Abstract
Background
Identifying alternative sustainable feed sources with high nutritional values is crucial for the future of environmentally and socially responsible aquaculture. In this regard, microalgae have been proven to have positive effects on fish health, which overwhelmed our interest in this study.
Methods
Pediastrum boryanum (P. boryanum) was incorporated into Nile tilapia feed at concentrations of 0, 0.75, and 1.5 mg/kg, as control, PbExt0.75, and PbExt1.5 groups to assess its effects on growth and biochemical indices, oxidant/antioxidant activities, immune and stress-related gene expression, and intestinal morphology.
Results
After 8 weeks, fish fed P. boryanum supplemented feed exhibited significant increases in final weight, length, condition factor, body weight gain, and specific growth rate, while the spleen-somatic index (SSI) and hepatosomatic index (HSI) showed no significant differences compared to the control group. Dietary P. boryanum supplementation also enhanced IgM levels and lysozyme activity, along with no marked effect on markers of liver function enzymes (alanine aminotransferase/ALT and aspartate aminotransferase/AST) or protein status (total protein and albumin). Furthermore, P. boryanum addition increased the activity of superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) enzymes, highlighting its antioxidant potential, whereas malondialdehyde (MDA) concentrations showed no significant differences among the groups. Gene expression analysis revealed that tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), and transforming growth factor-β1 (TGF-β1) expression notably increased in groups fed P. boryanum containing feed, while no significant difference was observed in hepatic Heat Shock Protein 70 (HSP70) mRNA expression. Histopathological examination revealed no adverse effects of P. boryanum supplementation on the liver, spleen, or intestinal tissues. Villous height and villous surface area were notably increased in the high P. boryanum supplementation group, suggesting improved intestinal integrity and nutrient absorption.
Conclusion
Dietary P. boryanum supplementation can potentially improve growth performance, immune response, antioxidant status, and intestinal health of Nile tilapia, making it a promising candidate for sustainable aquaculture.
Similar content being viewed by others
Introduction
The practice of aquaculture, involving the farming of fish, crustaceans, mollusks, and aquatic plants, has been experiencing significant growth driven by the rising demand for aquatic food due to the expanding global population [1]. The volume of global fish production amounted to 186.6 million metric tons in 2023, up from 184.6 million metric tons in 2022 [2]. Aquaculture now provides over 50% of fish for human consumption and is projected to overtake wild-caught fisheries as the primary source of seafood in the coming years [3]. However, the continued expansion and intensification of aquaculture faces sustainability challenges that threaten its long-term viability.
Microalgae, which are microscopic photosynthetic organisms, have emerged as promising feed additives and partial fishmeal substitutes owing to their well-documented nutritional properties [4]. Microalgae are rich sources of proteins, lipids, polysaccharides, pigments, minerals, and vitamins [5,6,7]. They also contain bioactive compounds such as carotenoids, polyunsaturated fatty acids, and polysaccharides, which can enhance growth, health, and immune responses in farmed aquatic species [8, 9]. Additionally, microalgae production systems have ecological advantages over other feed sources, as microalgae can be cultivated efficiently on non-arable land using non-potable water without the need for fertilizer or pesticide input [10]. Therefore, microalgae are gaining interest for supporting the nutritional, environmental, and economic sustainability of aquaculture.
The green microalga Pediastrum boryanum (P. boryanum) of the Hydrodictyaceae family is particularly interesting. P. boryanum is characterized by flat oval-shaped coenobia comprising 4, 8, 16, or 32 cells surrounded by marginal spines [11]. This ubiquitous freshwater microalga found in lakes and ponds has a rigid cell wall composed of pectin and cellulose [12, 13]. The rigid cell wall confers stability and protects the nutritional content from degradation in the fish digestive system [14]. Valuable nutritional characteristics have spurred interest in exploring P. boryanum as a sustainable feed additive to enhance the growth, health, and product quality of farmed animals [15,16,17,18,19,20]. Therefore, the current study provides evidence for the benefits of dietary P. boryanum supplementation on the growth performance and immune responses of key farmed finfish species tilapia (Oreochromis niloticus). Overall, this study provides a basis for understanding the diverse benefits conferred by dietary P. boryanum and its promising role in supporting sustainable intensification in the aquaculture industry.
Materials and Methods
Ethics approval statement
The experiment was conducted according to a protocol involving the use of animals approved by the Mansoura University Animal Care and Use Committee (VM. PhD.23.10.25). All fish handling procedures and regulations followed the ARRIVE guidelines for Animal Care and Use. Furthermore, all relevant organizational and government rules and regulations governing the ethical use of the experimental animals were followed. Written informed consent was obtained from all the participants in this study.
Diet preparation
The fish feed was prepared in the laboratory of the Department of Nutrition, Faculty of Veterinary Medicine, Mansoura University. Three diets were formulated according to NRC [21], as described in Table 1. An adaptation methodology [22], was used to extract the active components from P. boryanum, in accordance with a previous report [23]. P. boryanum was acquired from the National Research Center of Cairo, Egypt. Algal powder (100 g) was extracted using 1 L of distilled magnetized water at 70 °C for two hours. Three distinct diets were designed based on the initial diet, incorporating doses of 0, 0.75, and 1.5 mg/kg, and designated as follows. Control, PbExt0.75, and PbExt1.5, respectively. All ingredients were mixed with gelatin, and water was added until stiff dough was formed. The paste was pelleted into 3-mm-diameter pellets using a meat mincer (ME605131 1600-Watt, Moulinex, Groupe SEB, France). The resulting strands were shadow-dried, broken up, sieved into pellets, and stored in plastic bags at 4 °C until use.
