Abstract
Background
Spirulina (SP) is widely used as a nutritional supplement to enhance child nutrition in low-income countries. We assessed Spirulina’s efficacy of the current dose supplied by institutions in Cambodia on improving growth and anemia in a cross-over randomized controlled trial in preschool underprivileged children from similar settings.
Methods
Preschool children cared by a not-for-profit institution were randomly and blindly allocated (2 to 1) to spirulina or placebo: 100 g in total, given in 2 g per day. After 5 weeks of wash-out, participants were crossed-over to the other group. Anthropometric gain and selected hematological data (blood cell count, ferritin, and C-reactive protein) were assessed at each phase.
Results
A total of 179 children completed the trial, 149 (83.2%) completed all the anthropometrics, and 99 (55.3%) all hematological measures. Mean BMI was 14.18 (95%CI: 14.00–14.37) and 31(20.8%) children had thinness. Mean blood hemoglobin was 11.9 g/dL (95%CI: 11.8–12.1). The weight gain of the SP group showed a modest higher trend compared to placebo (0.63 kg; 95%CI: 0.54–0.72 and 0.46 kg; 95%CI: 0.33–0.58, respectively; p = 0.07). Height increased similarly in both groups. The number of anemic children decreased by 6 (6.06%) and 11 (11.11%) on Placebo or SP, respectively (p = 0.004). Tolerance was good.
Conclusion
SP may be recommended to improve childhood anemia. The analysis of the usual daily dose (2 g) provided by organizations in Cambodia shows a tendency to improve weight gain in the group supplemented with SP very close to significance, but no trend in height. Increased doses and longer supplementation should be evaluated further.
Trial registration
The study was retrospectively registered at ISRCTN under number 11696165 on 12/12/2018.
Similar content being viewed by others
What is already known on this topic?
Spirulina (SP) is a rich nutrient widely used as a nutritional supplement. There is some evidence for the effectiveness of SP (1 g per day) in alleviating vitamin A deficiency, and some debated evidence for improving the health of malnourished children.
Controlled studies of MS in children are very rare and the effectiveness of low daily doses has not been evaluated in improving growth in disadvantaged children.
What this study adds?
Daily small doses of SP alleviated anemia.
The usual daily dose (2 g) provided by institutions in Cambodia shows a tendency to improve weight gain in the SP supplemented group without obtaining any significance (p = 0.07).
The results suggest to further evaluate doses and longer supplementation together with more subtle biological parameters of nutrition and immune function in a similar context of underprivileged children.
Introduction
Spirulina (Arthrospira platensis) (SP), a filamentous microalga (Cyanobacterium) grows naturally in alkaline water bodies in subtropical waters [1]. Its extreme popularity [2, 3] as nutritional supplement is due to its improved bioavailability [4] and high contents content of balanced proteins (55–70%) polyunsaturated fatty acids, microelements, β-carotene, and other vitamins [5,6,7,8,9,10,11,12,13,14].
SP has been considered “the best food for the future”, to combat malnutrition in low-income countries and to improve overall health [13,14,15,16]. This resulted in extended scientific interest [5, 17] and its use by many institutions in developing countries [18,19,20,21,22,23,24,25]. SP is recommended for use as an ingredient in foods, at levels ranging from 0. 5 to 3. 0 g per serving [14, 25,26,27].
The rationale for using SP to correct anemia is based on SP’s high content of iron with high bioavailability, content of porphyrin, and its content of phycocyanin which boost the erythropoietic response [28,29,30,31]. Animal testing and clinical studies, mostly uncontrolled [32, 33], suggest a beneficial effect of SP [27,28,29,30,31,32,33]. However, control studies remain scarce.
In Cambodia, SP is provided to children by NGOs under the assumption that using low doses of SP daily (2 g) will improve the children’s growth and health. We assessed the impact of SP supplementation on growth, and anemia using a cross-over design in a single-blinded trial (Trial ID: ISRCTN11696165) and attempted to present this study according to the consort check-list (S1. Cohort check-list).
