Abstract
Background
Few studies have assessed lung function in Hispanic subjects recovering from mild COVID-19. Therefore, we examined the prevalence of impaired pulmonary diffusing capacity for carbon monoxide (DLCO) as defined by values below the lower limit of normal (< LLN, < 5th percentile) or less than 80% of predicted in Hispanics recovering from mild COVID-19. We also examined the prevalence of a restrictive spirometric pattern as defined by the ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) being ≥ LLN with the FVC being < LLN. Finally, we evaluated previous studies to find factors correlated to impaired DLCO post-COVID-19.
Methods
In this observational study, adult patients (n = 146) with mild COVID-19 were recruited from a long-term follow-up COVID-19 clinic in Yucatan, Mexico, between March and August 2021. Spirometry, DLCO, and self-reported signs/symptoms were recorded 34 ± 4 days after diagnosis.
Results
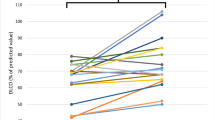
At post-evaluation, 20% and 30% of patients recovering from COVID-19 were classified as having a restrictive spirometric pattern and impaired DLCO, respectively; 13% had both. The most prevalent reported symptoms were fatigue (73%), a persistent cough (43%), shortness of breath (42%) and a blocked/runny nose (36%). Increased age and a restrictive spirometric pattern increased the probability of having an impaired DLCO while having a blocked nose and excessive sweating decreased the likelihood. The proportion of patients with previous mild COVID-19 and impaired DLCO increased by 13% when the definition of impaired DLCO was < 80% predicted instead of below the LLN. When comparing previous studies, having severe COVID-19 increased the proportion of those with impaired DLCO by 21% compared to those with mild COVID-19.
Conclusions
One-third of patients with mild COVID-19 have impaired DLCO thirty-four days post-diagnosis. The criteria that define impaired DLCO and the severity of COVID-19 disease affects the proportion of those with impaired DLCO at follow-up. One-fifth of patients have a restrictive spirometric pattern.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) is a virus that originated in Wuhan City, China, in December of 2019 and is responsible for the coronavirus disease (COVID-19) [1]. Between December 29, 2019, and February 16, 2020, deaths increased from 1 to 1666 in China.1 By October 1, 2021, over 4.79 million people worldwide have died from COVID-19 [2]. As such, COVID-19 has become one of the fatal pandemics ever recorded in human history.
As the number of patients recovering from COVID-19 increases worldwide, there is an urgent need to keep analysing pulmonary sequelae to facilitate optimal clinical treatments. COVID-19 is a heterogeneous disease with several long-term sequelae [3]. Patients recovering from the acute phase may report long-term multi-system symptoms, including various pulmonary function abnormalities [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22], psychological sequelae [3], and reduced physical functioning [10, 23, 24]. Specifically, pulmonary diffusing capacity for carbon monoxide (DLCO) is significantly impaired 30 to 180 days after the onset of (SARS-CoV-2) (Additional file 1: Table S1). However, most of these studies evaluated lung function in hospitalised patients due to the severity of their condition; very few studies focused on patients recovering from mild COVID-19 [4, 7, 10, 20]. Furthermore, even fewer studies focus on the effects of COVID-19 in Latino populations [6, 25].
Recent reports indicate the presence of racial and ethnic disparities with a disproportionate burden of COVID-19-related severity infections and mortality [26, 27]. These disparities may be partly attributable to higher comorbidities that worsen COVID-19 outcomes [28]. Specifically, there is limited research on the physiological effects of COVID-19 in the Mexican Latino population. Few studies have analysed the Latino community's persistent symptoms and lung function post-COVID-19 [28].
Our main objectives were to (1) determine factors associated with an impaired DLCO in Hispanic patients with mild COVID-19; and (2) evaluate data from previous studies to determine which factors predicted the proportion of patients with an impaired DLCO at follow-up.
Materials and methods
Study design
This is an observational cross-sectional study from the Long-term follow-up COVID-19 clinic at the High Specialty Regional Hospital of Yucatan, Mexico, from March 2021 to August 2021. We consecutively enrolled one hundred and fifty patients during the period. Inclusion criteria were adults over 18 years old recovering from mild COVID-19, defined as symptomatic patients meeting the case definition for COVID-19 without evidence of pneumonia or hypoxia (current WHO diagnostic criteria) [29]. All patients were scheduled after 4–6 weeks (34.4 ± 3.8 days) of baseline symptoms to perform pulmonary function tests and were evaluated for persistent symptoms at the clinic. The Ethics Committee of the High Specialty Regional Hospital in Yucatan, Mexico, approved this study (Protocol number 2020–024). All patients provided written informed consent to participate in this study, in compliance with the Helsinki declaration. This study followed the Guidelines for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).