Experimental design and rearing Fish
A total of 120 apparently healthy Nile tilapias, with an average body weight of 57.5 ± 2.5 g, were selected for this study. The study was conducted as a field trial at a private fish farm in Manzala City, Dakahlia Governorate, Egypt. Fish were randomly assigned to three experimental groups in duplicates (20 fish/hapa). Nile tilapia were allocated to six hapas (200 × 500 × 100 cm3, 10 m3) for the trial. Water quality parameters were checked on the farm and were maintained within the normal range as follows: 26 -27 °C for water temperature, 6.7 for D.O., 6.5–7.6 for pH, and 0.02–0.15 mg/L for ammonia. Fish were fed at 3% of their body weight throughout the experimental period, divided into two equal rations at 09.00 h and 16.00 h.
Growth performance assessment
Three fish from each hapa group (N = 6) were sampled at the end of the trial. One fish was sampled at a time from each hapa; the three fish were sedated with 30 mg/L of buffered tricane (MS-222®ARGENT) [24], and each was weighed and measured to determine the final body weight (FBW), length, body weight gain (BWG), condition factor (k), and specific growth rate (SGR) [25].
Body weight gain (BWG) = FW − IW, where FW is the final weight and IW is the initial weight.
Condition factor (K) = (W/L3) × 100; where: W = weight of fish in grams and L = total length of fish in "cm.”
Specific growth rate (SGR) = 100 × (LN(FW) − LN(IW))/duration (days), where LN = Length, FW = final weight, and IW = initial weight.
All measurements were taken carefully to minimize stress and potential harm to the fish.
Sample collection
After the trial, fish (six fish per group) were randomly selected and euthanized using buffered tricaine methanesulfonate (E10521-10G, Sigma-Aldrich, UK). Blood samples from the caudal veins was then collected and transferred into sterile tubes and left to clot at room temperature for 4 h before centrifugation at 1198 × g for 10 min to collect serum for biochemical and immunological parameter analysis as well as antioxidant status. The fish were then immediately dissected. The liver and spleen were weighed to determine the hepatosomatic index (HSI) and spleen-somatic index (SSI) according to standard AOAC methods [26], respectively, using the following formulae: HSI = Wt of fish liver/fish BWt × 100, SSI = Wt of fish spleen/fish BWt × 100. A liver sample was placed in RNAlater® (Sigma Aldrich, USA) for the purpose of analyzing immune gene expression. Portions of the liver, spleen, and intestine were preserved in 10% neutral formaldehyde until histopathological examination.
Evaluation of serum immunological parameters
Serum lysozyme activity was measured using fish lysozyme (LZM) ELIZA kits (MyBioSource, Inc., USA) according to the manufacturer's instructions. The optical density was measured at a wavelength of 450 nm. The concentration of lysozyme in the samples was then determined by comparing the O.D. of the samples to the standard curve, and the lysozyme concentration was reported as µg/mL. In addition, serum IgM levels were determined using fish IgM ELIZA kits (CSBE-12045Fh, CUSABIO BIOTECH CO., Ltd, China), according to the manufacturer's instructions. The optical density was measured at a wavelength of 450 nm. The optical density was measured spectrophotometrically at a wavelength of 450 nm. The concentration of IgM in the samples was then determined by comparing the O.D. of the samples to the standard curve, and the IgM concentration was reported as µg/mL.
Determination of antioxidant status and oxidative stress markers
Malondialdehyde (MDA) levels and the activities of reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) were spectrophotometrically measured (6745 UV/Vis. Spectrophotometer) using a colorimetric method. MDA levels, GSH, and SOD activities were measured at 534 nm, 405 nm, and 560 nm, respectively, using diagnostic kits (Biodiagnostics, Egypt), as described elsewhere [27, 28], and were expressed as nmol/L serum. CAT activity was measured using an Elabscience® biochemical assay kit (Elabscience Biotechnology Inc., USA) according to [29], by measuring the decrease in hydrogen peroxide concentration at 240 nm, and was expressed as U/L serum.
Determination of serum biochemical parameters
Total serum protein and albumin levels were measured spectrophotometrically using test kits (VitroScient, Egypt, Germany). Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were estimated using commercial kits (Spinreact, Spain).
RNA Extraction, Complementary DNA synthesis, and qRT-PCR
Total RNA from each group was extracted from 100 mg of liver tissue in Genzol™ (Geneaid Biotech Ltd, Taiwan) using a manual homogenizer without DNase treatment, followed by dissolving of the pellet in TE buffer (pH 8.0), as described previously [30]. A Nanodrop spectrophotometer (Q5000/Quawell, Massachusetts, USA) was used to determine RNA concentration. Following the manufacturer’s instructions, 1 µg of total RNA was used to synthesize complementary DNA (cDNA) using a TOPscript™ RT DryMIX(dT18) cDNA Synthesis Kit (Enzynomics Co. Ltd., Daejeon, Republic of Korea), according to the manufacturer's protocol. The specific primers used to amplify the selected genes of Nile tilapia with stress marker genes, heat shock protein-70 (HSP-70), proinflammatory genes, tumor necrosis factor-alpha (TNF-α), anti-inflammatory genes, Transforming Growth Factor-β (TGF-β1), and Interleukin-10 (IL-10), in addition to β-actin as a housekeeping gene, have been previously described [31, 32]. Solg™ 2X Real-Time PCR Smart mix (Including SYBR® Green) was used in conjunction with the QuantStudio1™ Real-Time PCR System (Applied Biosystems™ Thermo Fisher Scientific, USA) to quantitatively analyze gene expression (SolGent Co., Ltd. Yuseong-gu, Daejeon, Korea). The thermocycling conditions were as follows: 95°C for 20 s, followed by 40 cycles of denaturation at 60°C for 40 s, and elongation at 72°C for 30 s. Relative gene expression levels were evaluated in triplicate on template controls using the 2−ΔΔCT formula [33].
