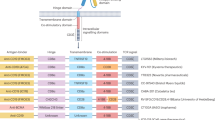
Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that can affect multiple organ systems and is heterogenous in its presentation and response to therapy. When diagnosed in childhood, SLE is associated with increased morbidity and mortality compared to adult SLE, often requiring substantial immunosuppression with the risk of significant side effects. There remains a significant unmet need for new therapies that can improve disease control and reduce glucocorticoid and other toxic medication exposure for patients with severe or refractory disease. The pathogenesis of SLE involves B cell dysregulation and autoantibody production, which are a hallmark of the disease. Currently approved B cell directed therapies often result in incomplete B cell depletion and may not target long-lived plasma cells responsible for SLE autoantibodies. It is hypothesized that by persistently eliminating both B cells and plasmablasts, CAR T therapy can halt autoimmunity and prevent organ damage in patient’s refractory to current B cell-depleting treatments. Herein we summarize the current preclinical and clinical data utilizing CAR T cells for SLE and discuss the future of this treatment modality for lupus.
Similar content being viewed by others
Background
Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that can affect multiple organ systems and is heterogenous in its presentation and response to therapy. SLE remains incurable and has significant negative impacts on the function and quality of life of affected individuals. While the current therapies used to treat SLE have led to an improvement in morbidity and mortality, many patients with SLE remain refractory to available treatment regimens [1, 2]. Additionally, when diagnosed in childhood, SLE is associated with increased morbidity and mortality compared to adult SLE [3, 4]. SLE is known to be more aggressive in non-white ethnicities [5]. Lupus nephritis (LN) in particular carries significant morbidity and has an increased prevalence and severity in children and adolescents compared to adults [6, 7].
Despite advances in therapy [8, 9], the survival rates for SLE and LN have plateaued over recent decades [10]. In the United States, SLE is the tenth leading cause of death and the number one cause of death from a chronic inflammatory disease for females in the 15–24 year age group [11]. Complete remission of LN is achieved in only 40–60% of youth despite aggressive therapy [12]. Children who develop end stage kidney disease (ESKD) due to LN have a 5-year mortality of approximately 22% [13]. Compared to other children on hemodialysis, those with LN have an increased mortality rate and are less likely to receive renal transplant despite the fact that post-transplant, graft survival and infection rates are comparable [14].
Due to increased disease severity, most children with SLE require prolonged treatment with high dose glucocorticoids and aggressive immunosuppression often with cyclcophosphamide (CYC). They can have high cumulative exposure to these therapies that carry significant morbidity and mortality, particularly from infection [15]. Glucocorticoids carry a significant morbidity risk in SLE in particular with increased risk of lupus-related damage as well as adverse effects like cataracts, hypertension, hyperglycemia, dyslipidemia, avascular necrosis, obesity and poor growth, and osteoporosis [16]. Therefore, there remains a significant unmet need for new therapies that can improve disease control and reduce glucocorticoid and other toxic medication exposure for these patients.
Disease pathogenesis and targeted treatments
Both the innate and adaptive immune responses are implicated in the pathogenesis of SLE [17,18,19]. The innate immune system includes complement proteins, macrophages, neutrophils, antigen presenting cells, and various antimicrobial molecules and support cells. In SLE, there is poor clearance of apoptotic material allowing for the presentation of intracellular substances that then trigger the inflammatory cascade [20,21,22,23]. Abnormalities and defects in the complement pathway [24] and the Fas ligand pathway [25,26,27] are also involved in its pathogenesis. Defects in neutrophil apoptosis result in increased antigen production and triggering of inflammation [28]. The adaptive immune system includes T cells, B cells, and natural killer cells. In SLE, B cells are dysregulated and produce autoantibodies, which are a hallmark of SLE [29,30,31]. Autoantibodies are thought to contribute to SLE pathogenesis in many ways, including from deposition as well as direct damage to tissues, stimulation of interferon production and signaling, and binding to and increasing the immunogenicity of neutrophil extracellular traps [32, 33].
Despite a multimodal approach to target the different aspects of the immune system involved in SLE pathogenesis, many patients have refractory disease, defined as a “failure to improve within 3–4 months or the inability to achieve partial remission after 5–12 months or complete remission after 2 years of treatment” [34, 35]. In most studies, SLE is also considered refractory when patients do not respond to 1–3 immunosuppressive medications in addition to corticosteroids [35]. Given that so many therapeutic options are exhausted in these patients, they are often reliant on glucocorticoids for disease control.
