Abstract
Background
Anopheles stephensi is recognized as the main malaria vector in Iran. In recent years, resistance to several insecticide classes, including organochlorine, pyrethroids, and carbamate compounds, has been reported for this medically important malaria vector. The main objective of the present study was to evaluate the insecticide susceptibility status of An. stephensi collected from the southern part of Iran, and to clarify the mechanism of resistance, using bioassay tests and molecular methods comparing the sequence of susceptible and resistant mosquitoes.
Methods
Mosquito larvae were collected from various larval habitats across six different districts (Gabrik, Sardasht, Tidar, Dehbarez, Kishi and Bandar Abbas) in Hormozgan Provine, located in the southern part of Iran. From each district standing water areas with the highest densities of Anopheles larvae were selected for sampling, and adult mosquitoes were reared from them. Finally, the collected mosquito species were identified using valid keys. Insecticide susceptibility of An. stephensi was tested using permethrin 0.75%, lambdacyhalothrin 0.05%, deltamethrin 0.05%, and DDT 4%, following the World Health Organization (WHO) test procedures for insecticide resistance monitoring. Additionally, knockdown resistance (kdr) mutation in the voltage-gated sodium channel (vgsc) gene was sequenced and analysed among resistant populations to detect possible molecular mechanisms of observed resistance phenotypes.
Results
The susceptibility status of An. stephensi revealed that resistance to DDT and permethrin was found in all districts. Furthermore, resistance to all tested insecticides in An. stephensi was detected in Gabrik, Sardasht, Tidar, and Dehbarez. Analysis of knockdown resistance (kdr) mutations at the vgsc did not show evidence for the presence of this mutation in An. stephensi.
Conclusion
Based on the results of the current study, it appears that in An. stephensi from Hormozgan Province (Iran), other resistance mechanisms such as biochemical resistance due to detoxification enzymes may be involved due to the absence of the kdr mutation or non-target site resistance. Further investigation is warranted in the future to identify the exact resistance mechanisms in this main malaria vector across the country.
Similar content being viewed by others
Background
Iran is a malaria-endemic country. Seven species of Anopheles mosquitoes are recognized as malaria vectors, namely Anopheles culicifacies sensu lato (s.l.), Anopheles fluviatilis s.l., Anopheles stephensi, Anopheles superpictus s.l., Anopheles maculipennis s.l. Anopheles dthali, and Anopheles sacharovi. Among these, An. stephensi is notably recognized as the main malaria vector [1,2,3,4]. The majority of malaria cases are concentrated in the southern and southeastern provinces of Iran including Hormozgan, Sistan, and Baluchestan, and southern parts of Kerman. These regions are characterized by refractory malaria, and have suitable conditions for the reproduction of Anopheles mosquitoes; most of the aforementioned malaria vectors are present there [4,5,6]. Various insecticides, such as malathion, mirimiphos-methyl, DDT, dieldrin, propoxur, lamdacyhalothrin and deltamethrin, belonging to different groups, have been utilized for controlling Anopheles mosquitoes in malaria-endemic areas. This control is implemented through different intervention tools, including indoor residual spraying (IRS) and insecticide-treated nets (ITNs). However, the extensive use of insecticides has led to the emergence of resistance in An. stephensi against most of these insecticides [7, 8]. Reports from different districts of Hormozgan province, such as Siahoo, Geno, Bandar Abbas, Bashagard, and Jask, have indicated resistance in An. stephensi to DDT and lambdacyhalothrin through the World Health Organization (WHO) insecticide susceptibility protocol [9,10,11,12]. This resistance is attributed to mutations in the voltage-gated sodium channel (vgsc), resulting in insecticide target site insensitivity, known as knockdown resistance (kdr) mutations. Additionally, metabolic resistance due to detoxification enzymes, such as cytochrome P450 monooxygenases (P450s), is implicated in pyrethroid metabolic resistance in An. stephensi [13,14,15]. For instance, biochemical and molecular investigations conducted on An. stephensi from Afghanistan revealed the presence of both kdr mutation and metabolic mechanisms responsible for insecticide resistance [16]. Although there are several reports about emergence of resistance to insecticide in An. stephensi from different parts of Hormozgan province, but no study has been conducted on this species to detect the mechanisms of resistance to insecticides therefore the objective of the current study was to characterize the susceptibility status of the main malaria vector, An. stephensi, as well as its resistance mechanisms, utilizing bioassay tests and molecular methods in the southern part of Iran.