Histopathological examination
The Liver, spleen, and intestine tissue samples were fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin wax, and sectioned at 5 µm. Hematoxylin and Eosin (H&E) was consistently applied to specific slides in accordance with the protocol outlined in [34], to examine their morphology and integrity. Histomorphometric measurements were obtained by examining the stained slides under a light microscope (Olympus CX 31) and capturing images using a connected camera (Olympus DP 21 digital camera) (Olympus Corporation, Tokyo, Japan). This procedure was performed as previously described [35]. The five highest villi per section were identified and selected for measurement. The length from the tip to the bottom and height per section of each villus were determined. The mean values of the measurements were denoted as the villus height per section [36].
Statistical analysis
Before conducting one-way analysis of variance (ANOVA), Kolmogorov–Smirnov and Levene's tests were used to assess normality and homogeneity, respectively. Analyses were performed using GraphPad® statistics package version 8.4.2. (GraphPad Software, Inc., USA). To examine the differences between the means, Tukey's honestly significant difference test was applied. The significance level was set at P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***). All data are presented as mean ± SEM.
Results
Growth performance assessment
The bar graphs in Fig. 1 illustrate the growth performance parameters. FW and BWG showed similar trends, where they displayed a significant increase in PbExt0.75 and PbExt1.5 groups compared to the control group, with no statistical differences between the PbExt-supplemented groups. However, fish length increased significantly in the PbExt0.75 group compared to the control, with no variation (P > 0.05) compared to PbExt1.5. The SGR, K factor, and SSI showed a significant increase in PbExt1.5, compared to the control group. The hepatosomatic index (HSI) showed no significant differences across all groups.
The growth indices, including final weight, length (A), K factor, hepatosomatic index (HSI), and spleen-somatic index (SSI) (B), of Nile tilapia fed on control or diets supplemented with P. boryanum at 0.75 or 1.5 mg/kg. Data were expressed as Mean ± SD. Values with a different letter superscript are significantly different between groups
Evaluation of serum immunological parameters
A notable increase in the activity of lysozyme was observed in both PbExt0.75 (P < 0.01) and PbExt1.5 (P < 0.001) groups compared to the control group, with a statistically significant increase in lysozyme (P < 0.05) between the fish fed the high level of P. boryanum group compared to the other groups (Fig. 2B). The IgM levels in Nile tilapia displayed a noticeable increase in the Nile tilapia-fed PbExt0.75 (P < 0.05) and PbExt1.5.(P < 0.001) compared to that in the control group, with a statistically significant increase in both PbExt doses (P < 0.01) (Fig. 2A).
A Lysozyme activity and (B) IgM level of Nile tilapia fed on control or diets supplemented with P. boryanum at 0.75 or 1.5 mg/kg. Data were expressed a Mean ± SD. Values with a different letter superscript are significantly different between groups. Asterisks indicate levels of significance (ANOVA with post hoc Tukey test, *P < 0.05; **P < 0.01; ***P < 0.001)
Determination of antioxidant status and oxidative stress markers
The oxidant/antioxidant parameters are shown in Fig. 3 A-D. The activities of SOD, CAT, and GSH increased in the groups fed PbExt0.75 (SOD, CAT, P < 0.05; and GSH, P < 0.001) and PbExt1.5 (GSH, CAT, P < 0.001; and SOD, P < 0.01) compared to the control. Only SOD and CAT showed a significant rise in the PbExt1.5 (P < 0.05) group compared to the PbExt0.75 group. Interestingly, the MDA levels (Fig. 3A) were not significantly different among the groups.
A Catalase (CAT), (B) glutathione reductase (GSH), and (C) Malondialdehyde (MDA) activities of Nile tilapia fed on control or diets supplemented with P. boryanum at 0.75 or 1.5 mg/kg. Data were expressed as a Mean ± SD. Values with a different letter superscript are significantly different between groups. Asterisks indicate levels of significance (ANOVA with post hoc Tukey test, *P < 0.05; **P < 0.01; ***P < 0.001)
Determination of serum biochemical parameters
Serum biochemical parameters are presented in Fig. 4 A-D. Liver function enzyme (ALT and AST) and protein profiles (total protein and serum albumin levels) showed no statistical differences between the supplemented and non-supplemented groups.