Methods
Survey participants
The survey was conducted at the preschool “Pour un Sourire d’Enfant (PSE)” in Phnom Penh, Cambodia from October 2014 until July 2015. Children (4–7 years) were included if they had no current disease, or allergy to SP, were able to attend the PSE school, and with the informed oral consent of their parents/guardians after a medical screening at PSE health center. All PSE children are fed once daily at school.
Randomization and blinding
A randomized list using computer generated numbers by blocks of four was prepared before the trial and secured in an external office outside PSE. Envelops with the study number and group name inside were prepared by an external assistant, a pharmacist trainee who was not involved in the distribution of the envelopes, and then sealed. A number corresponding to the identification code and class number of the child was recorded on the envelope following the randomization list. As a result, no connection between these codes and the name of the children was possible for the people preparing the envelopes. Individual envelopes were given to each class according to the number of participants and opened by the teachers under the supervision of the team. SP and placebo were provided as sprinkles which had no attractive taste. The placebo was brown unsweetened dietary sprinkles available locally. Due to this small minimal difference, the study was downgraded from double to single blind trial. Everything was done to be close to a double-blind clinical trial. The authors had no information on the individual status of children on SP or placebo before and during the study. In addition, blinding was ensured for the trial team during data entering and preliminary analysis using a code number 0 or 1 based on the nutrient allocation.
SP was obtained from Antenna Cambodia, a non-for- profit organization highly active in SP field which conducts regular quality control [23,24,25, 28]. Assessment for potential bacterial contamination content was conducted by Pasteur Institute, Phnom Penh, and for iron and vitamin A content by Laboratoire Developpement Méditerranée AQMC lab Saint-Aunes France. Both analyses were satisfactory. Iron and vitamin A contents were at 666 mg/100 g and 1 μg/100 g, respectively.
Baseline assessments
Baseline anthropometric measures and hematological assessments were conducted before the survey, then 4 weeks after the first treatment the week just before Phase 2, and after the second supplementation. Anthropometric assessment (weight and height, using UNICEF tools with 100 g and 0. 1 precision, respectively) was conducted by the same person after rigorous training and testing. Weight for height (Wasting) score and Height for age score (undernutrition) were calculated using WHO Anthro software for children below 5 years. Body mass index (BMI) for children using WHO 2007 standards for children below and above 5 years was calculated.
Laboratory blood samples (4 ml) were taken by nurses from the Cambodian Pasteur Institute. The samples were stored and assayed for blood cell count, hemoglobin level (HB, mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) using Pentra 80XL (HORIBA), ferritin using Elecsys E411(ROCHE), and C-reactive protein (C-RP) using Pentra C400 (HORIBA) at Pasteur Institute laboratory.
Supplementation and follow up
Two grams of SP or Placebo was administered daily by the children’s teacher during the first hour of the morning 5 times a week for 8 weeks or until the end of the individual bag of 100 g.
After 5 weeks of the wash-out period, participants were crossed over to the other group (Phase 2). The intake and tolerance of supplements were directly observed (DOT) by the teachers and events were recorded daily on a standardized pre-tested one-sheet form during Phase 1. This form recorded events such as nausea, vomiting, or any signs of potential allergy or side effects. The form was revised daily during the first weeks of the trial. Children with non-minor events had to be presented directly to the school health center.
Outcome
The main outcome was the change in nutritional status, specifically a difference in weight and height before and at the end of the trial. Secondary outcomes were the tolerance and the evolution of hematological data.
Definitions
Anemia was defined as hemoglobin below 11 g/l using WHO categories[46]. Eosinophil levels were defined as increased > 500 per mm3. C-RP was defined as normal if < 6 mg / l. Ferritin was defined as low if < 30 μg / L. Malnutrition was appreciated using weight for height and height for age z-score in children below 5 years.
