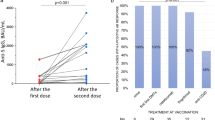
Abstract
Background
To investigate the safety (adverse events [AEs] and post-vaccination multiple sclerosis [MS] activity within 6 weeks), clinical efficacy (protection against coronavirus disease 2019 [COVID-19]), and vaccine-induced humoral immunogenicity (SARS-CoV-2 neutralizing antibody, anti-nucleocapsid IgG, and anti-spike IgG) of the Sinopharm (BBIBP-CorV) vaccine among people with MS (PwMS) receiving different disease-modifying therapies (DMTs).
Methods
This prospective cohort study was conducted between November 2021 and May 2022. PwMS were followed for six months after the 2nd dose of vaccination. Antibody responses were measured 2–16 weeks after the 2nd dose injection. Multivariate logistic regression was employed to assess the impact of each DMT on dichotomous antibody responses, adjusting for age, sex, MS phenotype, expanded disability status scale, disease duration, and vaccination-antibody titration interval.
Results
Among the 261 screened PwMS, 209 (aged 38.23 ± 9.73 years, female: 70.8%; relapsing-remitting MS: 80.4%) were included. The frequencies of experiencing non-serious AEs and post-vaccination MS activity were 66.0% and 4.8%, respectively. Breakthrough COVID-19 infection was observed in 14.8% of the PwMS. A subcohort of 125 PwMS was assessed for antibody responses. Positive neutralizing antibodies, anti-nucleocapsid IgG, and anti-spike IgG were detected in 36.8%, 35.2%, and 52.0% of the PwMS, respectively. Multivariate regression indicated a 96% (OR: 0.04 [95% CI: 0.00, 0.51], P = 0.013), 93% (OR: 0.07 [0.01, 0.64], P = 0.019), and 89% (OR: 0.11 [0.01, 0.96], P = 0.045) reduced odds of positive neutralizing antibody, anti-nucleocapsid IgG, and anti-spike IgG, respectively, among fingolimod-receivers. Additionally, anti-CD20s-receivers had 88% (OR: 0.12 [0.02, 0.85], P = 0.034) lower odds of being positive for anti-nucleocapsid IgG.
Conclusions
BBIBP-CorV appeared to be well tolerated in PwMS, with promising clinical efficacy. However, a suboptimal humoral response was observed in PwMS receiving fingolimod and anti-CD20s. Future research should investigate the relationship between humoral responses and the frequency and severity of COVID-19 infection across various DMTs.
Similar content being viewed by others
Introduction
Multiple sclerosis (MS) is a multifactorial chronic inflammatory autoimmune disease of the central nervous system (CNS) that is mediated by both the innate and adaptive immune systems [1]. According to the most recent version of the Multiple Sclerosis International Federation’s Atlas of MS, based on a 2020 global study, 2.8 million people worldwide have MS [2].
The worldwide pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has claimed millions of lives (WHO Coronavirus Dashboard, accessed: https://covid19.who.int/). A recent meta-analysis estimated that among people with MS (PwMS), the prevalence of suspected COVID-19 is 4% [3]. Furthermore, the prevalence of hospitalization and death following COVID-19 infection among this group is estimated to be 10% and 4%, respectively [3]. Although after the Omicron variant, the number of worldwide daily cases of COVID-19 declined in the third year of the pandemic and it seems that SARS-CoV-2 will move toward endemicity, it will remain a potential seasonal risk for immunosuppressed individuals such as PwMS [4, 5]. Hence, all PwMS for whom there are no other contraindications are recommended to receive and stay up to date with approved SARS-CoV-2 vaccines [5, 6]. Additionally, the Centers for Disease Control and Prevention (CDC) recommends that individuals who are moderately or severely immunocompromised, including PwMS, might require additional doses of updated SARS-CoV-2 vaccines [6, 7].
While vaccination is crucial for preventing infection in PwMS, there are unique considerations for this population compared to the general population, both in terms of disease risk and vaccine response. First, PwMS are more likely to contract specific bacterial and viral infections [5]. Second, these infections might be associated with a greater risk of relapses or pseudo-relapses [5]. Third, some disease-modifying therapies (DMTs) frequently described in PwMS, including anti-CD20 therapies (e.g., rituximab and ocrelizumab), are suggested to increase the risk of infection-related hospitalization and mortality [5]. Moreover, some DMTs, such as anti-CD20s or sphingosine-1-phosphate receptor (S1P) modulators (e.g., fingolimod), may decrease SARS-CoV-2 vaccine-induced immunity [5, 8]. On the other hand, other DMTs (e.g., interferon [IFN]-β and teriflunomide) might exert antiviral effects by preventing excessive host immune responses [5].