Widespread implementation of treatments such as CYC has improved outcomes for patients with refractory disease, but gains have been more modest in patients with severe kidney disease [10, 36].
B cell-directed therapies
Recent research has turned to B cell therapies to try and improve outcomes in SLE and LN [37, 38]. B cells produce autoantibodies, which are known to play a critical role in SLE pathogenesis [39, 40] In addition, B lymphocytes indirectly affect antigen-presenting activity in SLE [41]. Rituximab, a monoclonal antibody that targets CD20-expressing B cells, is used to treat lupus with mixed effects [42]. In the LN Assessment with Rituximab (LUNAR) trial for adults with SLE, rituximab was not shown to be effective in reaching the endpoints of improved clinical renal outcomes after 1 year of therapy [43]. The Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial sought to evaluate if there was a major or partial clinical response for moderate to severe extrarenal manifestation of adult SLE [44]. Likewise, it did not reach its end goal. Despite these studies not reaching their endpoints, rituximab has continued to be used clinically for the treatment of lupus due to real-world experience supporting its efficacy. Trial design may have contributed to the LUNAR and EXPLORER studies not meeting their primary endpoints– including poorly targeted patient selection, inclusion of patients on excessively high doses of corticosteroids, and inadequate study time to reach ambitious endpoints [35]. In a meta-analysis that included 31 studies involving over a thousand SLE patients, the global response to rituximab was 72% with a complete response in 46% and a partial response rate of 32% [41].
Belimumab is a fully humanized monoclonal antibody against B lymphocyte stimulator (BAFF), an essential survival factor for B cells, that is used as an adjunct B cells depleting agent for lupus treatment. Two international phase III clinical trials, BLISS-52 [45] and BLISS-76 [46], of adults with SLE led to the U.S. Food and Drug Administration FDA approval of belimumab for lupus in 2011, the first new treatment for lupus in over 50 years. Subanalysis of those studies suggested its benefit in LN. BLISS-LN was conducted and randomized 448 adult SLE patients with active, biopsy-proven class III, IV and/or V LN 1:1 to receive belimumab or placebo, plus standard of care therapy, for 104 weeks. The primary endpoint was met by 43% of patients receiving belimumab compared to 32% on placebo. A pediatric trial of belimumab, PLUTO [47], showed similar efficacy compared to the adult data with 41.2% achieving SRI response rate [48]. Thus, numerous studies have shown that anti-B cell therapies may serve as a potent treatment for SLE [49], even though we do not always see an adequate response in every patient. It has been postulated that the failure of current B cell therapies is largely related to transient or incomplete depletion of B cells, as has been seen in both rheumatoid arthritis and in SLE [50, 51].
Chimeric antigen receptor T Cells
Chimeric antigen receptor T cells (CAR T) are T cells that are genetically engineered to express a CAR to target specific surface antigens without the need for MHC presentation. A CAR combines an antibody derived scFV extracellular domain with an intracellular domain designed to stimulate T cell activation. The most common clinically utilized CAR T cells have an intracellular domain composed of a CD3𝛇 stimulatory domain combined with either 4-1BB or CD28 costimulatory domains. CAR T cells were first developed in the 1980s, however, the major clinical breakthrough occurred over 20 years later with the first human trials of anti-CD19 CAR T cells for B cell malignancies [52]. Tisagenlecleucel, an autologous anti-CD19 CAR T cell product, was the first CAR T cell product utilized in pediatric cancer. In phase I/II clinical trials of tisagenlecleucel in pediatric patients with relapsed/refractory acute lymphoblastic leukemia, complete response rates were > 80% after a single dose [53]. These dramatic response rates led to FDA approval of tisagenlecleucel in 2017, and since then 5 other CAR T cell products have been approved for adults with B cell lymphomas and multiple myeloma. The majority of CAR T cell studies utilize autologous T cells collected via apheresis. CAR T cell manufacturing times vary but may take up to 2 weeks for CAR transduction and T cell expansion. Prior to infusion, patients undergo lymphodepleting chemotherapy to enhance CAR T cell in vivo expansion and persistence. Typical regimens for lymphodepletion include both CYC and fludarabine.