Methods
Study area
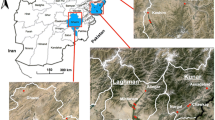
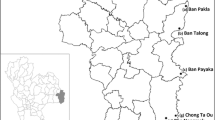
Hormozgan Province is localized in the southern region of Iran, bordering the Persian Gulf. It encompasses 13 major cities and lies between latitude 25°24′–28°53′N and longitude 52°44′–59°14′ E [17]. For the present study, six cities (Gabrik, Sardasht, Tidar, Dehbarez,Kishi and Bandar Abbas) within Hormozgan Province, which have a history of implementing malaria control programs using IRS and ITNs, were selected for entomological studies (Fig. 1).
Sample collection
Six distinct sampling locations were chosen across five counties: Jask, Bashagard, Bandar Abbas, Khamir, and Rudan. These areas have a historical record of insecticide use in malaria control programs. The topographical and climatic conditions of these districts present a diverse environmental landscape. Jask, with its hot desert climate, is indicative of its arid summers and mild winters. Bashagard, characterized by its mountainous terrain, endures cold winters and hot, dry summers, with a climate moderated by its proximity to the mountains. Bandar Abbas and Khamir, situated at a lower elevation, are subject to a subtropical desert climate, reflecting it’s hotter and more humid conditions. Rudan's climate mirrors that of Bandar Abbas but with longer, sweltering summers and short, cool winters, mostly clear skies (Fig. 2).
Mosquito larvae were collected from various larval habitats using the standard dipper method [18]. From each district standing water areas with the highest densities of Anopheles larvae were selected for sampling. Collected samples were transferred to the insectary and placed in a holding container for rearing under standard conditions with a temperature range of 25–29 °C, a photoperiod of 12:12 h (light: dark), and a humidity level of 50–70%. The emerged adult mosquitoes were identified using valid keys [19] and were fed with 10% aqueous sucrose solution and subsequently utilized in both bioassay and molecular investigations.
Adult susceptibility tests procedure
Adult susceptibility tests of mosquitoes were conducted following WHO guidelines, utilizing standard insecticide-impregnated filter paper: permethrin 0.75%, lambdacyhalothrin 0.05%, deltamethrin 0.05%, and DDT 4%. Each bioassay involved 100 test mosquitoes (four replicates of 25), non-blood-fed female mosquitoes aged between 3 and 5 days, Additionally, 50 female control mosquitoes (2 replicates) were exposed to the insecticide-impregnated filter paper for 1 h. After a 24-h recovery period, the mortality rates were recorded, moreover, mosquitoes were supplied with 10% fresh sugar solution during this time. Correction of the mortality rate in the test samples was corrected using the Abbott formula when the mortality rate of control ranged between 5 and 20%. Tests with a control mortality rate exceeding 20% were repeated [20]. Based on WHO criteria, the susceptibility level of the mosquitoes was categorized into three classes: mortality between 98–100% indicated susceptibility; mortality between 90 and 97% suggested a candidate for resistance or tolerance requiring further investigation to confirm resistance; and mortality less than 90% was classified as resistance [21].
Molecular analysis of the voltage-gated sodium channel (vgsc) gene
The genomic DNA from mosquito legs was extracted using the Collins extraction method [22]. Polymerase chain reaction (PCR) assays were developed for amplifying the S6 segment of domain II of the vgsc gene. This fragment contains a region in the exon that leads to kdr type resistance in case of mutation. The forward and reverse primers used were St-F (5′- GAT TGT GTT CCG TGT GCT GT -3′) and St-L/SR (5′- GCG GGC AGGGCG GCG GGG GCG GGG CCC GAT CGG AAA GTA AGT TAC TTA CGT CT -3′), respectively.