Methods
Patients received a comprehensive medical assessment with a detailed medical history. Data including demographics, persistent symptoms from surveys, and pulmonary function test results were collected during the follow-up visit. Demographic data included age, sex, body mass index (BMI), previous cardiovascular disease risk factors for which regular pharmacological treatment was incorporated (including systemic hypertension, cardiac disease, diabetes mellitus), tobacco use (current or former smoker vs never smoker), obesity (defined as body mass index > 30 kg/m2). Patients were asked if they received oral corticosteroids and/or anticoagulants during the disease and to recount the presence or absence of symptoms at the time of the visit, including fatigue, shortness of breath on effort, cough, chest tightness, chest pain, sore throat, blocked and/or runny nose, loss of smell, loss of taste, diarrhoea, abdominal pain, muscle or joint pain, headache, tachycardia, sore or red eyes, excessive sweating (over a 24 h period, including night sweats), hair loss, and weight loss [30, 31].
Spirometry and measurements of DLCO were performed by a well-trained respiratory therapist who is also a Registered Pulmonary Function Technologist certified by The National Board for Respiratory Care. All tests were performed under physician supervision. The equipment used for lung function measurements was the Easy One Pro®, NDD Medical Technologies, Switzerland. Spirometry reference equations were obtained from Hankinson (1999) [32]. The technical quality of spirometry was adhered to per 2019 spirometry standards [33]. The reference equations for pulmonary diffusing capacity for carbon monoxide (DLCO) were obtained from Vazquez-Garcia and colleagues [32]. The 2017 Technical standards for DLCO were followed for technical quality [34].
Analysis
Continuous variables are expressed ad mean (S.D.), and categorical variables are expressed as absolute values and percentages. A Fisher’s exact test compared the number of males versus females with an impaired DLCO defined as below the lower limit of normal (< LLN, < 5th percentile). A Fisher’s exact test also compared the number of males versus females with a restrictive spirometric pattern as defined by the ratio of the pre-bronchodilator forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) being ≥ LLN with the FVC being less than the LLN [35]. The Fisher's Exact Test would allow an examination of sex differences in the proportion of males versus females with abnormally low lung function. Several N-1 Chi-Squared Tests were used to determine whether the percentage of several signs and symptoms present post-COVID-19 were different between those with a DLCO < LLN and those with a DLCO ≥ LLN [36]. A Benjamini–Hochberg procedure was used to control the false discovery rate [37], which we set to 0.05.
Binary logistic regression was performed using the backward: likelihood ratio method. This stepwise method enters all independent variables at once and then removes each variable one at a time according to the probability of the likelihood-ratio statistic until only the significant variables remain in the model. Binary logistic regression was used to determine the factors associated with an impaired DLCO (DLCO < LLN, less than a z-score of -1.645, or < 5th percentile) in patients with previous mild COVID-19. Cardiovascular disease (CVD) risk factors [1 = yes, 0 = no for smoking status, high blood pressure, cardiac arrhythmia, and obesity], true cardiac disease (yes = 1, no = 0), fatigue (yes = 1, no = 0), dyspnoea (yes = 1, no = 0), cough (yes = 1, no = 0), headache (yes = 1, no = 0), chest tightness (yes = 1, no = 0), sore throat (yes = 1, no = 0), persistent loss of smell (yes = 1, no = 0), dysfunction in the sense of taste (yes = 1, no = 0), conjunctivitis (yes = 1, no = 0), blocked and/or runny nose (yes = 1, no = 0), use of oral corticosteroids during the disease (yes = 1, no = 0), use of anticoagulant medications (yes = 1, no = 0), sex (male = 1, female = 0), age (years old), height (cm), weight (kg), body mass index (BMI, kg/m2), a restrictive spirometric pattern (forced vital capacity or FVC below the lower limit of normal, LLN, and FEV1/FVC ≥ LLN, yes = 1, no = 0), forced expiratory volume in 1 s (FEV1, L), forced expiratory flow rate over the middle half of expiration (FEF25-75, L/s) and peak expiratory flow (PEF, L/s).