The liver function enzyme activities (A) AST, (B) ALT, and protein profile [(C) total protein and (D) Albumin] of Nile tilapia fed on control or diets supplemented with P. boryanum at 0.75 or 1.5 mg/kg. Data were expressed as a Mean ± SD. Values with a different letter superscript are significantly different between groups. Asterisks indicate levels of significance (ANOVA with post hoc Tukey test, *P < 0.05; **P < 0.01; ***P < 0.001)
Gene expression of Hepatic mRNA level of Nile tilapia
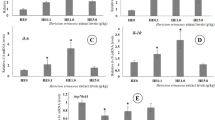
The bar graphs are shown in Fig. 5. represents the relative expression of proinflammatory and stress genes, including TNF-α, IL-10, TGF-β1, and HSP70. There was no significant difference in the expression of HSP70 (Fig. 5B) between the control and supplemented groups. A significant increase in TNF-α (Fig. 5A) was observed in both PbExt0.75 (P < 0.05) and PbExt1.5 (P < 0.01) groups compared to the control, with no statistical changes between the PbExt supplemented doses. A significant increase was observed in IL-10 (Fig. 5C) in favor of PbExt1.5 compared to both the control (P < 0.05) and PbExt0.75 (P < 0.05) groups, with no statistical significance between the latter. A significant increase was observed in TGF-β1 (Fig. 5D) in both the PbExt0.75 (P < 0.01) and PbExt1.5 (P < 0.01) groups compared to the control group.
Histopathological examination
Histopathological examination of the control and supplemented groups revealed a normal histological appearance of hepatocytes, sinusoids, and hepatopancreas in all groups. Similarly, in the spleen, both white and red pulps, including melanomacrophage centers, maintained their histological appearance across all groups. Similarly, in the proximal intestine, there was no observable structural damage in the groups supplemented with the microalgae extract compared with that in the control group (Fig. 6). Interestingly, a marked increase in villous height (VH) was observed in the PbExt1.5 group compared to the control. Villous surface area (VSA) and the VH/crypt depth (CD) ratio showed the same trend, whereas VSA increased significantly in both PbExt0.75 (P < 0.05) and PbExt1.5 (P < 0.01), and the same VH/CD ratio (P < 0.001) compared to the control group, without notable changes between the former. Villous width (VW) and CD did not show any variation (P > 0.05) across all groups (Fig. 7).
Villous height (VH), villous width (VW), crypt depth (CD), VH/CD ratio, and villous surface area (VSA) in the proximal intestine of Nile tilapia fed on control or diets supplemented with P. boryanum at 0.75 or 1.5 mg/kg. Data were expressed as a Mean ± SD. Values with a different letter superscript are significantly different between groups. Asterisks indicate levels of significance (ANOVA with post hoc Tukey test, *P < 0.05; **P < 0.01; ***P < 0.001)
Discussion
The freshwater microalga P. boryanum is an excellent fish feed additive because of its exceptional nutritional composition, growth-promoting effects, pigmentation benefits, immunomodulatory properties, and potential for sustainable production [37]. These properties make it a promising fish feed additive. However, there is a lack of research on these comprehensive properties in literature. To the best of our knowledge, this study is the first to investigate comprehensive arrays of parameters to evaluate P. boryanum benefits. Therefore, in our discussion, we will point out similar microalgal extracts used in fish, and we will also cite studies using P. boryanum microalgae in other animal species.
In the present study, P. boryanum extract supplementation improved the growth indices at both doses, while displaying no significant changes in the HSI across all groups, indicating a degree of consistency in liver size relative to total body mass. Similar, the addition of mixed microalgal extracts of Chlorella vulgaris (C. vulgaris), Euglena viridis (E. viridis), and Spirulina platensis (S. platensis) at 0.01, 0.02% of basal diet exhibited a marked increase in the growth efficacy of Labeo rohita fish after 28 days of supplementation [38]. However, no noticeable change was found in the growth of Nile tilapia juveniles fed diets containing an extract obtained from the green alga Ulva clathrata at higher doses (0.5–1%) [39]. These discrepancies could be related to the type of extract used, the dose of supplementation, the fish spp and its life stage, and the rearing conditions, either in indoor tanks or farm ponds. Another study conducted by Ahmed et al. [40], reported that rabbits fed a diet enriched with 10 ml/kg P. boryanum extract exhibited notable enhancements in final body weight, daily weight gain, and feed conversion ratio. These findings could be attributed to the nutritional composition of P. boryanum, as it is a good source of proteins, essential fatty acids, minerals, carotenoids, chlorophylls, and phenolic compounds with antioxidant properties [15,16,17,18,19]. For instance, P. boryanum contains 45–60% protein, 9–14% lipids including linoleic and α-linolenic acids, and carotenoids such as violaxanthin, lutein, and β-carotene at concentrations ranging from 4.4 to 29.2 mg/g dry weight [15, 18, 20]. Therefore, its high protein content reflects an increase in growth performance, and the rigid cell wall of P. boryanum confers stability and protects its nutritional content from degradation in the fish digestive system [14]. These findings highlight the potential of P. boryanum as a dietary supplement that influences the growth performance of Nile tilapia.
Lysozyme activity and IgM levels were significantly elevated in the PbExt0.75 and PbExt1.5 groups compared to the control. Coincided with our findings, rohu (Labeo rohita) fed with a mixed algal extract (C. vulgaris, E. viridis, and S. platensis) at 0.01 and 0.02% of the basal diet showed significantly higher activity of lysozyme after 28 days of experimental trial [38]. Also, It has been reported that dietary inclusion of Ulva clathrata (U. clathrata) extract at a lower level of 0.1% of the diet displayed a noticeable amelioration in the innate immunity parameters, such as lysozyme and complement activities, after 60 days of feeding [39]. Furthermore, the dietary addition of 0.5% of red algal extract (Laurencia caspica) to the Nile tilapia showed a significantly increased in lysozyme and IgM activities after 50 d [41]. It has been suggested that P. boryanum supplementation elicits B lymphocytes owing to its polysaccharide content, thereby augmenting both innate and adaptive immune responses [13]. Additionally, arachidonic acid, a precursor of biologically active prostaglandins and leukotrienes, promotes leukocyte chemotaxis, reactive oxygen species formation, and other pro-inflammatory effects [42].