The cycling conditions consisted of an initial denaturation step at 95 °C for 5 min, followed by 35 cycles at 95 °C for 30 s, 48 °C for 30 s, and 72 °C for 45 s, and a final extension step at 72 °C for 7 min [23]. From each resistant population extracted DNA with high quality send for sequencing after that sequences obtained were submitted as queries to the National Center for Biotechnology Information’s (NCBI) Basic Local Alignment Search Tool (BLAST) to confirm correct loci were amplified. A comparison of the sequence in susceptible and resistant mosquitoes was conducted on all samples to detect the presence or absence of kdr type resistance in vgsc, referencing sequence details from a previous study by Singh et al. [23] on An. stephensi. Finally, all sequences were deposited in the gene bank with accession numbers (MN868413.1- MN868424.1).
Results
Insecticide susceptibility status
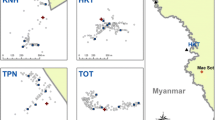
The mortality rate of An. stephensi following exposure to the insecticides was calculated after a 24-h recovery period and is illustrated in Fig. 3. According to the criteria for insecticide resistance outlined by the WHO, resistance to DDT and permethrin have been observed across all districts. However, it is noteworthy that while resistance to all tested insecticides in An. stephensi was detected in Gabrik, Sardasht, Tidar, Dehbarez, a different pattern emerged in Kishi and Bandar Abbas. In Kishi and Bandar Abbas, mosquito mortality was observed between 90–97% for lambdacyhalothrin and deltamethrin, indicating the population of these areas as resistance candidates. Across all districts, DDT exhibited the lowest toxicity in An. stephensi, suggests a high level of resistance to this insecticide.
Analysis of vgsc sequence
Based on adult susceptibility test results in Gabrik, Sardasht, Tidar, and Dehbarez, resistance to all tested insecticides was detected. Subsequently, molecular investigations were carried out on their population. The sequences of vgsc in all populations exhibited a high degree of similarity (99%) with the sequences from India (accession number: JF304954.1), moreover, these sequences correspond to a susceptible strain that was deposited in Gene Bank [23]. The comparison of the region containing kdr mutations at the locus L1014 (blue highlighted in Fig. 4) due to the amino acid substitution of leucine (TTA) with phenylalanine (TTT) or serine (TCA) in the vgsc gene. kdr mutations L1014F and L1014S [23, 24] were not found in resistant populations across all districts.
Discussion
The findings of the current study indicated resistance to DDT detected in An. stephensi across all studied areas. This corroborates with previous studies conducted in the southern part of Iran, where a high level of resistance to DDT in An. stephensi has been observed [25,26,27], including in Hormozgan Province [7, 9,10,11,12]. Moreover, resistance to DDT in An. stephensi has been reported in neighboring countries of Iran, such as Pakistan, Saudi Arabia, Iraq, Oman, United Arab Emirates, and Afghanistan [12, 13, 15]. Although resistance to all pyrethroid insecticides, as well as DDT, was detected in An. stephensi in Gabrik, Sardasht, Tidar and Dehbarez, this species exhibited tolerance to lambdacyhalothrin and deltamethrin in Bandar Abbas and Kishi. In a previous study conducted by Zare et al. in Jask County, recognized as an active malaria focus in Hormozgan Province, insecticide susceptibility tests revealed that An. stephensi was resistant to lambda-cyhalothrin and tolerance to Deltamethrin. Additionally, they reported that this observation might be attributed to the utilization of these mentioned insecticides for IRS and LLINs. Although concerning permethrin, the results differed, as An. stephensi was found to be susceptible to it [12].