As CD19 CAR T cells can deplete both normal and pathogenic B cells, as well as plasmablasts, there is promise in utilizing this novel therapeutic avenue for SLE [52]. It is hypothesized that by potently eliminating both B cells and plasmablasts, CAR T therapy can halt autoimmunity and prevent organ damage in patients refractory to current B cell-depleting treatments.
To that end, CD19 CAR T cells have demonstrated efficacy in proof-of-concept murine models of lupus [54, 55]. MRL-lpr is a spontaneous SLE murine model with severe lupus nephritis. Infusion of syngeneic anti-mouse CD19 CAR T cells into 13-week-old MRL-lpr mice after low dose irradiation resulted in nearly complete eradication of circulating CD19 + B cells in the blood, which correlated with improved survival and less active disease [54]. Importantly, the mice spared total body irradiation prior to the CD19 CAR T infusion did not exhibit such robust B cell depletion. Similarly, it was demonstrated that infusion of anti-CD19 CD8 + T cells into MRL-lpr and NZB/W mice led to profound and prolonged depletion of CD19 + B cells with aplasia lasting more than one year post therapy [55]. Anti-DNA IgG and IgM antibodies that were detectable before CAR T cell injections declined to undetectable levels after treatment and remained low to undetectable in most of the CAR T cell-treated mice for at least 19 weeks post infusion. These results correlated to a drastic improvement in survival and reversal of high-grade proteinuria in the CAR T treated mice [55].
CAR T cells in human SLE
Given this promising pre-clinical data and substantial clinical experience with CD19 CAR T cells for hematologic malignancies, there is a large interest in utilizing CD19 CAR T cells for autoimmune disease. Early clinical success has been demonstrated in patients with SLE, anti-synthetase syndrome, and myasthenia gravis [56,57,58]. In 2023, Schett and colleagues, published a comprehensive review about CAR T therapy in autoimmune disease including lupus. The first report of CAR T to treat human SLE was published by a group in Germany in 2021. Mougiakakos, D et al., reported a case of a 20-year-old woman with severe and refractory SLE who presented with active LN (class IIIA), nephrotic syndrome, pericarditis, pleurisy, rash, arthritis, and a history of Libman–Sacks endocarditis whose lupus was inadequately controlled despite treatment with hydroxychloroquine, high-dose glucocorticoids, CYC, mycophenolate mofetil (MMF), tacrolimus, belimumab, and rituximab [59]. After lymphodepletion with fludarabine and CYC, a single dose of 1.1 × 106 CD19 CAR T cells/kg (CD4 + to CD8 + T cell ratio of 3:1) was administered. The common side effects of CAR T therapy including cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and prolonged cytopenias were not observed in this patient. She had complete depletion of circulating B cells that was maintained for more than 44 days. Her double-stranded DNA (dsDNA) autoantibodies and complement levels normalized. Clinically, she had improvement in proteinuria and her SLEDAI score improved from 18 to 0 post treatment [59].
Five young adult patients with refractory lupus were enrolled in a compassionate use trial of autologous CD19 CAR T cell therapy after previously failing management with conventional immunosuppressives [60]. The patients, aged 18 to 24 years, had disease durations ranging from 1 to 9 years and high baseline lupus disease activity as measured by the SLEDAI (scores 8 to 16). All had LN (stages III, IV, or III/V). In brief, the patients received lymphodepleting chemotherapy with fludarabine and CYC from day − 5 to day − 3 followed by 1 × 106 CAR T cells/kg. All five patients had an in vivo expansion of the infused CD19 CAR T cells and rapid and sustained depletion of circulating B cells. All patients achieved control of LN and SLEDAI scores decreased to inactive levels (range 0–2) by 3 months. Nephritis resolved in all five of the patients, as demonstrated by normalization of proteinuria. Other manifestations including arthritis, fatigue, and fibrosis of cardiac and pulmonary valves resolved after the administration of CD19 CAR T cells. Complement C3 levels normalized, and anti-dsDNA and ANA levels significantly declined in all patients. The patients were able to discontinue both corticosteroids and hydroxychloroquine and demonstrated a durable mean remission of 9.8 months (5–17 months). Severe CRS was not observed in any of the patients, although 3 patients developed grade 1 CRS (fever alone) lasting 2–3 days that was successfully treated by administration of methimazole in four of the patients and a single infusion of tocilizumab (8 mg/kg) in one patient. No patients had signs of ICANS, or infections reported during the short-term period of the study (5–17 months). No SLE flare has been reported in any of the patients thus far despite reconstitution of patients peripheral B cells [61]. More recently, long-term follow up data up to 29 months post infusion demonstrated a lasting remission in all patients without any immunosuppressive medications [62].