The results of this study provide intriguing insights into the effects of P. boryanum microalgal extract on oxidative stress markers and antioxidant status in Nile tilapia, in which antioxidant enzyme activities were enhanced without oxidative stress. Our results are consistent with Khanzadeh et al. [41], who reported a significant increase in SOD and CAT after the dietary addition of 0.5% of red algal extract (Laurencia caspica) to the Nile tilapia for 50 days. Similarly, Abdel-Latif and Khalil [43], reported that dietary supplementation with S. platensis (2.5%) for eight weeks significantly enhanced the activities of CAT, SOD, GSH-Px in Nile tilapia. Furthermore, myeloperoxidase activity displayed no significant changes in Labeo rohita fed mixed algal extract at 0.1 and 0.2% of basal diet [38], and in hybrid red tilapia fed Haematococcus pluvialis- microalgal extract at 0.5. 1, and 1.5 mg/kg for 60 days [44], which was attributed to the biological constituents of P. boryanum microalga, such as carotenoids and canthaxanthin, which play a remarkable role as antioxidant compounds and free radical scavengers. Furthermore, polysaccharide content enhances antioxidant defenses [45]. P. boryanum biosynthesizes and accumulates high levels of carotenoids, chlorophylls, and phenolic compounds with antioxidant properties [15,16,17,18,19].
There were no adverse effects on liver function enzymes and protein profiles, as indicated by the lack of statistical changes. Previous studies showed similar results, finding that Labeo rohita fed a 0.08% diet of mixed algae extract (C. vulgaris, E. viridis, and S. platensis) for 28 days had no significant albumin-globulin ratio than the control group [38]. Similarly, protein profile, ALT and AST levels did not change in Nile tilapia fed 0.5% of red algal extract (Laurencia caspica) for 50 days [41]. The same findings were observed in Gibel carp upon feeding algal extract at (Ulva lactuca and Solieria chordalis) 0.2% [46]. Other studies concerning P. boryanum in other animal species revealed same effects as well, Ahmed et al. [40], showed no statistical changes in liver enzyme levels (ALT and AST) or plasma albumin in New Zealand white rabbits fed Pediastrum spp. Silva et al. [13] showed that the administration of various P. boryanum extracts did not alter the serum levels of the liver enzymes ALT and AST in rats. Fonseca et al. [47] reported no significant increases in liver ALT and AST enzymes in mice orally administered 300 mg/kg or 2000 mg/kg freeze-dried P. boryanum biomass suspended in carboxymethyl cellulose. These findings suggested that there were no adverse effects on liver function or disruption of protein homeostasis. The lack of significant changes in these biochemical parameters coupled with the MDA, HSP70 expression level, and histopathology results (see below) support its safety on fish health, and Fonseca et al. [47] classified P. boryanum as having minimal toxicity or security. Our findings contribute to the overall understanding of the safety and physiological compatibility of P. boryanum supplementation in Nile tilapia, encouraging its potential application in aquaculture, without apparent negative effects on liver health or protein metabolism.
P. boryanum dietary supplementation showed no significant hepatic gene expression of HSP70, whereas TNF-α, IL-10, and TGF-β1 expression was significantly increased in groups fed with P. boryanum. Tumor Necrosis Factor-alpha (TNF-α) functions as a pro-inflammatory cytokine and serves as a valuable prognostic indicator for immune responses in fish [48], whereas Heat Shock Protein 70 (HSP70) functions as a molecular chaperone, safeguarding fish cells against environmental stressors [49]. Interleukin-10 (IL-10) acts as an anti-inflammatory cytokine, preventing tissue damage and chronic inflammation by reducing unnecessary T-cell responses to microbial infections [50]. Transforming Growth Factor Beta 1 (TGF-β1), a versatile cytokine, orchestrates cellular functions such as growth, differentiation, and immune responses and crucially contributes to tissue homeostasis and adaptive reactions during injury or inflammation [50]. The increase in TNF-α gene expression upon P. boryanum extract administration showed the immunostimulatory property of the microalga as the polysaccharide content from the microalgal extract stimulated phagocytes to produce cytokines [39]. Similarly, dietary inclusion of Nannochloropsis gaditana microalgal extract at 10 ml/kg of diet in zebra fish (Danio rerio) [51], and Haematococcus pluvialis microalgal extract at 0.5. 1, and 1.5 mg/kg in hybrid red tilapia fed for 60 days [44], and mixed algal extract at 0.01 and 0.02% of basal diet in Labeo rohita for 28 days displayed cytokine expression by upregulating the expression of TNF-α and IL-10 genes. Similarly, increased transcripts TNF-α mRNAs were significantly modified in the senegalese sole post-larvae administered with crude extract of N. gaditana (0.2 mg dry mass equivalent mL−1 sea water) [52]. To date, there are no reports on the effect of the microalgal extract on the expression of hepatic cytokines, so the upregulation of both pro-and anti-inflammatory cytokines in Nile tilapia-fed P. boryanum diets might be an effort to balance the excess production of anti-inflammatory cytokines, namely IL-10, TGF-β1 which can compromise the host's ability to remove microorganisms through suppression of immune cell function, proposing immunomodulatory effects [51].