The susceptibility status of An. stephensi to tested insecticides underwent alterations, particularly with an increase in resistance to deltamethrin, a change also noted for permethrin, which had been previously considered susceptible. In the current investigation, insecticide susceptibility tests revealed that An. stephensi demonstrated resistance to Permethrin in Gabrik district as one of the regions in Jask county. Furthermore, this emergence of insecticide resistance was also attributed to the aforementioned intervention measures for controlling malaria vectors. Reports of resistance to pyrethroid compounds have also been documented in Southeast Iran concerning An. stephensi against cyfluthrin and lambdacyhalothrin, mirroring this study. The usage of IRS and ITNs has been implicated in the escalation of this resistance status [1]. Resistance to pyrethroids insecticides in a malarious area where IRS and ITNs are used should be considered an alarm signal, as the development of resistance to insecticides leads to a reduction of the impact of vector control actions [28, 29]. Analysis of the vgsc gene indicated the absence of kdr L1014F and L1014S mutations in all studied areas. This suggests the presence of other resistance mechanisms, such as metabolic resistance due to detoxification enzymes, in An. stephensi. Similar findings have been reported in the Somali region of eastern Ethiopia, where researchers observed the absence of kdr L1014F and L1014S mutations in the collected samples of An. stephensi. These researchers have suggested that the resistance to pyrethroids observed in the species may be due to metabolic or other mechanisms [14].
In two previous studies in Sistan and Baluchestan, the neighbouring province of Hormozgan, different types of resistance mechanisms were identified in An. stephensi populations. Initially, biochemical analyses revealed metabolic mechanisms involved in cyfluthrin and DDT resistance in An. stephensi from Chabahhar region [6]. Subsequently, molecular assays were conducted in Saravan region, on An. stephensi populations with tolerance to deltamethrin, permethrin and resistance to DDT, which provided evidence for kdr mutation among examined samples. Furthermore, following the initial detection of kdr allele from a pyrethroid-selected strain in Dubai, the presence of the L1014F mutation, the same mutation previously described, was also reported from the Saravan region, in Iran [28, 30]. In eastern Afghanistan, regarding An. stephensi[16] and in West Africa concerning An. gambiae [31], the findings indicated that both of these mechanisms are involved in resistance. Similar to these findings, it is plausible that the same mechanisms may also exist in the aforementioned populations from Sistan and Baluchestan. However, because each of these studies examined only one resistance mechanism separately, their results differed.
It is noteworthy that most studies in Iran have primarily focused on biochemical analyses to elucidate resistance mechanisms in malaria vectors, with only a few examining both biochemical and molecular mechanisms together. Additionally, the overactivity of detoxification enzymes has been frequently implicated in resistance in malaria vectors [6, 32]. For instance, in a temephos-resistant strain of An. stephensi from Chabahar, molecular analysis did not reveal evidence of G119S mutation in the acetylcholinesterase gene, but biochemical assays indicated enzymatic involvement in resistance [33].
Conclusion
In the present study, resistance against all tested insecticides was observed in An. stephensi in most of the studied areas. The absence of the kdr mutation in resistant populations suggests that the observed resistance may be attributed to biochemical or metabolic mechanisms. This main malaria vector in Iran has demonstrated resistance to all tested insecticides, indicating the need for further biochemical studies to precisely identify the resistance mechanisms in An. stephensi.
Availability of data and materials
The data supporting the findings of the study must be available within the article and/or its supplementary materials, or deposited in a publicly available database.
References
Gorouhi MA, Vatandoost H, Oshaghi MA, Raeisi A, Enayati AA, Mirhendi H, et al. Current susceptibility status of Anopheles stephensi (Diptera: Culicidae) to different imagicides in a malarious area, Southeastern Iran. J Arthropod Borne Dis. 2016;10:493–500.
Azari-Hamidian S, Norouzi B, Harbach RE. A detailed review of the mosquitoes (Diptera: Culicidae) of Iran and their medical and veterinary importance. Acta Trop. 2019;194:106–22.
Hanafi-Bojd AA, Azari-Hamidian S, Hassan V, Zabihollah C. Spatio-temporal distribution of malaria vectors (Diptera: Culicidae) across different climatic zones of Iran. Asian Pac J Trop Med. 2011;4:498–504.