Using mean data from 22 previous studies (including the current study), multiple linear regression analysis using forward selection was used to identify which of the five following factors would predict the proportion of patients who had previous COVID-19 and impaired DLCO at follow-up. The mean age (years old), mean body mass index (kg/m2), the mean number of days between receiving the COVID-19 diagnosis and follow-up, and history of mild vs severe COVID-19 disease (i.e. patients that were either hospitalised, intubated, presented with fibrotic C.T. changes in the lung were labelled as 1 or severe, compared to those that were not, and labelled as 0 or mild), and the criteria used to define impaired DLCO (DLCO < 80% predicted labelled as 1, vs DLCO < LLN labelled as 0) were predictors used in the model.
All data were analysed by a statistical software package (IBM SPSS Statistics, Version 27, Chicago IL). A p-value of < 0.05 was used to signify statistical significance. Any case with a standardised residual ≥ 3.0 was removed from any model.
Results
One hundred and fifty subjects were recruited from the Long-term follow-up COVID-19 clinic. Four patients were removed due to the reference equations not fitting the age range of the subjects, leaving 146 patients for the analysis. The anthropometric characteristics are presented in Table 1. Approximately 50% of the subjects were obese (BMI ≥ 30 kg/m2). The gas exchange parameter, DLCO, was reduced compared to predicted values (Table 2). In fact, 30% of the sample had a DLCO value below the LLN, and the percentages were similar between males and females (Table 3). Twenty-one percent of the sample had a restrictive spirometric pattern (FVC < LLN and an FEV1/FVC ≥ LLN) (Table 3), and when coupled with a DLCO < LLN, about 13% of the patients had both impaired DLCO and lung restriction.
For the regression analysis, two subjects had missing data, and then data from an additional five subjects were removed from the analysis, with their data being outliers (standardised residuals ≥ 3.0). Thus 139 subjects remained for the binary logistic regression analysis. The analysis revealed an overall model of four predictors that were statistically reliable in distinguishing between those with a DLCO below the LLN and those with normal DLCO [-2 Log-Likelihood = 101.7, Nagelkerke R2 = 0.51; Omnibus tests of model coefficients χ2 = 60.0, df = 4, p < 0.001, Akaike Information Criterion (AIC) = 111; Bayesian Information Criterion (BIC) = 126]. Increased age (from 25 to 83 years of age) and a restrictive spirometric pattern increased the probability of an impaired DLCO, while a blocked / runny nose and excessive sweating reduced the probability of having an impaired DLCO. The model was a good fit [Hosmer and Lemeshow Test, χ2 = 6.7, df = 8, p = 0.57], correctly identifying 81% of the cases (Table 4).
Using mean data from 21 previous studies (including the current study, see Additional file 1: Table S1), multiple linear regression analysis was used to identify which factors would predict the proportion of previously infected SARS-CoV-2 patients with impaired DLCO at follow-up. Regression results indicate an overall model of four predictors (previous history of severe COVID-19, criteria used to define impaired DLCO, mean age of the group, and number of days between diagnosis of COVID-19 and testing) that significantly determined the percentage of previously infected COVID-19 patients with an impaired DLCO at follow-up [R2adj = 0.46, F(4,31) = 8.4, residual standard deviation = 15.6%, p < 0.001, AIC = 306, BIC = 316]. The model accounted for 46% of the variance defining those with DLCO impairment. A summary of the regression model is presented in Table 5. Having a previous severe case of COVID-19 increases the proportion of those with impaired DLCO by 21% at follow-up. When using the more liberal definition of impaired DLCO (< 80% of predicted), the proportion of those with impaired DLCO increased by 13% in a given study. Finally, when the patient's mean age for a study increased by one year (from 41 to 69 years), the proportion of those with impaired DLCO increased by about 1%. Interestingly, the time of follow-up (20 to 180 days) did not seem to affect the percentage of those with impaired DLCO in a particular study.