Additionally, a 32 year old woman with SLE was successfully treated with CD19-targeted CAR T cells [63]. Lupus was diagnosed during pregnancy. Clinical manifestations included serositis, nephritis, cytopenias, immunologic findings (ANA, dsDNA, hypocomplementemia), and concern for central nervous system involvement. She remained refractory to treatment with multiple agents including tacrolimus, belimumab, CYC, and rituximab. Prior to undergoing conditioning treatment for cell retrieval, she was weaned off immunosuppression three weeks prior to leukapheresis except for glucocorticoids, which were tapered to 5 mg daily. She tolerated CAR T cell treatment with no reported adverse effects, and after three months she achieved lupus low disease activity per low lupus disease activity state (LLDAS) criteria, proteinuria normalized, and she no longer had detectable circulating anti-dsDNA antibodies three months after treatment. Of note, B cells returned to the peripheral blood within 2 months without associated disease recurrence.
Importantly, the feasibility of manufacturing autologous CAR T cells from patients with lupus has been demonstrated to be achievable [64]. Manufacturing failures can occur in patients with active malignancy who are heavily immunosuppressed and are often cytopenic. Similar concerns exist in patients with SLE. All SLE patients studied were on glucocorticoids and received a wide array of immunosuppressive treatment prior to cell collection for CAR T generation. Glucocorticoids were tapered to a maximum of 10 mg/day one week prior to apheresis while other immunosuppressive agents were held two weeks beforehand. Despite patients being on low dose glucocorticoids, enough autologous T cells were successfully collected to generate ten times the minimum amount of CAR T cells needed for a single clinical infusion [64].
CAR T cells targeting the B cell Maturation Antigen (BCMA) receptor are FDA approved in patients with multiple myeloma and have a similar safety profile to CD19 CAR T cells. One strategy is to deplete both pathogenic B cells and antibody producing plasma cells by targeting both BCMA and CD19. To this end, Zhang et al. developed a CD19-BCMA compound CAR T cell product that has been successfully to eliminate donor specific alloantibodies prior to stem cell transplantation [65]. The use of the compound CD19-BCMA CAR T cells in a patient with SLE and stage IV diffuse large B cell lymphoma was reported [66]. She received fludarabine and CYC lymphodepleting chemotherapy followed by an infusion of 5.3 × 106 CD19-BCMA CAR T cells/kg. B cell depletion was rapid and complete through 198 days after treatment. The patient was able to discontinue glucocorticoid therapy and achieved normal complement levels and undetectable ANA levels after treatment. She remained in remission 23 months after treatment despite B cell reconstitution, but specific immunophenotyping of the reconstituted B cells was not reported [66]. Safety and preliminary efficacy of a combination of CD19 and BCMA CAR T cells in SLE has also been reported [67]. 12 patients with SLE refractory to multiple lines of immunosuppressive therapies including rituximab and belimumab were enrolled in a clinical trial. Patients received 1 or 2 × 10^6 of each CD19 and BCMA CAR T cells. The infusions were safe with only mild grade 1 CRS and no neurologic toxicity. Anemia was reported in all (Table 1). Mild infections were reported in 4 patients within 6 months of infusion [67]. After infusion of CD19/BCMA CAR T cells all patients met LLDAS criteria and were able to discontinue all immunosuppression including glucocorticoids. B cell aplasia was noted for approximately 3 months after CAR T cell therapy and no SLE flares have occurred at a median follow up of 118.5 (45–524) days [67].
Discussion and conclusions
In conclusion, despite recent expansion of available treatments for SLE, remission rates remain unacceptably low, and morbidity and mortality rates remain unacceptably high. This is especially true for patients with SLE onset in childhood who have worse disease severity and outcomes when compared to adult-onset SLE. B cell directed CAR T cell therapy offers significant promise for improving outcomes for refractory pediatric disease in particular. As described above, CAR T cell therapy has demonstrated safety and efficacy in treating refractory SLE in young adults with durability reported to last 2 years after receiving a single infusion. Novel therapeutics have recently been approved to treat lupus nephritis, including in the induction phase of therapy; however, most of these agents serve as add-on therapies [68]. The potential of CAR T cell therapies to reduce the need for other potentially toxic medications including glucocorticoids in children and adolescents with SLE is therefore particularly encouraging. The safety profile to date for the SLE population is more favorable compared to the oncology population due to a smaller target T cell population, and therefore a lower underlying risk of CRS.