Recent studies have evaluated the safety and efficacy of SARS-CoV-2 vaccines among PwMS [9,10,11,12,13]. However, it is crucial to bear in mind that the safety and efficacy data of vaccines can differ across various vaccine platforms, DMTs, and diverse populations [5, 14]. Thus, to better understand how different DMTs affect vaccine response and guide individualized vaccination strategies, there is still a strong need to continue reporting data on clinical and immunological efficacy, as well as the safety profile of SARS-CoV-2 vaccines among PwMS across different DMTs [15]. The existing scientific literature on the safety and efficacy of SARS-CoV-2 vaccines among PwMS from Middle Eastern countries is notably limited. Furthermore, specific studies on the safety and efficacy of SARS-CoV-2 vaccines such as the Sinopharm (BBIBP-CorV) vaccine for PwMS are scarce [16]. Therefore, this study investigated the clinical effectiveness, vaccine-induced antibody (Ab) response (humoral immunogenicity), and safety of the Sinopharm (BBIBP-CorV) vaccine in Iranian PwMS receiving different DMTs.
Materials and methods
Study design and ethics statement
This prospective cohort study is reported in accordance with the guidelines issued by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [17] (Supplemental Table 1). The study was conducted between November 2021 and May 2022 at an academic hospital complex affiliated with the Tehran University of Medical Sciences, Tehran, Iran, and was approved by the local Institutional Review Board (IR.TUMS.IKHC.REC.1400.322). The study endpoint was six months after receiving the 2nd dose of the vaccine. Patient anonymity was carefully protected, and participants provided verbal and written informed consent for participation and publication according to the Declaration of Helsinki [18].
At the time of this study, the dominant SARS-CoV-2 variant in Iran was the Delta variant [19, 20]. Having been vaccinated, patients were prospectively followed up on their routine visit schedule for up to six months after receiving the 2nd dose. Additionally, at 2 to 16 weeks after the 2nd dose injection, blood samples were taken from the patients to measure the levels of SARS-CoV-2 neutralizing Ab, SARS-CoV-2 anti-nucleocapsid IgG, and SARS-CoV-2 anti-spike IgG. Possible serious adverse events (AEs) following vaccination, as well as the symptoms of possible relapse or pseudo-relapse (transient worsening), were explained to the patients, and they were instructed to visit the hospital sooner if they experienced these symptoms. In the case of any symptoms of relapse or pseudo-relapse, thorough medical examination and neuroimaging studies (as appropriate) were performed by a skilled neurologist. This approach aimed to confirm the diagnosis, differentiate between a true relapse and a pseudo-relapse, and distinguish between an exacerbation and the natural progression of the disease in patients with progressive MS.
Study population
Using consecutive sampling, this study enrolled individuals with MS confirmed by a neurologist using the McDonald criteria 2017 [21]. The participants were aged 16 to 65 years. Both newly diagnosed individuals and individuals with established MS were eligible regardless of their disease course. All participants received at least one dose of the Sinopharm (BBIBP-CorV) SARS-CoV-2 vaccine and attended the clinic between November 2021 and May 2022. They were actively followed up with at least two clinic visits in the year before vaccination and six months after vaccination. Finally, participants were willing to participate and provided written and verbal informed consent.
To ensure accurate and generalizable results, the study excluded the following groups: (a) pregnant or lactating women due to maternal immune system changes associated with pregnancy, as well as the effects of pregnancy on the clinical course of MS [22]; (b) individuals with other neurological or psychological diagnoses, such as neuromyelitis optica, optic neuritis, transverse myelitis, acute disseminated encephalomyelitis, myasthenia gravis, conversion, seizure, and neurodegenerative diseases; (c) individuals experiencing unstable medical conditions (excluding MS relapse or transient worsening), defined as changes in the medication type, medication dose, or disease severity within the past three months; (d) individuals with recent changes in DMTs; and (e) those who experienced “rebound syndrome” after cessation of medications such as fingolimod or natalizumab [23]. Finally, (f) immunocompromised individuals with malignancies, organ transplants, or inflammatory rheumatic diseases were excluded because studies suggest that their vaccine efficacy and immunogenicity may be diminished [5].