Study size
A required sample size of 119 people was calculated to detect a treatment difference of 0. 3 with alpha risk = 0. 05 and power 90%, based on the assumption that the standard deviation of the difference in the response variables is 1 with A ratio of 2:1 (SP)/(Control).
One of the constraints of the trial was the anticipated dropping out of preschool children. Finally, it was decided to include 50% more children in case of drop-out or incomplete data.
Analysis
Data was entered using Epidata 7 and analyses were conducted using Stata11. Analysis was per protocol (PP) for the main outcome and anemia and by intention to treat (ITT) for the secondary outcome of tolerance. For comparison of means, an analysis of equality of variance and an analysis of normality of variables (Shapiro-Wilk W test) were conducted. Students’ t-test for unpaired variables or the Wilcoxson test were used as appropriate. Fisher’s exact test and Pearson chi-square were used to comparing frequency. The pairwise comparisons between Placebo and SP group were done using paired Students’ t-test and Wilcoxon signed-rank test, and the McNemar test for frequency. P < 0. 05 was deemed as statistically significant.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Initial characteristics of children
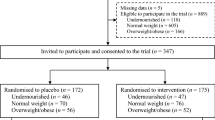
A total of 194 children were included in the survey (Fig. 1). No refusal from parents was recorded. A total of 15 children withdrawn from the survey, 14 during Phase 1 and 1 during Phase 2, leaving 179 (92. 3%) for safety analysis. Of them, 149 (83. 2%) completed the three anthropometrics satisfactorily and were analyzed. Of 129 children present for initial blood sampling, 99 underwent the three laboratory measurements and were analyzed.
No significant difference was encountered at baseline between the initial randomized groups 0 and 1 of children who completed the study (Table 1). The initial mean weight and height were 14. 2 kg (95%CI: 13. 9–14. 6) and 100. 2 cm (95%CI: 99. 0–101. 3), respectively. The mean BMI was 14. 18 (95%CI: 14. 00–14. 37) and 31 (20. 8%) children had thinness. Of the 102 children aged below 5 years, 16 (15. 7%) were suffering from wasting and 23 (22. 5%) from stunting. The mean blood hemoglobin was 11. 9 g/dL (95%CI: 11. 8–12. 1), 11 children had light anemia (11. 11%), 32 (32. 32%) microcytosis and 82 (82. 8%) children had hypereosinophilia, 9 (9. 1%) has a low ferritin and C-Reactive Protein (CRP) above 6 mg/L. The initial characteristics of enrolled children are presented (Supplementary Tables 1 and 2).
Changes during the administration of placebo and Spirulina
The absolute changes within the Placebo and Spirulina groups are shown in Table 2. The weight gain of the SP group showed a modest benefit compared to Placebo, which was close to the significance level (0. 63 kg; 95%CI: 0. 54–0. 72 and 0. 46 kg; 95%CI: 0. 33–0. 58, respectively; p = 0. 07). Height increased similarly in both groups (2. 6 cm 95%CI: 1. 7–3. 4 and 2. cm 95%CI: 1. 9–2. 2, respectively; p = 0. 8). Thinness was appreciated for the whole population while wasting and stunting were appreciated among the 60 children aged below 5 years at the end of the study. The proportion of children with thinness decreased more in the SP group without being significant (− 15% versus − 2. 14%, respectively; p = 0. 17). The proportion, of children aged below 5 years affected with wasting and stunting did not change between placebo and SP groups.
Few children had anemia in both groups (15, 15. 1% and 12, 12. 1% in Placebo and SP groups, respectively). There was a significant decrease of the number of children with anemia in the SP group compared to Placebo group. The number of anemic children decreased by 6 (6. 0%) and 11 (11. 1%) in the placebo and spirulina groups, respectively (p = 0. 004).