Discussion
The main purpose of this study was to identify possible predictors of impaired DLCO in patients that had mild COVID-19 within the first 4 to 6 weeks. We found that patients who are recovering from mild COVID-19 also have persistent symptoms (Additional file 1: Table S2) as well as pulmonary function abnormalities (Table 3). For each one-year increase in age, the odds of having an impaired DLCO increased by 10%, while having a restrictive spirometric pattern increased the risk of having an impaired DLCO by 12-fold (Table 4). Regarding symptoms, having reported a blocked/runny nose and excessive sweating at follow-up reduced the odds of having an impaired DLCO by about 90% (Table 4). When using the logistic model, with a mean age of 50.7 years, having no lung restriction (0), no blocked nose (0), and no excessive night sweats (0), the probability of an impaired DLCO in this population is 24%. The chance increases to 79% when a restrictive spirometric pattern is evident (Table 4). Thus, having a restrictive spirometric pattern increases the odds of having impaired DLCO at follow-up by 12-fold. While 20% per cent of the patients had a restrictive spirometric pattern, only 3% of patients showed an obstructive pattern (as defined by an FEV1/FVC below LLN), demonstrating the after-effects COVID-19 are likely to result in lung restriction and poor pulmonary diffusion. A binary logistic regression model was chosen because it determined how well the measured variables predicted impaired DLCO while providing a summary of the accuracy of the classification of cases. The data collected had many variables, and the logistic regression analysis allowed the determination of the most important factors that predicted impaired DLCO in these patients.
The most common lung function parameter that is impaired in COVID-19 survivors is DLCO [38]. We have identified, in our population, 3 out of 10 patients without pneumonia or reduced SpO2 beyond 90% on room air at sea level (Yucatán, México) have an abnormal lung diffusion during the follow-up. Current available information is focused on severe cases and less attention has been paid to mild cases. Yet, these patients might have lung function abnormalities since mild cases usually develop ground glass opacities instead of lung consolidation. Ground glass opacities are associated with local dysregulations involving endothelial and epithelial injury markers suggesting some degree of venous thromboembolism, endothelial dysfunction, and abnormalities in cardiopulmonary circulatory physiology, which in turn may reflect DLCO abnormalities [39]. In a recent meta-analysis of 12 studies, being female, altered chest computerised tomography, age, higher D-dimer levels, and urea nitrogen were identified as factors for impaired DLCO [38]. This current study demonstrated similar odds ratios for age as the meta-analysis [38]; however, unlike the meta-analysis, sex was not a predictor of impaired DLCO in this study. Furthermore, the meta-analysis did not report that blocked / runny noses or excessive sweating were negative predictors. The results demonstrating that excessive sweating and runny noses were protective against impaired gas exchange are unique and puzzling and could be spurious outcomes. From the data presented in Additional file 1: Table S2, the proportion of those with a runny nose and abnormal sweating was statistically significant between those with impaired DLCO compared to those with normal DLCO. However, when the Benjamini–Hochberg procedure was used to control the false-discovery rate for 25 paired comparisons, these two variables became non-significant.
Another purpose of this study was to determine the variables that predict the percentage of previously infected SARS-CoV-2 patients who had a DLCO impairment during follow-up. Patients with previous severe COVID-19 disease at diagnosis would increase the likelihood of impaired DLCO by nearly 21% compared to those with previous mild COVID-19 disease. When studies used the usual cut-off < 80% of predicted to define DLCO impairment, then 13% more patients would be classified as having an abnormal gas exchange compared to if DLCO impairment was defined as below the LLN. Thus, if using the stricter definition of DLCO impairment as being below the LLN (i.e., below the 5th percentile) for height, age, sex, and ethnicity, 13% fewer patients would be classified as having a reduced DLCO. The definition of a low DLCO being < 80% of predicted is not correct and may misclassify patients, as the per cent of the predicted value at the LLN (5th percentile) decreases beginning at about 40 years of age [40, 41]. Patients with the same height, sex, and ethnicity have a DLCO of about 79% predicted at 40 years of age at the LLN (5th percentile) compared to about 73% predicted at 85 years of age at the LLN [41]. Therefore, using z-scores is preferred instead of using an absolute cut-off of less than 80% predicted to define a clinically low DLCO.