Study objectives
This study has two efficacy objectives:
-
1.
The clinical efficacy of the BBIBP-CorV vaccine, which was defined as the prevention of confirmed COVID-19 of any severity ≥ 7 days after the 1st or 2nd dose of vaccination. The 7-day duration was chosen according to previous literature and the incubation period of SARS-CoV-2 [24, 25].
-
2.
Humoral immunogenicity induced by the BBIBP-CorV vaccine, as assessed by the levels of SARS-CoV-2 neutralizing Ab, SARS-CoV-2 anti-nucleocapsid IgG, and SARS-CoV-2 anti-spike IgG at least 14 days after the 2nd dose injection. We compared the level of vaccine-induced Ab response (humoral immunogenicity) between patients receiving different DMTs and those not receiving any DMTs. Further analysis explored differences in immunogenicity across different DMT types.
There are two safety objectives of this study:
-
1.
The BBIBP-CorV vaccine solicited and unsolicited serious and non-serious AEs.
-
2.
Post-BBIBP-CorV-vaccination MS activity, defined as new onset or clinical relapse/exacerbation of MS ≤ 6 weeks (typical time frame) or 6–12 weeks (plausible but not typical time frame) following vaccination, provided that the patient was not infected with COVID-19 during this interval. We selected 6 weeks and 6–12 weeks based on the suggested criteria for labeling causality in neurological AEs following immunization [26, 27].
Study measures and definition of terms
The following independent variables were collected: (a) patients’ baseline characteristics (age, sex, past medical history, and habitual history), (b) MS-related characteristics (MS phenotype, age of disease onset, disease duration, expanded disability status scale [EDSS], and DMT type), (c) COVID-19-related characteristics (history of confirmed/suspected COVID-19 infection prior to vaccination and severity of disease), and (d) SARS-CoV-2 vaccine-related characteristics (type and dose of the vaccine, date of receiving each vaccine).
The prospectively assessed outcome variables included (a) SARS-CoV-2 vaccine safety-related characteristics (AEs and new MS onset or MS relapse/exacerbation ≤ 6–12 weeks following vaccination) and (b) SARS-CoV-2 vaccine clinical and immunological efficacy-related characteristics (being afflicted with COVID-19 at least 7 days after receiving the 1st or 2nd dose of vaccine, and the level of vaccine-induced Ab response at least 14 days after receiving the 2nd dose).
A definite history of COVID-19 was defined as a positive microbiological test or physician diagnosis based on a compatible clinical/imaging presentation in the context of an exposure risk in circumstances where testing was not available. Self-reported symptoms of new-onset fever and/or respiratory symptoms, such as cough, dyspnea, sore throat, rhinorrhea, nasal congestion, smell, or taste disturbances, myalgias, diarrhea, etc., were considered to indicate a history of suspected COVID-19 [28]. COVID-19 severity was classified as asymptomatic, mild, moderate, severe, or critically ill according to the National Institute of Health guidelines [28]. Non-serious AEs after vaccination were defined as injection site reactions, fever, fatigue, headaches, chills, muscle pain, joint pain, dizziness, diarrhea, nausea, vomiting, etc. Serious AEs were defined as anaphylaxis, thrombosis with thrombocytopenia syndrome, Guillain‒Barré syndrome, myocarditis, pericarditis, and events requiring hospital admission (except for MS activity, which was separately evaluated) [29]. COVID-19 after the 1st or 2nd dose of vaccination was confirmed by polymerase chain reaction (PCR) and physician diagnosis. Acute MS relapse was defined as a monophasic clinical episode with patient-reported symptoms and objective neurologic findings attributed to focal or multifocal CNS inflammatory demyelinating events that developed acute or subacute and lasted at least 24 h, with or without recovery, and in the absence of fever or infection [21]. Notably, paroxysmal or fleeting symptoms, as well as pseudo-relapses -defined as a temporary worsening of existing MS symptoms caused by increased body temperature, underlying infection, metabolic disturbance, or medical illness- were considered a lack of relapse [30].