Vatandoost H, Raeisi A, Saghafipour A, Nikpour F, Nejati J. Malaria situation in Iran: 2002–2017. Malar J. 2019;18:200.
Vatandoost H, Zahirnia AH. Responsiveness of Anopheles maculipennis to different imagicides during resurgent malaria. Asian Pacific J Trop Med. 2010;3:360–3.
Gorouhi MA, Oshaghi MA, Vatandoost H, Enayati AA, Raeisi A, Abai MR, et al. Biochemical basis of cyfluthrin and DDT resistance in Anopheles stephensi (Diptera: Culicidae) in malarious area of Iran. J Arthropod Borne Dis. 2018;12:310–20.
Abadi YS, Sanei-Dehkordi A, Paksa A, Gorouhi MA, Vatandoost H. Monitoring and mapping of insecticide resistance in medically important mosquitoes (Diptera: Culicidae) in Iran (2000–2020): a review. J Arthropod Borne Dis. 2021;15:21–40.
Edrissian GH. Malaria past and pre-sent situation in Iran. Iran J Parasitol. 2006;1:1–14.
Vatandoost H, Oshaghi MA, Abai MR, Shahi M, Yaaghoobi F, Baghaii M, et al. Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan Province, Southern Iran, 2002. Acta Trop. 2006;97:196–203.
Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, Haghdoost AA, Shahi M, Sedaghat MM, et al. Entomological and epidemiological attributes for malaria transmission and implementation of vector control in southernIran. Acta Trop. 2012;121:85–92.
Soleimani-Ahmadi M, Vatandoost H, Shayeghi M, Raeisi A, Abedi F, Eshraghian MR, et al. Vector ecology and susceptibility in a malaria endemic focus in southern Islamic Republic of Iran. East Mediterr Health J. 2012;18:1034–41.
Zare M, Soleimani-Ahmadi M, Davoodi SH, Sanei-Dehkordi A, et al. Insecticide susceptibility of Anopheles stephensi to DDT and current insecticides in an elimination area in Iran. Parasit Vectors. 2016;9:571–5.
Enayati A, Hanafi-Bojd AA, Sedaghat MM, Zaim M, Hemingway J. Evolution of insecticide resistance and its mechanisms in Anopheles stephensi in the WHO Eastern Mediterranean region. Malar J. 2020;19:258.
Yared S, Gebressielasie A, Damodaran L, Bonnell V, Lopez K, Janies D, et al. Insecticide resistance in Anopheles stephensi in Somali region, Eastern Ethiopia. Malar J. 2020;19:180.
Ahmad M, Buhler C, Pignatelli P, Ranson H, Nahzat SM, Naseem M, et al. Status of insecticide resistance in high-risk malaria provinces in Afghanistan. Malar J. 2016;15:98.
Safi NHZ, Ahmadi AA, Nahzat S, Warusavithana S, Safi N, Valadan R, et al. Status of insecticide resistance and its biochemical and molecular mechanisms in Anopheles stephensi (Diptera: Culicidae) from Afghanistan. Malar J. 2019;18:249.
Hormozgan Province (2024). https://en.wikipedia.org/wiki/Hormozgan_Province.
Sattler MA, Mtasiwa D, Kiama M, Premji Z, Tanner M, Killeen GF, et al. Habitat characterization and spatial distribution of Anopheles sp. mosquito larvae in Dar es Salaam (Tanzania) during an extended dry period. Malar J. 2005;4:4.
Azari-Hamidian S, Harbach RE. Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae). Zootaxa. 2009;2078:1–33.
Abbot WS. A method of computing the efectiveness of an insecticide. J Econ Entomol. 1925;18:265–7.
WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Geneva: World Health Organization; 2016.
Collins FH, Mendez MA, Rasmussen MO, Mehaffey PC, Besansky NJ, Finnerty V. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am J Trop Med Hyg. 1987;37:37–41.