No significant change was observed in the change of mean hemoglobin, mean MCV, mean eosinophils or, in the number of children with low ferritin or microcytosis between Placebo and SP. The number of children with hyper eosinophils decreased slightly similarly since the beginning of the trial and remains high despite a deworming treatment given to all children by the medical health Centre before Phase 2.
Two changes were close to the significance level of 0. 05. The mean ferritin increased in placebo group while it decreased in SP group (p = 0. 06). The number of children with elevated C-RP which was low at the beginning of the trial, decreased in the SP groups from 8 to 1 and increased from 4 to 8 in the Placebo group (p = 0. 09).
Acceptability and tolerance of supplements
The acceptability of both supplements was good. No refusal was reported by the teachers. Tolerance was good. Only a few transient and benign side effects of transient nausea or some vomiting were reported by the teachers during the first days in less than ten children with no detectable differences between the groups.
Discussion
This survey provides the findings of a cross-over blind randomized control trial of 2 g of SP daily compared to a placebo given in underprivileged children cared for by PSE preschools in the Phnom Penh area of Cambodia. The trial was set up to explore the potential of SP as a booster to improve growth and hematological profile including anemia status. It shows a modest gain of weight, close to the significance level and a significant decrease in the number of children with anemia in the SP group compared to the placebo group. Two other outcomes changed and were close to significance level: the mean ferritin change and the number of children with elevated C-RP.
Frequent shortcomings of SP studies in children have been previously described, particularly the small size or the absence of a control group in most studies [16,17,18, 25]. We attempted to compensate for these shortcomings by using a cross-over design.
The gain in weight in the SP group compared to the Placebo group was modest. The short duration and minimal input of calories and proteins by 2 g of SP can be suspected to be the cause of the modest specific impact of SP on weight gain as previously discussed [21, 26]. The fact that all children benefited from a daily meal provided by the school probably decreased the interest of supplementing low doses of SP nutrients. There are no comparable controlled studies of SP supplementation in young underprivileged children attending preschool. Most studies in children were conducted on children with known health problems or older children [20, 27, 34,35,36]. A study in India among older schoolgirls used 0.9 g daily of SP for 3 months and suggested nutritional benefits, but this was not a controlled trial [32]. A controlled study in young Chinese schoolgirls showed the effectiveness of 2 and 4 g of daily SP for 10 weeks to improve vitamin A status [37]. In the context of severe malnutrition, the objectives are quite different and daily dosages of SP ranged from 1 g daily for 1 year for vitamin A deficiency, 3 g daily in a controlled study in Gaza, and up to 10 g daily for severe malnutrition [17,18,19,20,21,22, 26, 34,35,36,37,38,39,40,41]. In addition to doses, another question is the duration of SP supplementation. In Burkina Faso, a study compared a group of children receiving nutritionally enhanced flour and deworming treatment with two groups of children receiving an additional 5 g of SP for 90 days [21]. This study led to an unsatisfactory result of SP supplementation according to the author. The conclusions were highly criticized by various organizations [38,39,40]. Looking back at the results, we found that some of the interesting findings of this study and their implications might have been missed in the passionate debate. At 90 days, a weight gain persisted in the SP group while decreasing in the SP-free group. The persistence of weight gain at 90 days suggests that prolongation of supplementation could be beneficial for children when weight gain is the main objective. From these studies and our findings, it may be suggested that higher doses of SP can be considered in future studies in children from deprived settings beginning with 3 g daily of SP.
A significant decrease in the number of children with anemia was observed while on SP compared to the placebo group. The study shows a clear benefit of SP on the reduction of anemic compared to placebo and can be recommended. This result is supported by animal studies [28,29,30,31, 42, 43] and clinical studies [32, 33, 43, 44].
Further studies should consider assessing higher doses and longer supplementation periods together with more subtle biological parameters for nutrition and immunity, which we were unable to address due to limited funds.
Limitations of the study
This study had to face an expected high rate of loss to follow up on this group of underprivileged children. We tried to compensate for this using a larger number of children than calculated.