Immune response metrics
The antigenic targets most examined in clinical settings are those targeting the spike (S) protein, the receptor binding domain (RBD) of the spike protein, and the nucleocapsid (N) core [31]. The presence of any of these antigens may lead to the development of IgM, IgA, or IgG isotypes [31]. Current evidence, based on vaccine efficacy trials, suggests that the quantitative titers of anti-spike IgG, anti-RBD IgG, and SARS-CoV-2 neutralizing Ab tests all correlate with protection against symptomatic infection, with neutralizing antibodies having the strongest correlation [32, 33]. Nevertheless, there is currently no specific Ab test or threshold to definitely estimate a person’s risk of contracting a subsequent infection [31]. In this study, we evaluated the production of SARS-CoV-2 neutralizing Ab, SARS-CoV-2 anti-nucleocapsid IgG, and SARS-CoV-2 anti-spike IgG, 2–16 weeks after the 2nd dose of SARS-CoV-2 vaccination.
SARS-CoV-2 neutralizing ab
This test is used to measure the level of neutralizing Abs in people who have recovered from COVID-19 or in people vaccinated with the SARS-CoV-2 vaccine [31]. Notably, neutralizing Ab levels are strongly predictive of immune protection and vaccine efficacy [34]. In our center, the cutoff for a positive SARS-CoV-2 neutralizing Ab was ≥ 2.5 µg/ml (© PISHTAZTEB DIAGNOSTICS).
SARS-CoV-2 anti-nucleocapsid IgG
This test is used to assess the presence of IgG Abs against the SARS-CoV-2 nucleocapsid core [31]. Values higher than 1.1 were considered seropositive, and values lower than 0.9 were considered seronegative. Values between 0.9 and 1.1 were considered suspicious (© PISHTAZTEB DIAGNOSTICS).
SARS-CoV-2 anti-spike IgG
This test is used to measure the level of IgG Abs against the spike antigen of SARS-CoV-2 [31]. A cutoff level of ≥ 8 RU/ml was considered a positive result (© PISHTAZTEB DIAGNOSTICS).
Statistical analysis
Descriptive statistics are reported as the mean ± standard deviation (SD) and median (interquartile range [IQR]) for symmetric and asymmetric numeric variables, respectively. Categorical variables are expressed as frequencies (percentages). Multivariate logistic regression was employed to assess the impact of each DMT on dichotomous Ab responses. The model was adjusted for covariates, including age, sex, MS phenotype, EDSS score, disease duration, and the interval between vaccination and Ab titration. Comparative analyses of variable distributions, such as age, EDSS score, age of disease onset, and disease duration, were conducted between patients with negative and positive levels of neutralizing Ab, anti-nucleocapsid IgG, and anti-spike IgG using bar plots. Additionally, comparisons of neutralizing Ab, anti-nucleocapsid IgG, and anti-spike IgG levels were performed according to sex, MS phenotype, DMT use, and specific types of DMTs, and the results are presented in bar plots. The relationships between the levels of neutralizing Ab, anti-nucleocapsid IgG, and anti-spike IgG and numeric variables were explored using the locally weighted least squares regression (loess) method. All analyses were conducted using R (version 4.3.2), and p-values less than 0.05 were considered to indicate statistical significance. No imputation was performed for missing data.
Results
Baseline characteristics of the study participants
Figure 1 illustrates the flow diagram of the study participants. Among the 261 PwMS who were screened during the study period, 52 were excluded for the following reasons: pregnancy or lactation (n = 6), presence of comorbid neuropsychological conditions (n = 9), immunocompromised status (n = 7), any unstable medical condition (n = 11), changes in DMT less than three months before enrollment (n = 16), or presence of rebound syndrome (n = 3). Therefore, a total of 209 PwMS (mean age: 38.23 ± 9.73 years, female: 70.8%) were included. To assess vaccine-induced humoral immunogenicity, three groups of patients were further excluded from the primary cohort: patients with a definite or suspected history of COVID-19 (n = 71), those who did not undergo blood testing (n = 7), and those who were afflicted with COVID-19 during the interval between vaccination and Ab titration (n = 6). Subsequently, a subcohort of 125 patients (mean age: 38.90 ± 9.04 years, female: 68.8%) was analyzed for Ab assessments (the immunogenicity subcohort).
Table 1 presents the baseline demographic, clinical, MS-related, and COVID-19-related characteristics of the study participants in the total cohort (N = 209) and the immunogenicity subcohort (N = 125). Most of the patients (80.4%) had relapsing-remitting MS (RRMS), with a mean disease duration of 8.57 ± 7.13 years and a mean EDSS of 1.85 ± 1.87. Of the 209 PwMS, 195 (93.3%) were on DMTs. IFN-β (26.3%), rituximab (21.5%), and fingolimod (17.7%) were the most frequently described DMTs. A similar pattern was observed in the immunogenicity subcohort. Notably, 71 (34.0%) patients had a suspected/definite history of COVID-19 prior to vaccination, with the majority experiencing mild symptoms (71.8%).