We were not able to measure total lung capacity (TLC) using a body plethysmograph to verify lung restriction; thus, a restrictive spirometric pattern (FEV1/FVC ≥ LLN and FVC < LLN, pre-bronchodilator) was used instead as a surrogate of true lung restriction. The sensitivity to identify true pulmonary restriction (TLC < LLN) with a restrictive spirometric pattern is about 34%, but the specificity is nearly 98% [42]. The negative predictive value (NPV) means that the percentage of patients who do not have a restrictive spirometric pattern and do not have restrictive lung disease is 97% [39]. The prevalence of a restrictive spirometric pattern (FEV1/FVC ≥ LLN and FVC < LLN, pre-bronchodilator) in populations is about 3 to 9% [35, 42]. In this group of patients with mild COVID-19, we found the restrictive spirometric pattern to be about 20% which is more than double the population average.
Among persistent symptoms, fatigue and shortness of breath on effort are the most prevalent descriptors included in Long COVID-19, and these were not different in ambulatory patients recovering from mild COVID-19 [30]. About 74% of the patients experienced undue fatigue, and nearly half experienced shortness of breath on effort and/or a significant cough.
Limitations
Our study has some limitations. In addition to not having measured lung volumes with plethysmography, we do not have any baseline lung function data in these patients prior to SARS-CoV-2 infection or a control group due to the pandemic to perform further analysis. However, most studies involving lung function analysis among COVID-19 survivors were done with the same limitation. Indeed, it is difficult to determine with certainty that these patients with poor diffusing capacity and/or a restrictive spirometric pattern are due to them having mild COVID-19. Nonetheless, these patients are compared against established reference norms. The prevalence of an impaired DLCO in our sample was five times more than expected in a normal population and at least two-fold more than expected for a restrictive spirometric pattern. To reduce this bias, we analysed all risk factors related to lung function abnormalities, including published studies. Also, there is a gap in implementing regular lung function assessments in suspected obstructive lung disease cases. Instead, chest x-ray studies are more frequently requested, especially in countries with limited-resource settings; therefore, it is expected that patients were not subjected to pulmonary function tests prior to SARS-CoV-2 infection.
Conclusions
Nearly one-third of patients with mild COVID-19 have impaired DLCO 34 days post-diagnosis, and one-fifth of patients have lung restriction. The odds of having an impaired DLCO at follow-up increased by 10% for every one-year increase in age (from 25 to 83) and increased 12-fold if a restrictive spirometric pattern was evident. However, having excessive night sweats and a blocked/runny nose each reduced the probability of an impaired DLCO at follow-up by about 90%, demonstrating a protective effect against an impaired gas exchange. In a summary of 22 studies, having severe COVID-19 disease at diagnosis increased the percentage of those with impaired DLCO by 21%. And, if the study used < 80% of predicted to define DLCO impairment, then 13% more patients would be classified as having a poor gas exchange.
Availability of data and materials
All data generated or analysed during this study are included in this article and/or its supplementary material files. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SARS-CoV2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus disease 2019
- DLCO:
-
Pulmonary diffusing capacity for carbon monoxide
- LLN:
-
Lower limit of normality
- WHO:
-
World Health Organization
- BMI:
-
Body mass index
- FEV1 :
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- FEF25-75 :
-
Forced expiratory flow rate over the middle half of expiration
- PEF:
-
Peak expiratory flow
- TLC:
-
Total lung capacity
- CVD:
-
Cardiovascular disease
References
Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. https://doi.org/10.1016/j.jaut.2020.102433.
Our World in Data. Cumulative COVID-19 tests, confirmed cases and deaths, world Global Change Data Lab; 2021 [cited 2021 October 2.]. Available from: https://ourworldindata.org/grapher/covid-tests-cases-deaths?country=~OWID_WRL.
Bellan M, Soddu D, Balbo PE, Baricich A, Zeppegno P, Avanzi GC, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. https://doi.org/10.1001/jamanetworkopen.2020.36142.
Abdallah SJ, Voduc N, Corrales-Medina VF, McGuinty M, Pratt A, Chopra A, et al. Symptoms, pulmonary function and functional capacity four months after COVID-19. Ann Am Thorac Soc. 2021. https://doi.org/10.1513/AnnalsATS.202012-1489RL.
Morin L, Savale L, Pham T, Colle R, Figueiredo S, Harrois A, et al. Writing committee for the COMEBAC study group four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325(15):1525–34. https://doi.org/10.1001/jama.2021.3331.
Gochicoa-Rangel L, Hernandez-Morales AP, Salles-Rojas A, Madrid-Mejia W, Guzman-Valderrabano C, Gonzalez-Molina A, et al. Gas exchange impairment during COVID-19 recovery. Respir Care. 2021;66(10):1610–7. https://doi.org/10.4187/respcare.09114.