Nutritional intake at home was unknown. This was mitigated by the fact that all children received their lunch at PSE. No stool testing was conducted, and the high level of parasitism reflected by the rate of hyper eosinophil persisted during the study despite a general deworming after the first phase. This was probably affecting the normal absorption of iron in children.
Conclusion
The tolerance and acceptability of SP were good. SP 2 g daily may be recommended to improve childhood anemia. The usual dose of 2 g provided by institutions in Cambodia has only a modest impact on weight and no trends in height. Increased doses and longer supplementation should be evaluated to improve the growth of children, together with more subtle biological parameters of nutrition and immune function in a similar context of underprivileged children.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Soudy ID, Minet-Quinard R, Mahamat AD, et al. Vitamin A status in healthy women eating traditionally prepared SP (Dihe) in the Chad Lake area. PLOS ONE 201813(1):e0191887. https://doi.org/10.1371/journal.pone.0191887
Spolaore P, Joannis-Cassan C, Duran E, et al. Commercial applications of microalgae. J Biosci Bioeng. 2006;101:87–96 PMID:16569602.
García JL, Vicente M, Galán B. Microalgae, old sustainable food and fashion nutraceuticals. Microbial Biotechnology 10:1017–1024.
Ciferri O. Spirulina, the edible microorganism. Microbiol Rev. 1983;47:551–78.
Khan Z, Bhadouria P, Bisen PS. Nutritional and therapeutic potential of Spirulina. Curr Pharm Biotechnol. 2005;6:373–9.
Kent M, Welladsen HM, Mangott A, et al. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS One. 2015;10(2):e0118985. https://doi.org/10.1371/journal.pone.0118985.
Habib MAB, Parvin M, Huntington TC, et al. A review on culture, production and use of Spirulina as food for humans and feeds for domestic animals and fish. FAO Fish Aquac Circ. 2008;1034:33 Accessed 20/09/2018 available http://www.fao.org/3/a-i0424e.pdf.
Ku CS, Yang Y, Park Y. Lee J health benefits of blue-green algae: prevention of cardiovascular disease and nonalcoholic fatty liver disease. J Med Food. 2013;16:103–11. https://doi.org/10.1089/jmf.2012.2468 PMID:23402636.
Nicoletti M. Microalgae Nutraceuticals. 2016; 5(3) pii: E54. doi: https://doi.org/10.3390/foods5030054.
Dagnelie PC, van Staveren WA, van den Berg H. Vitamin B-12 from algae appears not to be bioavailable. Am J Clin Nutr. 1991;53:695–7.
Watanabe F, Katsura H, Takenaka S, et al. Pseudovitamin B12 is the predominant cobamide of an algal health food, spirulina tablets. J Agric Food Chem. 1999;47:4736–41.
Watanabe F. Vitamin B12 sources and bioavailability. Exp Biol Med. 2007;232:1266–74.
Michaelsen KF, Hoppe C, Roos N, et al. Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food Nutr Bull. 2009;30:S343–404.
American Dietetic Association; Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada:Vegetarian diets. J Am Diet Assoc. 2003;103:748–65.
Gumbo JR, Nesamvuni CN. A Review: Spirulina a source of bioactive compounds and nutrition. Journal of Chemical and Pharmaceutical Sciences 3:1317–1325.
Karkos PD, Leong SC, Karkos CD, et al. Spirulina in clinical practice:evidence-based human applications. Evid Based Complement Alternat Med 2011:531053. 10.1093/ecam/nen058 [doi];nen058 [pii].
Siva Kiran R,. R,Madhu G. M,Satyanarayana S. V Spirulina in combating protein energy malnutrition (PEM) and protein energy wasting (PEW)—a review. Journal of Nutr Res 2015;3:62–79.