Our findings shed light on the antioxidative effect of the microalga P. boryanum, which reflected the stable expression of HSP70 among groups, together with the MDA results herein, confirming that no stresses were revealed upon P. boryanum feeding on Nile tilapia. Furthermore, bioactive compounds in microalgae, such as flavonoids, polysaccharides, and polyunsaturated fatty acids (arachidonic acid), have been associated with anti-inflammatory activity by reducing neutrophil infiltration and modulating proinflammatory cytokines [13], thus maintaining fish health and homeostasis [45].
Our previous analyses herein were histopathologically evident, where no alteration in tissues architecture had been found, besides, favorable histomorphometric indices, particularly the VH, VH/CD, and VSA were observed, which positively influenced nutrient absorption with subsequent growth improvement. Similarly, Zebrafish juveniles supplemented with 10 g/kg of Nannochloropsis gaditana showed no inflammatory changes in intestinal histopathological examination and positively affected their morphometry [51]. In the same context, found no negative influences of feeding 1.2% microalgae (Schizochytrium sp.) in Nile tilapia [53]. In the same context, hybrid red tilapia fed Haematococcus pluvialis microalgal extract at 1 g/kg for 60 days enhanced the intestinal villi length and width, and no histopathological lesions were induced in liver or intestine fed the microalgal extract [44]. The data obtained as mentioned earlier are due to the bioactive constituents of microalgae, which contribute to the suppression of oxidative damage and inflammatory changes, thus maintaining fish growth, immune status, and overall health.
Conclusion
In conclusion, this study provides evidence that P. boryanum microalgal extract has beneficial effects as a feed additive for Nile tilapia (Oreochromis niloticus). Fish fed P. boryanum extract demonstrated improved growth performance and innate immune and antioxidant status, and induced no oxidative stress or inflammatory changes. Furthermore, P. boryanum maintains intestinal histomorphology and health. Overall, this study provides a strong basis for utilizing P. boryanum microalgal extract to develop efficient and sustainable feed that can enhance the productivity and quality of farmed tilapia.
Availability of data and materials
Data is provided within the manuscript.
Abbreviations
- BWG:
-
Body weight gain
- FW:
-
Final weight
- IW:
-
Initial weight
- K:
-
Condition factor
- SGR:
-
Specific growth rate
- MSS-222:
-
Tricaine methanesulfonate
- HSI:
-
Hepatosomatic index
- SSI:
-
Spleenosomatic index
- LZM:
-
Lysozyme
- MDA:
-
Malondialdehyde
- GSH:
-
Reduced glutathione
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AOAC:
-
Association of Official Analytical Chemists
- DW:
-
Distilled water
- NRC:
-
National Research Council
- P. boryanum :
-
Pediastrum boryanum
- ROS:
-
Reactive oxygen species
- TNF-α :
-
Tumor necrosis factor-α
- HSP70 :
-
Heat Shock Protein 70
- TGF-β1 :
-
Transforming Growth Factor-β
- IL-10 :
-
Interleukin-10
References
Norman R, Crumlish M, Stetkiewicz S. The importance of fisheries and aquaculture production for nutrition and food security. Revue scientifique et technique (International Office of Epizootics). 2019;38(2):395–407.
Verdegem M, Buschmann AH, Latt UW, Dalsgaard AJ, Lovatelli A. The contribution of aquaculture systems to global aquaculture production. J World Aquaculture Soc. 2023;54(2):206–50.
FAO. FAO yearbook. Fishery and Aquaculture Statistics 2018/FAO annuaire. 2020.
Ahmad A W, Hassan S, Banat F. An overview of microalgae biomass as a sustainable aquaculture feed ingredient: food security and circular economy. Bioengineered. 2022;13(4):9521–47.
Ampofo J, Abbey L. Microalgae: Bioactive composition, health benefits, safety and prospects as potential high-value ingredients for the functional food industry. Foods. 2022;11(12):1744.
Verni M, Demarinis C, Rizzello CG, Pontonio E. Bioprocessing to Preserve and Improve Microalgae Nutritional and Functional Potential: Novel Insight and Perspectives. Foods. 2023;12(5):983.
Eze CN, Onyejiaka CK, Ihim SA, Ayoka TO, Aduba CC, Nwaiwu O, Onyeaka H. Bioactive compounds by microalgae and potentials for the management of some human disease conditions. AIMS microbiology. 2023;9(1):55.
Zhou L, Li K, Duan X, Hill D, Barrow C, Dunshea F, Martin G, Suleria H. Bioactive compounds in microalgae and their potential health benefits. Food Bioscience. 2022;49:101932.
Saha S, Shukla SK, Singh HR, Singh B, Jha SK. Bioactive Compounds from Microalgae. In An Integration of Phycoremediation Processes in Wastewater Treatment. Amsterdam: Elsevier. 2022. pp. 337–58.
Tibbetts SM, Milley JE, Lall SP. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J Appl Phycol. 2015;27:1109–19.
Komárek J, Jankovská V. Review of the Green Algal Genus Pediastrum;-Implication for Pollenanalytical Research. Blumea. 2003;48(2):288.
Blokker P, Schouten S, van den Ende H, de Leeuw JW, Hatcher PG, Sinninghe Damsté JS. Chemical structure of algaenans from the fresh water algae Tetraedron minimum, Scenedesmus communis and Pediastrum boryanum. Org Geochem. 1998;29(5):1453–68.