Barisione G, Brusasco V. Lung diffusing capacity for nitric oxide and carbon monoxide following mild-to-severe COVID-19. Physiol Rep. 2021;9(4):e14748. https://doi.org/10.14814/phy2.14748.
Ekbom E, Frithiof R, Emilsson O, Larson LM, Lipcsey M, Rubertsson S, et al. Impaired diffusing capacity for carbon monoxide is common in critically ill Covid-19 patients at four months post-discharge. Respir Med. 2021;182: 106394. https://doi.org/10.1016/j.rmed.2021.106394.
Nunez-Fernandez M, Ramos-Hernandez C, Garcia-Rio F, Torres-Duran M, Nodar-Germinas A, Tilve-Gomez A, et al. Alterations in respiratory function test three months after hospitalisation for COVID-19 pneumonia: value of determining nitric oxide diffusion. J Clin Med. 2021;10(10):2119. https://doi.org/10.3390/jcm10102119.
van den Borst B, Peters JB, Brink M, Schoon Y, Bleeker-Rovers CP, Schers H, et al. Comprehensive health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2021;73(5):e1089–98. https://doi.org/10.1093/cid/ciaa1750.
Lerum TV, Aalokken TM, Bronstad E, Aarli B, Ikdahl E, Lund KMA, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57(4):2003448. https://doi.org/10.1183/13993003.03448-2020.
Guler SA, Ebner L, Aubry-Beigelman C, Bridevaux PO, Brutsche M, Clarenbach C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021;57(4):2003690. https://doi.org/10.1183/13993003.03690-2020.
Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299(1):E177–86. https://doi.org/10.1148/radiol.2021203153.
Sonnweber T, Sahanic S, Pizzini A, Luger A, Schwabl C, Sonnweber B, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57(4):2003481. https://doi.org/10.1183/13993003.03481-2020.
Blanco JR, Coboeballos MJ, Navarro F, Sanjoaquin I, de Arnaiz Las Revillas F, Bernal E, et al. Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin Microbiol Infect. 2021;27(6):892–6. https://doi.org/10.1016/j.cmi.2021.02.019.
Qin W, Chen S, Zhang Y, Dong F, Zhang Z, Hu B, et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. Eur Respir J. 2021;58(1):2003677. https://doi.org/10.1183/13993003.03677-2020.
Shah AS, Wong AW, Hague CJ, Murphy DT, Johnston JC, Ryerson CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2021;76(4):402–4. https://doi.org/10.1136/thoraxjnl-2020-216308.
Huang Y, Tan C, Wu J, Chen M, Wang Z, Luo L, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):163. https://doi.org/10.1186/s12931-020-01429-6.
Liang L, Yang B, Jiang N, Fu W, He X, Zhou Y, et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci. 2020;35(47):e418. https://doi.org/10.3346/jkms.2020.35.e418.
Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6):2001217. https://doi.org/10.1183/13993003.01217-2020.
Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomised controlled study. Complement Ther Clin Pract. 2020;39:101166. https://doi.org/10.1016/j.ctcp.2020.101166.
Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. https://doi.org/10.1016/j.eclinm.2020.100463.
Baricich A, Borg MB, Cuneo D, Cadario E, Azzolina D, Balbo PE, et al. Midterm functional sequelae and implications in rehabilitation after COVID-19: a cross-sectional study. Eur J Phys Rehabil Med. 2021;57(2):199–207. https://doi.org/10.23736/S1973-9087.21.06699-5.
van der Sar-van der Brugge S, Talman S, Boonman-de Winter L, de Mol M, Hoefman E, van Etten RW, et al. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir Med. 2021;176:106272. https://doi.org/10.1016/j.rmed.2020.106272.
Cortes-Telles A, Lopez-Romero S, Figueroa-Hurtado E, Pou-Aguilar YN, Wong AW, Milne KM, et al. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir Physiol Neurobiol. 2021;288:103644. https://doi.org/10.1016/j.resp.2021.103644.
Buikema AR, Buzinec P, Paudel ML, Andrade K, Johnson JC, Edmonds YM, et al. Racial and ethnic disparity in clinical outcomes among patients with confirmed COVID-19 infection in a large U.S. electronic health record database. EClinicalMedicine. 2021;39:101075. https://doi.org/10.1016/j.eclinm.2021.101075.