Halidou DM, Degbey H, Daouda H, et al. Supplémentation en spiruline dans le cadre de la réhabilitation nutritionnelle: revue systématique. Rev Epidemiol Sante Publique. 2008;56:425–31.
Venkatasubramanian K, Edwin N. In collaboration with Antenna technologies, Geneva and Antenna trust Madurai (1999). A study on preschool nutrition supplementation family income booster by Spirulina. Madurai Medical College; p. 20.
Branger B, Cadudal J, Delobel M, et al. La spiruline comme complément alimentaire dans la malnutrition du nourrisson au Burkina Faso. Arch Pediatr. 2003:424–31.
Simpore J, Kabore F, Zongo F, et al. Nutrition rehabilitation of undernourished children utilizing Spiruline and Misola. Nutr J 2006;5:3. 1475–2891-5-3 [pii];https://doi.org/10.1186/1475-2891-5-3 [doi].
Falquet J, Hurni JP. Spiruline, aspects Nutritionnels. Antenna Technologies. 2006:1–22.
Kapoor R, Mehta U. Iron status and growth of rats fed different dietary Iron sources. Plant Foods Hum Nutr. 1993;44:29–34.
Hug C, Weid D. Spirulina in the Fight Against Malnutrition: Assessment and Prospects 2011, Fondation Antenna Technologies, www.antennaindia.org/Spirulina_spirulina%20assesment%20and%20procespects.PDF. (Accessed Sept 2021)
Hosseini SM, Khosravi-Darani K, Mozafari MR. Nutritional and medical applications of spirulina microalgae. Mini Rev Med Chem. 2013;13:1231–7 MRMC-EPUB-20130401-12 [pii].
Masuda K, Inoue Y, Inoue R, et al. Spirulina effectiveness study on child malnutrition in Zambia. 2014 Published by Institute of Development Studies, Brighton. https://opendocs.ids.ac.uk/opendocs/handle/20.500.12413/4398 (Accessed Sept 2021).
Seshadri CV. Large-scale nutritional supplementation with spirulina-alguae. Shri Amm Murugappa Chettiar research center. Madras: Shri Amm Murugappa Chettiar Research Center; 1993. http://el.doccentre.info/eldoc1/g79_/G79_B1061.pdf
Kapoor R, Mehta U. Supplementary effect of Spirulina on hematological status of rats during pregnancy and lactation. Plant Foods Hum Nutr. 1998;52:315–24.
Simsek N, Karadeniz A, Kalkan Y, et al. Spirulina platensis feeding inhibited the anemia- and leucopenia-induced lead and cadmium in rats. J Hazard Mater. 2008;164(263):1304–9.
Zhang CW, Zeng SQ, Zhang YZ. Effect of the C phycocyanin on the formation of mouse granulocytemonocyte progenitor cells. Chin J Mar Drugs. 1996;4:25–8.
Peng WM, Shang ST, Fu YL, et al. Properties of phycobili-proteins from Spirulina platensis. J China Agricultural Univ. 1999;4:35–8.
Sachdeva R, Kaur R, Sangha JK. Effect of supplementation of Spirulina on the haematological profile and intellectual status of school girls (7-9 years). J Hum Ecol. 2004;15:105–8.
Ramesh S, Manivasgam M, Sethupathy S, et al. Effect of Spirulina on anthropometry and bio-chemical parameters in school children. J Dent Med Sci. 2013;7:11–5.
Sall M, Dankoko B, Badiane M, et al. Résultats d'un essai de réhabilitation nutritionnelle avec la spiruline à Dakar (à propos de 59 cas). Med Afrique Noire. 1999:143–6.
Malam-Abdou B, Brah S, Andia A, et al. Interet d’une Supplementation en spiruline chez les enfants drepanocytaires homozygotes à l’hôpital national de Niamey. Eur Sci J. 2017;13(21):1857–7431.
Simpore J, Zongo F, Kabore F, et al. Nutrition rehabilitation of HIV-infected and HIV-negative undernourished children utilizing spirulina. Ann Nutr Metab. 2005;49:373–80.