Singh OP, Dykes CL, Lather M, Agrawal OP, Adak T. Knockdown resistance (kdr)-like mutations in the voltage-gated sodium channel of a malaria vector Anopheles stephensi and PCR assays for their detection. Malar J. 2011;10:59.
Carter TE, Gebresilassie A, Hansel S, Damodaran L, Montgomery C, Bonnell V, et al. Analysis of the knockdown resistance locus (kdr) in Anopheles stephensi, an arabiensis, and Culex pipiens s.l. for insight into the evolution of target-site pyrethroid resistance in Eastern Ethiopia. Am J Trop Med Hyg. 2022;106:632–8.
Vatandoost H, Mashayekhi M, Abai MR, Aflatoonian M, Hanafi-Bojd AA, Sharifi I. Monitoring of insecticides resistance in main malaria vectors in a malarious area of Kahnooj district, Ker-man province, Southeastern Iran. J Vector Borne Dis. 2005;42:100–8.
Borhani N, Vatandoost H. Susceptibility and irritability levels of main malaria vectors to synthetic pyrethroids in the endemic areas of Iran. Acta Med Iran. 2004;42:240–7.
Davari B, Vatandoost H, Ladonni H, Shaeghi M, Oshaghi MA, Basseri HR, et al. Comparative efficacy of different imagicides against different strains of Anopheles stephensi in the malaroius areas of Iran, 2004–2005. Pakistan J Biol Sci. 2006;9:885–92.
Hajibeygi R, Hejripour SZ, Taghavi N, Shahali H, Zarei S, Nouri M, et al. Evaluation of the knockdown resistance locus (kdr) in Anopheles stephensi (Diptera: Culicidae) in southeastern Iran. J Vector Borne Dis. 2023;60:444–8.
Syahrani L, Asih PB, Bowolaksono A, Dwiranti A, Zubaidah S, Rozi IE, et al. Impact of a spatial repellent intervention on Anopheles kdr insecticide resistance allele in Sumba, Indonesia. Malar J. 2024;22:31.
Enayati AA, Vatandoost H, Ladonni H, Townson H, Hemingway J. Molecular evidence for a kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi. Med Vet Entomol. 2003;17:138–44.
Corbel V, N’Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin. West Africa Acta Trop. 2007;101:207–16.
Abbasi E, Vahedi M, Bagheri M, Gholizadeh S, Alipour H, Moemenbellah-Fard MD. Monitoring of synthetic insecticides resistance and mechanisms among malaria vector mosquitoes in Iran: a systematic review. Heliyon. 2022;8: e08830.
Soltani A, Vatandoost H, Oshaghi MA, Ravasan NM, Enayati AA, Asgarian F. Resistance mechanisms of Anopheles stephensi (Diptera: Culicidae) to temephos. J Arthropod Borne Dis. 2015;9:71–83.
Acknowledgements
This study results from a research project approved by the Vice-Chancellor for Research and Technology of Hormozgan University of Medical Sciences.
Funding
This research has been funded by Hormozgan University of Medical Sciences Project No960079.
Author information
Authors and Affiliations
Contributions
ASD designed the study, interpreted the data, and drafted the manuscript; YS designed the study interpreted the data, and reviewed the manuscript; AP provided resources and facilitated the molecular analysis and reviewed the manuscript; MAG helped with the molecular analysis; MSA analysed the bioassay data, designed the study and reviewed the manuscript; SAJ collected samples, reared mosquitoes tested bioassay. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present investigation was approved by the ethics committee of Hormozgan University of Medical Sciences, Iran (IR.HUMS.REC.1396.41).
Consent for publications
All authors have read the manuscript and consented to its publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sanei-Dehkordi, A., Paksa, A., Gorouhi, M.A. et al. Preliminary monitoring of knockdown resistance (kdr) mutation in Anopheles stephensi: insights from a malarious area in Southeastern Iran. Malar J 23, 211 (2024). https://doi.org/10.1186/s12936-024-05042-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-05042-6