Silva MGCd, Hort MA, Hädrich G, Bosco LD, Vaz GR, Silva MMAd, Tavella RA, Badiale-Furlong E, Silva Júnior FMRd, Dora CL. Anti-inflammatory and Antioxidant Effects of the Microalga Pediastrum boryanum in Carrageenan-Induced Rat Paw Edema. Braz Arch Biol Technol. 2022;64:e21200748.
Torres Á, Fermoso FG, Rincón B, Bartacek J, Borja R, Jeison D. Chapter Challenges for Cost-Effective Microalgae Anaerobic Digestion. 2013.
Plodsomboon S, Sanoamuang L. Effects of Pediastrum boryanum and Dried Chlorella as Feeds on the Growth Performance and Carotenoid Content of the Fairy Shrimp Branchinella thailandensis (Branchiopoda, Anostraca). Tropical Natural History. 2023;23:73–81.
Jacinto GSS, Cruz G, Cabral AA, Bezerra GVP, Garcia RRP, Magalhães UN, Gomes WC. Biotechnological investigation of Pediastrum boryanum and Desmodesmus subspicatus microalgae species for a potential application in bioenergy. Algal Research. 2023;75:103266.
Merchant SS, Helmann JD. Elemental economy: microbial strategies for optimizing growth in the face of nutrient limitation. Adv Microb Physiol. 2012;60:91–210.
Corrêa da Silva MG. Pires Ferreira S, Dora CL, Hort MA, Giroldo D, Prates DF, Radmann EM, Bemvenuti RH, Costa JAV, Badiale-Furlong E: Phenolic compounds and antioxidant capacity of Pediastrum boryanum (Chlorococcales) biomass. Int J Environ Health Res. 2022;32(1):168–80.
Park JBK, Craggs RJ, Shilton AN. Investigating the life-cycle and growth rate of Pediastrum boryanum and the implications for wastewater treatment high rate algal ponds. Water Res. 2014;60:130–40.
Pasztaleniec A, Poniewozik M. Pediastrum species [Hydrodictyaceae, Sphaeropleales] in phytoplankton of Sumin Lake [Leczna-Wlodawa Lakeland]. Acta societatis botanicorum Poloniae. 2004;73(1):39–46.
NRC. Nutrient requirements of fish and shrimp. Washington, DC: National academies press. 2011.
Dent M, Dragović-Uzelac V, Penić V, Penić M, Bosiljkov T, Levaj B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage (Salvia officinalis L.) extracts. Food technology and biotechnology. 2013;51(1):84–91.
El-Ghamry A, El-Khateeb A, Mosa AA, El-Ramady H. Bio-Nano Fertilizers Preparation Using a Fully-Automated Apparatus: A Case Study of Nano-Selenium. Environment, Biodiversity and Soil Security. 2021;2021(5):171–83.
Noga EJ. Fish disease: diagnosis and treatment. USA: Wiley-Blackwell: Iowa State University Press; 2010.
Ricker W. Growth rates and models, vol. VIII Bioenergetics and Growth. New York: Academic Press; 1979.
Cunniff P, Washington D. Official methods of analysis of AOAC international. J AOAC Int. 1997;80(6):127A.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8.
Beutler E. Improved method for the determination of blood glutathione. J lab clin Med. 1963;61:882–8.
Aebi H. Catalase in vitro. In: Methods in enzymology. Vol. 105. edn. Amsterdam: Elsevier; 1984: p. 121–6.
Gorgoglione B, Zahran E, Taylor NG, Feist SW, Zou J, Secombes CJ. Comparative study of CXC chemokines modulation in brown trout (Salmo trutta) following infection with a bacterial or viral pathogen. Mol Immunol. 2016;71:64–77.
Zahran E, Elbahnaswy S, Ibrahim I, Khaled AA. Nannochloropsis oculata enhances immune response, transcription of stress, and cytokine genes in Nile tilapia subjected to air exposure stress. Aquacult Rep. 2021;21:100911.
Elbahnaswy S, Elshopakey GE, Ibrahim I, Habotta OA. Potential role of dietary chitosan nanoparticles against immunosuppression, inflammation, oxidative stress, and histopathological alterations induced by pendimethalin toxicity in Nile tilapia. Fish Shellfish Immunol. 2021;118:270–82.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–8.
Suvarna KS, Layton C, Bancroft JD. Bancroft’s theory and practice of histological techniques. Elsevier health sciences. 2018.
Islam SM, Rohani MF, Shahjahan MJAR. Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquaculture Reports. 2021;21:100800.
Pirarat N, Boonananthanasarn S, Krongpong L, Katagiri T, Maita M. Effect of activated charcoal supplemented diet on growth performance and intestinal morphology of Nile tilapia (Oreochromis niloticus). Thai J Vet Med. 2015;45(1):113–9.
Sathasivam R, Radhakrishnan R, Hashem A, Abd Allah EF. Microalgae metabolites: A rich source for food and medicine. Saudi J Biol Sci. 2019;26(4):709–22.
Sattanathan G, Liu W-C, Padmapriya S, Pushparaj K, Sureshkumar S, Lee J-W, Balasubramanian B, Kim IH. Effects of Dietary Blend of Algae Extract Supplementation on Growth, Biochemical, Haemato-Immunological Response, and Immune Gene Expression in Labeo rohita with Aeromonas hydrophila Post-Challenges. Fishes. 2022;8(1):7.
del Rocío Q-R, Fajer-Ávila EJ. The dietary effect of ulvan from Ulva clathrata on hematological-immunological parameters and growth of tilapia (Oreochromis niloticus). J Appl Phycol. 2017;29(1):423–31.
