on patients’ self-reporting will increase the accuracy and reliability of study findings. Second, ensuring an appropriate sample size will increase the study's statistical power and improve the generalizability of the results. Third, matched control groups based on age, sex, and comorbidities should be included to account for potential confounding factors. Fourth, to ascertain COVID exposure, both serology and PCR to detect past and current COVID-19 infections can be used, respectively. The serology of the negative control should be repeated at least a month after the outcome assessment to check if seroconversion occurred. Fifth, considering historical and contemporary control to determine whether non-specific stressors related to the pandemic (such as social isolation, economic stress, and uncertainty) have any effect on PCC.
Conclusion
In conclusion, the symptoms of PCC are non-specific and can be commonly reported among the general population and post-upper respiratory tract infections. In addition, many of these symptoms may also result from hospitalization for causes unrelated to COVID-19. Therefore, the exclusivity of PCC as a consequence of the SARS-CoV-2 infection is questioned.
Availability of data and materials
All data generated and or analyzed throughout this study were included either in this published article or its supplementary information file.
Abbreviations
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- COVID:
-
Coronavirus disease
- COVID-19:
-
Coronavirus disease 2019
- CT:
-
Computed tomography
- ICU:
-
Intensive care unit
- OR:
-
Odds ratio
- PC:
-
Prospective cohort
- PCC:
-
Post-COVID condition
- PCR:
-
Polymerase chain reaction
- PHS:
-
Post-hospital syndrome
- PICS:
-
Post-intensive care syndrome
- PRISMA:
-
Preferred reporting items for systematic reviewers and meta-analysis
- RC:
-
Retrospective cohort
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- URTI:
-
Upper respiratory tract infection
- WHO:
-
World health organization
References
Post COVID-19 condition. https://www.who.int/teams/health-care-readiness/post-covid-19-condition. Accessed 24 Jul 2023.
Coronavirus disease (COVID-19): Post COVID-19 condition. https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(COVID-19)-post-COVID-19-condition?gclid=CjwKCAjwh8mlBhB_EiwAsztdBOP5fOn28uuTeR3KMKWnwH85acS0AGoSUqTwnFM_rMJT6a6kYbxENhoClRYQAvD_BwE. Accessed 24 Jul 2023.
Post COVID-19 condition (Long COVID). https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition. Accessed 24 Jul 2023.
Post-COVID Conditions: Information for Healthcare Providers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html. Accessed 24 Jul 2023.
Post-COVID-19 condition (long COVID): Canada.ca. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/symptoms/post-covid-19-condition.html. Accessed 24 Jul 2023.
Recommendations|COVID-19 rapid guideline: managing the long-term effects of COVID-19|Guidance|NICE.
A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1. Accessed 24 Jul 2023.
Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–7.
Selvakumar J, Havdal LB, Drevvatne M, Brodwall EM, Lund Berven L, Stiansen-Sonerud T, et al. Prevalence and characteristics associated with post-COVID-19 Condition among nonhospitalized adolescents and young adults. JAMA Netw Open. 2023;6:E235763.
Alkodaymi MS, Omrani OA, Fawzy NA, Shaar BA, Almamlouk R, Riaz M, et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28:657–66.
Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593–607.
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:1–12.
O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. eClinicalMedicine. 2023;55:101762.
Amin-Chowdhury Z, Ladhani SN. Causation or confounding: why controls are critical for characterizing long COVID. Nat Med. 2021;27:1129–30.
Zheng YB, Zeng N, Yuan K, Tian SS, Yang YB, Gao N, et al. Prevalence and risk factor for long COVID in children and adolescents: a meta-analysis and systematic review. J Infect Public Health. 2023;16:660–72.
Tsampasian V, Elghazaly H, Chattopadhyay R, Debski M, Naing TKP, Garg P, et al. Risk factors associated with post-COVID-19 Condition a systematic review and meta-analysis. JAMA Intern Med. 2023;183:566–80.
Cochrane. 10.4.3.1 Recommendations on testing for funnel plot asymmetry. Database. 2008; Deeks 2005:2006–7. https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm. Accessed 24 Jul 2023.
Blankenburg J, Wekenborg MK, Reichert J, Kirsten C, Kahre E, Haag L, et al. Comparison of mental health outcomes in seropositive and seronegative adolescents during the COVID19 pandemic. Sci Rep. 2022;12:1–8.