BBIBP-CorV vaccine safety: adverse events and post-vaccination MS activity
As shown in Tables 2 and 138 (66.0%) individuals experienced at least one non-serious AE, with fever (20.6%), injection site pain (17.2%), fatigue (16.3%), and headaches (16.3%) being the most frequently reported AEs. None of the patients experienced any serious AEs. Within 12 weeks of vaccination, 14 individuals (6.7%) experienced MS activity, of whom 10 (4.8%) had symptoms initiated in less than six weeks. Among them, three individuals developed new-onset RRMS (1.4%), while seven (3.3%) experienced clinical relapses of RRMS. The individual characteristics of patients with post-vaccination MS activity are provided in Supplemental Table 2.
BBIBP-CorV vaccine clinical efficacy: COVID-19 infection after vaccination
As displayed in Table 3, a total of 31 individuals (14.8%) were infected (or re-infected) with COVID-19 after being vaccinated, with the majority being asymptomatic or experiencing only mild symptoms. Supplemental Table 3 presents individual patient data for those who developed COVID-19 after vaccination, including details on their antibody response development.
BBIBP-CorV vaccine-induced humoral immunogenicity: antibody responses
Ab responses were evaluated in a subcohort of 125 PwMS, of whom 46 (36.8%), 44 (35.2%), and 65 (52.0%) were seropositive for SARS-CoV-2 neutralizing Ab, anti-nucleocapsid IgG, and anti-spike IgG, respectively (Table 3).
Figure 2 displays the mean Ab levels across various levels of numeric study variables (age, EDSS score, age of MS onset, and disease duration). Figure 3 compares the Ab levels according to categorical study variables (sex, MS phenotype, DMT usage, and specific DMT types). To assess the association between DMTs and vaccine-induced humoral immunogenicity, Ab responses were compared between DMT receivers (n = 117) and non-receivers (n = 8), as well as between those receiving separate types of DMTs, including anti-CD20s (n = 31), fingolimod (n = 21), dimethyl fumarate (n = 16), natalizumab (n = 5), IFN-β (n = 31), glatiramer acetate (n = 12), and teriflunomide (n = 1).
Comparisons of variable distributions, including age, EDSS score, age of disease onset, and disease duration, between patients with negative and positive levels of SARS-CoV-2 (A) neutralizing Ab, (B) anti-nucleocapsid IgG, and (C) anti-spike IgG. Abbreviations: Ab: antibody; EDSS: expanded disability status scale; IgG: immunoglobulin G; ns: nonsignificant; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2. *P value < 0.05. **P value < 0.01. ***P value < 0.001. ****P value < 0.0001
Comparison of SARS-CoV-2 (A) neutralizing Ab, (B) anti-nucleocapsid IgG, and (C) anti-spike IgG levels according to sex, MS phenotype, receiving DMTs, and the specific type of DMTs. Abbreviations: Ab: antibody; DMF: dimethyl fumarate; DMT: disease-modifying therapies; FG: fingolimod; GA: glatiramer acetate; IFN: interferon; MS: multiple sclerosis; ns: nonsignificant; NTM: natalizumab; OCR: ocrelizumab; PPMS: primary progressive multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis; RTX: rituximab; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SPMS: secondary progressive multiple sclerosis; TFM: teriflunomide. Note: The small sample sizes for certain treatments (e.g., natalizumab and teriflunomide) may limit the robustness of comparison of their immunogenicity. *P value < 0.05. **P value < 0.01. ***P value < 0.001. ****P value < 0.0001
SARS-CoV-2 neutralizing ab
Patients who were positive for neutralizing Ab had significantly lower EDSS scores than their counterparts (Fig. 2A. B). Neutralizing Ab levels were significantly greater in patients with RRMS than in those with PPMS (Fig. 3A. B). The highest levels of neutralizing Ab were observed in patients receiving teriflunomide, glatiramer acetate, dimethyl fumarate, and IFN-β, while the lowest levels were attributed to natalizumab, anti-CD20s, and fingolimod (Fig. 3A. D). Patients on anti-CD20s, as well as those on fingolimod and natalizumab, exhibited significantly lower neutralizing Ab levels than those on IFN-β, dimethyl fumarate, and glatiramer acetate (Fig. 3A. D).