CAR T cells enable the elimination of tissue-resident B cells that may still contribute to disease pathogenesis after treatment with the other currently available B cell depleting agents such as Rituximab and Belimumab. However, the peripheral B cell compartment most often does recover, and no significant side effects have been reported to date in this patient population. Notably, the recently reported safety signal of T cell lymphomas from CAR T is rare and has not been reported in patients without an underlying primary malignancy [69]. As limited numbers of patients have underwent CAR T therapy for autoimmune diseases, further data is required to assess its long-term safety. Controlled clinical trials of CAR T therapy in both adult and pediatric lupus will provide further insight into the efficacy, safety, and durability of this therapy (Table 2).
Data availability
Not applicable.
Abbreviations
- CAR T:
-
Chimeric antigen receptor T cells
- Cyc:
-
Cyclophosphamide
- ESKD:
-
End stage kidney disease
- SLEDAI:
-
SLE disease activity index
- LLDAI:
-
Lupus low disease activity index
- SLE:
-
Systemic lupus erythematosus
References
Askanase A et al. New and future therapies: changes in the therapeutic armamentarium for SLE. Best Pract Res Clin Rheumatol, 101865 (2023).
Muñoz-Grajales C, Yilmaz EB, Svenungsson E, Touma Z. Systemic lupus erythematosus and damage: what has changed over the past 20 years? Best Pract Res Clin Rheumatol, 101893 (2023).
Huang X, et al. Differences in the clinical manifestations and mortality of systemic lupus erythematosus onset in children and adults: a systematic review and meta-analysis. Int Arch Allergy Immunol. 2022;183:116–26.
Smith EM, Lythgoe H, Hedrich CM. Current views on lupus in children. Curr Opin Rheumatol. 2023;35:68–81.
Liang MH et al. Choosing to end African American Health disparities in SLE. Arthritis Rheumatol (2024).
Pennesi M, Benvenuto S. Lupus Nephritis in Children: novel perspectives. Medicina. 2023;59:1841.
Yu C, et al. Lupus nephritis: new progress in diagnosis and treatment. J Autoimmun. 2022;132:102871.
Accapezzato D, et al. Advances in the pathogenesis and treatment of systemic lupus erythematosus. Int J Mol Sci. 2023;24:6578.
Morand EF, Fernandez-Ruiz R, Blazer A. & Niewold, T. B. Advances in the management of systemic lupus erythematosus. bmj 383 (2023).
Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971–2015: a systematic review and bayesian meta‐analysis. Arthritis Rheumatol. 2016;68:1432–41.
Yen EY, Singh RR. Brief report: lupus—an unrecognized leading cause of death in young females: a population-based study using nationwide death certificates, 2000–2015. Arthritis Rheumatol. 2018;70:1251–5.
Davidson JE, et al. Renal remission status and longterm renal survival in patients with lupus nephritis: a retrospective cohort analysis. J Rhuematol. 2018;45:671–7.
Hiraki LT, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthr Rhuem. 2011;63:1988–97.
Wasik H, Chadha V, Galbiati S, Warady B, Atkinson M. Dialysis outcomes for children with lupus nephritis compared to children with other forms of nephritis: a retrospective cohort study. Am J Kidney Dis. 2022;79:626–34.
Pan L, Yang S. Childhood-onset systemic lupus erythematosus: characteristics and the prospect of glucocorticoid pulse therapy. Front Immunol. 2023;14:1128754.
Enríquez-Merayo E, Cuadrado MJ. Steroids in Lupus: enemies or allies. J Clin Med. 2023;12:3639.
Crow MK. Pathogenesis of systemic lupus erythematosus: risks, mechanisms and therapeutic targets. Ann Rheum Dis. 2023;82:999–1014.
Loh JT, Lam K-P. Neutrophils in the pathogenesis of rheumatic diseases. Rheumatol Immunol Res. 2022;3:120–7.
Sutanto H, Yuliasih Y. Disentangling the pathogenesis of systemic lupus erythematosus: close ties between immunological, genetic and environmental factors. Medicina. 2023;59:1033.