Boscolo-Rizzo P, Hummel T, Hopkins C, Dibattista M, Menini A, Spinato G, et al. High prevalence of long-term olfactory, gustatory, and chemesthesis dysfunction in post-covid-19 patients: a matched case-control study with 1-year follow-up using a comprehensive psychophysical evaluation. Rhinology. 2021;59:517–27.
Castro VM, Rosand J, Giacino JT, McCoy TH, Perlis RH. Case-control study of neuropsychiatric symptoms in electronic health records following COVID-19 hospitalization in 2 academic health systems. Mol Psychiatry. 2022;27:3898–903.
Elkan M, Dvir A, Zaidenstein R, Keller M, Kagansky D, Hochman C, et al. Patient-reported outcome measures after hospitalization during the covid-19 pandemic: a survey among covid-19 and non-covid-19 patients. Int J Gen Med. 2021;14:4829–36.
Fjelltveit EB, Blomberg B, Kuwelker K, Zhou F, Onyango TB, Brokstad KA, et al. Symptom burden and immune dynamics 6–18 months following mild severe acute respiratory syndrome coronavirus 2 infection (SARS-CoV-2): a case-control study. Clin Infect Dis. 2023;76:E60-70.
Gorecka M, Jex N, Thirunavukarasu S, Chowdhary A, Corrado J, Davison J, et al. Cardiovascular magnetic resonance imaging and spectroscopy in clinical long-COVID-19 syndrome: a prospective case–control study. J Cardiovasc Magn Reson. 2022;24:1–11.
Joy G, Artico J, Kurdi H, Seraphim A, Lau C, Thornton GD, et al. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. JACC Cardiovasc Imaging. 2021;14:2155–66.
Liptak P, Duricek M, Rosolanka R, Ziacikova I, Kocan I, Uhrik P, et al. Gastrointestinal sequalae months after severe acute respiratory syndrome corona virus 2 infection: a prospective, observational study. Eur J Gastroenterol Hepatol. 2022;34:925–32.
Mizrahi B, Sudry T, Flaks-Manov N, Yehezkelli Y, Kalkstein N, Akiva P, et al. Long covid outcomes at 1 year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ. 2023. https://doi.org/10.1136/bmj-2022-072529.
Nersesjan V, Fonsmark L, Christensen RHB, Amiri M, Merie C, Lebech AM, et al. Neuropsychiatric and cognitive outcomes in patients 6 months after COVID-19 requiring hospitalization compared with matched control patients hospitalized for non-COVID-19 illness. JAMA Psychiat. 2022;79:486–97.
Pinto Pereira SM, Nugawela MD, Rojas NK, Shafran R, McOwat K, Simmons R, et al. Post-COVID-19 condition at 6 months and COVID-19 vaccination in non-hospitalised children and young people. Arch Dis Child. 2023;108:289–95.
Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA: J Am Med Assoc. 2021;326:869–71.
Rivera-Izquierdo M, Láinez-Ramos-Bossini AJ, de Alba IGF, Ortiz-González-Serna R, Serrano-Ortiz Á, Fernández-Martínez NF, et al. Long COVID 12 months after discharge: persistent symptoms in patients hospitalised due to COVID-19 and patients hospitalised due to other causes: a multicentre cohort study. BMC Med. 2022;20:1–10.
Søraas A, Kalleberg KT, Dahl JA, Søraas CL, Myklebust TÅ, Axelsen E, et al. Persisting symptoms three to 8 months after non-hospitalized COVID-19, a prospective cohort study. PLoS ONE. 2021;16(8):e0256142.
Stephenson T, Pinto Pereira SM, Shafran R, de Stavola BL, Rojas N, McOwat K, et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Heal. 2022;6:230–9.
Tarazona V, Kirouchena D, Clerc P, Pinsard-Laventure F, Bourrion B. Quality of life in COVID-19 outpatients: a long-term follow-up study. J Clin Med. 2022;11:6478.
van der Maaden T, Mutubuki EN, de Bruijn S, Leung KY, Knoop H, Slootweg J, et al. Prevalence and severity of symptoms 3 months after infection with SARS-CoV-2 compared to test-negative and population controls in the Netherlands. J Infect Dis. 2023;227:1059–67.
Funk AL, Kuppermann N, Florin TA, Tancredi DJ, Xie J, Kim K, et al. Post-COVID-19 conditions among children 90 days after SARS-CoV-2 infection. JAMA Netw Open. 2022;5:E2223253.