St Sauver JL, Lopes GS, Rocca WA, Prasad K, Majerus MR, Limper AH, et al. Factors associated with severe COVID-19 Infection among persons of different ages living in a defined Midwestern US population. Mayo Clin Proc. 2021;96:2528–39. https://doi.org/10.1016/j.mayocp.2021.06.023.
Parra-Rodriguez L, Gonzalez-Meljem JM, Gomez-Dantes H, Gutierrez-Robledo LM, Lopez-Ortega M, Garcia-Pena C, et al. The burden of disease in Mexican older adults: premature mortality challenging a limited-resource health system. J Aging Health. 2020;32(7–8):543–53. https://doi.org/10.1177/0898264319836514.
Geneva: WHO. COVID-19 Clinical management: living guidance. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1. Date last accessed: October 11, 2021.
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. https://doi.org/10.1038/s41598-021-95565-8.
Elliott J, Whitaker M, Bodinier B, Eales O, Riley S, Ward H, et al. Predictive symptoms for COVID-19 in the community: REACT-1 study of over 1 million people. PLoS Med. 2021;18(9):e1003777. https://doi.org/10.1371/journal.pmed.1003777.
Vazquez-Garcia JC, Perez-Padilla R, Casas A, Schonffeldt-Guerrero P, Pereira J, Vargas-Dominguez C, et al. Reference values for the diffusing capacity determined by the single-breath technique at different altitudes: The Latin American single-breath diffusing capacity reference project. Respir Care. 2016;61(9):1217–23. https://doi.org/10.4187/respcare.04590.
Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardisation of spirometry 2019 update. An official American thoracic society and European Respiratory society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–88. https://doi.org/10.1164/rccm.201908-1590ST.
Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1):1600016. https://doi.org/10.1183/13993003.00016-2016.
Toren K, Schioler L, Brisman J, Malinovschi A, Olin AC, Bergstrom G, et al. Restrictive spirometric pattern and true pulmonary restriction in a general population sample aged 50–64 years. BMC Pulm Med. 2020;20(1):55. https://doi.org/10.1186/s12890-020-1096-z.
Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26(19):3661–75. https://doi.org/10.1002/sim.2832.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B. 1995;57(1):289–300.
Zhi H, Ji X, Zhao Z, Liang H, Zhong S, Lou Y, et al. Risk factors for impaired pulmonary diffusion function in convalescent COVID-19 patients: a systematic review and meta-analysis. EClinicalMedicine. 2022;49:101473. https://doi.org/10.1016/j.eclinm.2022.101473.
Wichman D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–77. https://doi.org/10.7326/M20-2003.
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. https://doi.org/10.1183/09031936.00080312.
Stanojevic S, Graham BL, Cooper BG, Thompson BR, Carter KW, Francis RW, et al. Official ERS technical standards: Global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J. 2017;50(3):1700010. https://doi.org/10.1183/13993003.00010-2017.
Backman H, Eriksson B, Hedman L, Stridsman C, Jansson SA, Sovijarvi A, et al. Restrictive spirometric pattern in the general adult population: methods of defining the condition and consequences on prevalence. Respir Med. 2016;120:116–23. https://doi.org/10.1016/j.rmed.2016.10.005.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
ACT, EFH, DLOF, GSZ designed the study. ACT, EFH, DLOF collected the data and interpreted the patient data regarding lung function. ACT and GSZ analysed the data and prepared the manuscript. All authors made critical revisions to the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Ethics Committee of the High Specialty Regional Hospital in Yucatan, Mexico (No. CONBIOETICA- 31-CEI-002-20170731). The Ethics Committee assigned this study as protocol number 2020-024. All patients signed a written informed consent to participate in the scheduled visit in compliance with the Helsinki declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table S1.
Studies demonstrate impaired DLCO one to 180 days post- COVID-19 diagnosis. Table S2. Results from the medical assessment and detailed medical history obtained 34 (SD 4) days post-COVID-19 diagnosis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cortes-Telles, A., Figueroa-Hurtado, E., Ortiz-Farias, D.L. et al. Clinical predictors of lung function in patients recovering from mild COVID-19. BMC Pulm Med 22, 294 (2022). https://doi.org/10.1186/s12890-022-02086-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02086-9