Liu J, Zhang X, Cao X. Dendritic cells in systemic lupus erythematosus: from pathogenesis to therapeutic applications. J Autoimmun. 2022;132:102856.
Mevorach D. Systemic lupus erythematosus and apoptosis: a question of balance. Clin Rev Allergy Immunol. 2003;25:49–59.
Podestà MA, Faravelli I, Ponticelli C. Autophagy in lupus nephritis: a delicate balance between regulation and disease. Autoimmun rev. 2022;21:103132.
Liphaus BL, Kiss MH. B. The role of apoptosis proteins and complement components in the etiopathogenesis of systemic lupus erythematosus. Clinics. 2010;65:327–33.
Coss SL, et al. The complement system and human autoimmune diseases. J Autoimmun. 2023;137:102979.
Elkon K. Apoptosis in SLE–too little or too much? Clin Exp Rheumatol. 1994;12:553–9.
Lee YH, Bae S-C, Choi SJ, Ji JD, Song GG. Associations between the FAS – 670 A/G and – 1,377 G/A polymorphisms and susceptibility to autoimmune rheumatic diseases: a meta-analysis. Mol Biol Rep. 2012;39:10671–9.
Suzuki N, Ichino M, Mihara S, Kaneko S, Sakane T. Inhibition of FAS/FAS ligand-mediated apoptotic cell death of lymphocytes in vitro by circulating anti‐FAS ligand autoantibodies in patients with systemic lupus erythematosus. Arthritis Rheumatism: Official J Am Coll Rheumatol. 1998;41:344–53.
Ma S, Jiang W, Zhang X, Liu W. Insights into the pathogenic role of neutrophils in systemic lupus erythematosus. Curr Opin Rheumatol. 2023;35:82–8.
Gómez-Bañuelos E, Fava A, Andrade F. An update on autoantibodies in systemic lupus erythematosus. Curr Opin Rheumatol. 2023;35:61–7.
Kang N, et al. Aberrant B-cell activation in systemic lupus erythematosus. Kidney Dis. 2022;8:437–45.
Petty RE, et al. Textbook of pediatric rheumatology. Elsevier Health Sciences; 2020.
Kubota T. An emerging role for Anti-DNA antibodies in systemic Lupus Erythematosus. Int J Mol Sci. 2023;24:16499.
Qiu W, Yu T, Deng G-M. The role of organ-deposited IgG in the pathogenesis of multi-organ and tissue damage in systemic lupus erythematosus. Front Immunol. 2022;13:924766.
Bertsias GK, et al. Joint European League against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012;71:1771–82.
Cervera R, et al. Treatment for refractory lupus nephritis: rituximab vs triple target therapy. Autoimmun rev. 2019;18:102406.
Moroni G, et al. Changing patterns in clinical–histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann Rheum Dis. 2018;77:1318–25.
Arbitman L, Furie R, Vashistha H. B cell-targeted therapies in systemic lupus erythematosus. J Autoimmun. 2022;132:102873.
Plüß M, Piantoni S, Tampe B, Kim AH, Korsten P. Belimumab for systemic lupus erythematosus–focus on lupus nephritis. Hum Vaccines Immunotherapeutics. 2022;18:2072143.
Lou H, Ling GS, Cao X. Autoantibodies in systemic lupus erythematosus: from immunopathology to therapeutic target. J Autoimmun. 2022;132:102861.
Sciascia S, et al. Autoantibodies testing in autoimmunity: diagnostic, prognostic and classification value. Autoimmun rev. 2023;22:103356.
Alshaiki F, et al. Outcomes of rituximab therapy in refractory lupus: a meta-analysis. Eur J Rheumatol. 2018;5:118.
Abid N et al. The Safety and Efficacy of Rituximab and Belimumab in systemic lupus erythematosus: a systematic review. Cureus 15 (2023).
Rovin B, LUNAR Investigator Group, et al. Efficacy and safety of Rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64:1215–26.
Merrill J, et al. Assessment of flares in lupus patients enrolled in a phase II/III study of Rituximab (EXPLORER). Lupus. 2011;20:709–16.
Navarra SV, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–31.
Furie R, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthr Rhuem. 2011;63:3918–30.
Brunner HI, et al. Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial. Ann Rheum Dis. 2020;79:1340–8.
Brunner HI, et al. Efficacy and safety of belimumab in paediatric and adult patients with systemic lupus erythematosus: an across-study comparison. Rmd Open. 2021;7:e001747.