Larsson SB, von Feilitzen GS, Andersson ME, Sikora P, Lindh M, Nordén R, et al. Self-reported symptom severity, general health, and impairment in post-acute phases of COVID-19: retrospective cohort study of Swedish public employees. Sci Rep. 2022;12:1–12.
Lopez-Leon S, Wegman-Ostrosky T, del Ayuzo Valle NC, Perelman C, Sepulveda R, Rebolledo PA, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12:9950.
Welge-Lüssen A, Wolfensberger M. Olfactory disorders following upper respiratory tract infections. Adv Otorhinolaryngol. 2006;63:125–32.
Stankevice D, Fjaeldstad AW, Agergaard J, Ovesen T. Long-term COVID-19 smell and taste disorders differ significantly from other post-infectious cases. Laryngoscope. 2023;133:169–74.
Neo JE, Tan BJW, Tan EK. “Brain fog” and COVID-19. Am J Med Sci. 2023;365(5):472–4.
Symptoms of COVID-19|CDC. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 25 Jul 2023.
Islam MF, Cotler J, Jason LA. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue Biomed Heal Behav. 2020;8:61–9.
Wirth KJ, Scheibenbogen C. Dyspnea in post-COVID syndrome following mild acute COVID-19 infections: potential causes and consequences for a therapeutic approach. Medicina (B Aires). 2022;58:419.
Taverne J, Salvator H, Leboulch C, Barizien N, Ballester M, Imhaus E, et al. High incidence of hyperventilation syndrome after COVID-19. J Thorac Dis. 2021;13:3918.
Aparisi Á, Ybarra-falcón C, García-gómez M, Tobar J, Iglesias-echeverría C, Jaurrieta-largo S, et al. Exercise ventilatory inefficiency in post-covid-19 syndrome: Insights from a prospective evaluation. J Clin Med. 2021;10:2591.
Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail. 2021;9:927.
Motiejunaite J, Balagny P, Arnoult F, Mangin L, Bancal C, d’Ortho MP, et al. Hyperventilation: a possible explanation for long-lasting exercise intolerance in mild COVID-19 survivors? Front Physiol. 2020;11:1856.
Krumholz HM. Post-hospital syndrome: A condition of generalized risk. N Engl J Med. 2013;368:100.
Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Intern Med. 2017;5:90.
Quinn KL, Stukel TA, Huang A, Abdel-Qadir H, Altaf A, Bell CM, et al. Comparison of Medical and mental health sequelae following hospitalization for COVID-19, influenza, and sepsis. JAMA Intern Med. 2023. https://doi.org/10.1001/JAMAINTERNMED.2023.2228.
Grønkjær CS, Christensen RHB, Kondziella D, Benros ME. Long-term neurological outcome after COVID-19 using all SARS-CoV-2 test results and hospitalisations in Denmark with 22-month follow-up. Nat Commun. 2023;14:1–10.
Acknowledgements
None
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
AAz conceptualized this study. The authors: NR, SM, OE, ND, HAA, OAS, SM, HR, MM, AOIE, SMAR, AZ, MH, AA, AAE, and FEH, coped with the retrieval and screening of studies, which were further crosschecked by AAz and HK. Data collection was performed by NR, SM, and OE, which were further crosschecked by MH, AA, AAE, and FEH. Data analysis was performed by AAz and double-checked by HAA. All authors contributed to the data interpretation, discussion, and research conclusions. The manuscript was created by AAz with constructive feedback from all the authors. All the authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Table S1: Supplementary preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist; Table S2: Full search strategy; Table S3: Checklist items for quality assessment of the included studies; Table S4: List of excluded studies; Table S5: Quality assessment of the included studies; Table S6: Pooled odds ratios for clinical signs and symptoms across all included studies stratified by patients’ age category regardless the hospitalization state; Fig. S1: Funnel plot of dyspnea in non-hospitalized COVID-19 patients relative to negative control; Fig. S2: Funnel plot of fatigue in non-hospitalized COVID-19 patients relative to negative control; Fig. S3: Funnel plot of brain and memory deficits in non-hospitalized COVID-19 patients relative to negative control.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Azzam, A., Khaled, H., Refaey, N. et al. The burden of persistent symptoms after COVID-19 (long COVID): a meta-analysis of controlled studies in children and adults. Virol J 21, 16 (2024). https://doi.org/10.1186/s12985-024-02284-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02284-3