Ahmed S, Abousekkin M, El-Rahman A, Abo-Eid H. Effect of Green Algae and Natural Antioxidant Additioin to the Growing Rabbit’s Diet on Productive Performance and Economic Efficiency. International Journal of Environmental Studies and Researches. 2022;1(2):205–21.
Khanzadeh M, Beikzadeh B, Hoseinifar SH. The Effects of Laurencia caspica Algae Extract on Hemato-Immunological Parameters, Antioxidant Defense, and Resistance against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Aquac Nutr. 2023;2023(1):8882736.
Magalhães R, Guardiola FA, Guerreiro I, Fontinha F, Moutinho S, Olsen RE, Peres H, Oliva-Teles A. Effect of different dietary arachidonic, eicosapentaenoic, and docosahexaenoic acid content on selected immune parameters in gilthead sea bream (Sparus aurata). Fish Shellfish Immunol Rep. 2021;2:100014.
Abdel-Latif HM, Khalil RH. Evaluation of two phytobiotics, Spirulina platensis and Origanum vulgare extract on growth, serum antioxidant activities and resistance of Nile tilapia (Oreochromis niloticus) to pathogenic Vibrio alginolyticus. Int J Fish Aquat Stud. 2014;1(5):250–5.
Eldessouki EAA, Elshopakey GE, Elbahnaswy S. et al. Influence of astaxanthin-enriched Haematococcus pluvialis microalgae on the growth efficacy, immune response, antioxidant capacity, proinflammatory cytokines, and tissue histomorphology of hybrid red tilapia. Aquacult Int. 2024. p. 1–22. https://doi.org/10.1007/s10499-024-01524-1.
Kiran BR, Venkata Mohan S. Microalgal cell biofactory—therapeutic, nutraceutical and functional food applications. Plants. 2021;10(5):836.
Liang H, Kasiya HC, Huang D, Ren M, Zhang L, Yin H, Mi H. The Role of Algae Extract (Ulva lactuca and Solieria chordalis) in Fishmeal Substitution in Gibel Carp (Carrassius auratus gibeilo). Veterinary Sciences. 2023;10(8):501.
Fonseca AF. Corrêa da Silva M, da Silva M, Almeida K, Tavella R, Silva-Júnior F, Giroldo D, Dora C, Muccillo-Baisch A: Evaluation of acute toxicity of the microalgae Pediastrum boryanum. Vittalle. 2016;28:90–102.
Guzmán-Villanueva LT, Tovar-Ramírez D, Gisbert E, Cordero H, Guardiola FA, Cuesta A, Meseguer J, Ascencio-Valle F, Esteban MA. Dietary administration of β-1, 3/1, 6-glucan and probiotic strain Shewanella putrefaciens, single or combined, on gilthead seabream growth, immune responses and gene expression. Fish Shellfish Immunol. 2014;39(1):34–41.
Ming J, Xie J, Xu P, Liu W, Ge X, Liu B, He Y, Cheng Y, Zhou Q, Pan L. Molecular cloning and expression of two HSP70 genes in the Wuchang bream (Megalobrama amblycephala Yih). Fish Shellfish Immunol. 2010;28(3):407–18.
Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-β and interleukin-10. Immunity. 2008;28(4):468–76.
Monteiro M, Lavrador AS, Oliva-Teles A, Couto A, Carvalho AP, Enes P, Díaz-Rosales P. Macro-and microalgal extracts as functional feed additives in diets for zebrafish juveniles. Aquac Res. 2021;52(12):6420–33.
Carballo C, Mateus AP, Maya C, Mantecón L, Power DM, Manchado M. Microalgal extracts induce larval programming and modify growth and the immune response to bioactive treatments and LCDV in Senegalese sole post-larvae. Fish Shellfish Immunol. 2020;106:263–72.
Souza FPd, Lima ECSd, Urrea-Rojas AM, Suphoronski SA, Facimoto CT, Bezerra Júnior jdS, Oliveira TESd, Pereira UdP, Santis GWD, Oliveira CALd. Effects of dietary supplementation with a microalga (Schizochytrium sp.) on the hemato-immunological, and intestinal histological parameters and gut microbiota of Nile tilapia in net cages. PLoS One. 2020;15(1):e0226977.
Acknowledgements
The authors thank the organizations and individuals who provided in-kind support for this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
A.H.A Methodology, investigation, and writing of the original draft. S.E Investigation and co-supervision. E.R Investigation and co-supervision. E.Z conceptualization, review, editing, supervision, and correspondence. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experiment was conducted following the protocol involving the use of animals that was approved by the Mansoura University Animal Care and Use Committee (VM.PhD.23.10.25). All fish handling procedures and regulations followed the ARRIVE guidelines for Animal Care and Use. Furthermore, all relevant organizational and government rules and regulations governing the ethical use of the experimental animals were followed. Written informed consent was obtained from the owners of all animals involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Al-Wakeel, A.H., Elbahnaswy, S., Risha, E. et al. Dietary Pediastrum boryanum microalgal extract improves growth, enhances immunity, and regulates immune-related genes in Nile tilapia. BMC Vet Res 20, 321 (2024). https://doi.org/10.1186/s12917-024-04155-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-04155-z