Gómez-Urquiza JL, et al. Efficacy and safety of New B Cell-targeted Biologic Agent for the treatment of systemic lupus erythematosus: a systematic review and Meta-analysis. J Clin Med. 2023;12:4848.
Mendez LMG, et al. Peripheral blood B cell depletion after Rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrol. 2018;13:1502–9.
Vital EM, et al. Reduced-dose rituximab in rheumatoid arthritis: efficacy depends on degree of B cell depletion. Arthr Rhuem. 2011;63:603–8.
Mitra A, et al. From bench to bedside: the history and progress of CAR T cell therapy. Front Immunol. 2023;14:1188049. https://doi.org/10.3389/fimmu.2023.1188049.
Maude SL, et al. Tisagenlecleucel in Children and Young adults with B-Cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. https://doi.org/10.1056/NEJMoa1709866.
Jin X, et al. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell Mol Immunol. 2021;18:1896–903. https://doi.org/10.1038/s41423-020-0472-1.
Kansal R, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. 2019;11. https://doi.org/10.1126/scitranslmed.aav1648.
Blache U, Tretbar S, Koehl U, Mougiakakos D, Fricke. CAR T cells for treating autoimmune diseases. RMD open. 2023;9:e002907.
Pecher A-C, et al. CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome. JAMA. 2023;329:2154–62.
Sheng L, et al. Concurrent remission of lymphoma and Sjögren’s disease following anti-CD19 chimeric antigen receptor-T cell therapy for diffuse large B-cell lymphoma: a case report. Front Immunol. 2023;14:1298815.
Mougiakakos D, et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. 2021;385:567–9.
Mackensen A, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med. 2022;28:2124–32.
Schett G et al. (BMJ Publishing Group Ltd, 2022).
Müller F, et al. CD19 CAR T-Cell therapy in Autoimmune Disease - A Case Series with Follow-up. N Engl J Med. 2024;390:687–700. https://doi.org/10.1056/NEJMoa2308917.
Taubmann J et al. CD19 CAR-T cell treatment: unraveling the role of B cells in systemic Lupus Erythematosus. Arthritis Rheumatol (2023).
Kretschmann S, et al. Successful generation of CD19 Chimeric Antigen Receptor T Cells from patients with Advanced systemic lupus erythematosus. Transplantation Cell Therapy. 2023;29:27–33.
Zhang Z, et al. Immunotherapy targeting B cells and long-lived plasma cells effectively eliminates pre-existing donor-specific allo-antibodies. Cell Rep Med. 2023;4:101336. https://doi.org/10.1016/j.xcrm.2023.101336.
Zhang W, et al. Treatment of systemic lupus erythematosus using BCMA-CD19 compound CAR. Stem Cell Reviews Rep. 2021;17:2120–3.
Feng J, Yongxian H, Chang AH, Huang H. CD19/BCMA CAR-T cell therapy for refractory systemic lupus erythematosus-safety and preliminary efficacy data from a phase i clinical study. Blood. 2023;142:4835.
Fanouriakis A, et al. Multidisciplinary approach to lupus nephritis: clinical pearls, pitfalls, and positioning of newly-approved agents. Lupus. 2023;32:1155–63.
Prasad V, T-Cell Lymphoma. From CAR T-Cell Therapy—A New Safety Notice. JAMA (2024).
Acknowledgements
We have no acknowledgements.
Funding
There is no funding for this work.
Author information
Authors and Affiliations
Contributions
IS was the primary author and summarized the mouse data, the primary human data to date on CAR T for SLE, and the current clinical trials occurring. LH contributed to the background of SLE and rituximab. SC contributed to the immunologic background, citations, and in making Table 1. MK reviewed and provided feedback. KD reviewed and provided feedback. ML reviewed and provided feedback and contributed to the background of chimeric antigen receptor T cells. SPA reviewed and provided feedback. SA is the senior author and provided feedback and helped finalize the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors (IS, LH, SC, MK, KD, ML, SPA, and SA) have no competing interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Stojkic, I., Harper, L., Coss, S. et al. CAR T cell therapy for refractory pediatric systemic lupus erythematosus: a new era of hope?. Pediatr Rheumatol 22, 72 (2024). https://doi.org/10.1186/s12969-024-00990-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12969-024-00990-4