Li L, Zhao X, Wang J, et al. Spirulina can increase total-body vitamin a stores of Chinese school-age children as determined by a paired isotope dilution technique. J.Nutr.Sci. 2012;1:e19. https://doi.org/10.1017/jns.2012.21 PMID:25191548.
Falquet J, von der Weid D. Spiruline et nutrition. Arch Pediatr 2004;11:465. author reply 467–8.
Darcas C. Spiruline et nutrition. Arch Pediatr 2004;11(5):466–7;author reply 467–8.
Branger B. Spiruline et nutrition. Arch Pediatr. 2004;11(5):author reply:467–8.
Abed E, Ihab AN, Suliman E, et al. Impact of Spirulina on nutritional status, Haematological profile and Anaemia status in malnourished children in the Gaza strip: randomized clinical trial. Matern Pediatr Nutr 2016;2:110. doi:https://doi.org/10.4172/mpn.1000110 available on https://pdfs.semanticscholar.org/d1b2/845550c191f2f5dc69fd88cd2d28e8ea6379.pdf.
Nasirian F, Mesbahzadeh B, Maleki S. A, et al. The effects of oral supplementation of spirulina platensis microalgae on hematological parameters in streptozotocin-induced diabetic rats. Am J Transl Res 2017;12:5238–5244.
Mani U, Sadliwala A, Iyer U, et al. The effect of Spirulina supplementation on blood Haemoglobin levels of Anaemic adult girls. J Food Sci Technol. 2000;37:642–4.
Madhusnata De, Ajanta Halder, Tulika Chakraborty, et al. Incidence of anemia and effect of nutritional supplementation on women in rural and tribal populations of eastern and north-eastern. India Hematology 2011;20(6)3:190–2.
Acknowledgements
We thank the children and their families, teachers, medical doctors, and health staff at PSE for their kind participation and the staff from Antenna Cambodia for their dedication in managing the survey. We thank the lab team and Alexandra Kergueler from Pasteur Institute for conducting the blood sampling analyses. We thank UNICEF for kindly lending anthropometric measurement tools. We thank Corine Thore and Evelyne Mouillet for their help on bibliography research. A special thanks to Peter Odermatt, Leila N Sour and Eric Pussard for kindly revising and commenting on the manuscript.
Funding
This paper is independent research. PSE Cambodia (https://pse.ngo/pse-cambodia) supported the cost of Spirulina and the global care of children. Antenna France (https://www.antenna-france.org) supported the follow-up of children and project management by LH and the Antenna team. Volunteer contribution was provided for the conception, protocol, initiation, management, supervision of the study, and data analysis by HB. Volunteer contribution was provided by a pharmacist volunteer (CC) and a trainee (FB). Antenna Swiss (https://www.antenna.ch/en) supported the biological expenses. After the end of the study, HB received a lump sum for writing the initial reporting from Antenna France. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. The views expressed in this publication are those of the author(s) and not necessarily those of the PSE Cambodia or Antenna.
Author information
Authors and Affiliations
Contributions
1. HB had the original idea for this study, carried out the analyses and wrote the first and final draft of this manuscript. 2. LH, CC, and FB contributed to the study design, management of the study and data, CC prepared a draft for Fig. 1. 3. All authors reviewed the manuscript. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical agreement number 260 was obtained from the National Ethics Committee for Health Research (NEHRC) of Cambodia. Informed oral consent was obtained by children’sparents/guardians after a medical screening at PSE health center.
All methods were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
None declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Barennes, H., Houdart, L., de Courville, C. et al. Spirulina as a daily nutritional supplement of young pre-school Cambodian children of deprived settings: a single-blinded, placebo-controlled, cross-over trial. BMC Pediatr 22, 701 (2022). https://doi.org/10.1186/s12887-022-03766-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03766-5