SARS-CoC-2 anti-nucleocapsid IgG
The highest level of anti-nucleocapsid IgG was observed in DMT-non-receivers, as well as in patients receiving glatiramer acetate, dimethyl fumarate, and IFN-β, while the lowest levels were attributed to fingolimod and anti-CD20s (Fig. 3B. D). DMT receivers had lower anti-nucleocapsid IgG levels than those who did not receive DMTs (Fig. 3B. C). In particular, patients on anti-CD20s, as well as those on fingolimod, showed a reduced Ab response, compared to DMT-non-receivers, as well as IFN-β, dimethyl fumarate, and glatiramer acetate-receivers (Fig. 3B. D).
SARS-CoV-2 anti-spike IgG
Compared to their counterparts, patients with positive anti-spike IgG had considerably lower EDSS scores (Fig. 2C. B). Additionally, patients with RRMS had significantly higher levels of anti-spike IgG than those with PPMS (Fig. 3C. B). Patients receiving anti-CD20s, as well as those receiving fingolimod, showed a dampened Ab response compared to patients receiving IFN-β, dimethyl fumarate, and glatiramer acetate (Fig. 3C. D).
Correlations between ab levels and demographic and clinical characteristics in patients
Figure 4 shows the relationships between neutralizing Ab, anti-nucleocapsid IgG, and anti-spike IgG and numeric study variables. Accordingly, in patients who were positive for neutralizing Abs, older age (at vaccination) and older age at disease onset were significantly correlated with higher Ab levels. Additionally, a strong correlation was observed between positive anti-nucleocapsid IgG and disease duration, indicating that in individuals with a shorter duration of disease onset (at the time of vaccination), the concentration of this Ab was reduced.
The relationships between the levels of SARS-CoV-2 neutralizing Ab, anti-nucleocapsid IgG, and anti-spike IgG and numeric variables were determined using the Locally Weighted Least Squares Regression (loess) method. Abbreviations: Ab: antibody; EDSS: expanded disability status scale; IgG: immunoglobulin G; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
Factors affecting vaccine-induced humoral immunogenicity and the impact of DMTs
The unadjusted impact of variables on the development of positive Ab responses is shown in Supplemental Table 4. Accordingly, being female was associated with greater odds of having positive anti-nucleocapsid IgG (OR: 2.64; 95% CI: [1.15, 6.51], P = 0.027). Additionally, as the EDSS score increased, the odds of neutralizing antibody positivity and anti-spike IgG positivity significantly decreased (OR: 0.74 [0.59, 0.92], P = 0.10, and 0.82 [0.67, 0.99], P = 0.039, respectively). Anti-CD20s and fingolimod were associated with significantly lower odds of positive anti-nucleocapsid IgG (OR: 0.14 [0.02, 0.74], P = 0.024; OR: 0.10 [0.01, 0.60], P = 0.016, respectively).
Table 4 shows the adjusted impact of various DMT types on the development of positive Ab responses. After adjusting for age, sex, MS phenotype, EDSS score, disease duration, and vaccination-Ab titration interval, fingolimod-receivers had 96% (OR: 0.04 [0.00, 0.51], P = 0.013), 93% (OR: 0.07 [0.01, 0.64], P = 0.019), and 89% (OR: 0.11 [0.01, 0.96], P = 0.045) lower odds of positive neutralizing Ab, anti-nucleocapsid IgG, and anti-spike IgG than DMT-non-receivers, respectively. Additionally, individuals who received anti-CD20s had 88% (OR: 0.12 [0.02, 0.85], P = 0.034) lower odds of having positive anti-nucleocapsid IgG than those who did not receive DMTs.
Discussion
In this prospective study, we assessed the safety, clinical effectiveness, and humoral immunogenicity of the Sinopharm (BBIBP-CorV) SARS-CoV-2 vaccine in PwMS. The participants were predominantly female, in their 40s, and had a relapsing-remitting disease course. Approximately 66% of PwMS reported at least one non-serious AE post-vaccination. There were no serious AEs reported. The incidence of post-vaccination MS activity within 6 weeks was 4.8%. Approximately 15% of participants contracted a COVID-19 infection after vaccination, with the majority exhibiting asymptomatic or mild symptoms.
Approximately one-third of the PwMS tested positive for SARS-CoV-2 neutralizing Abs and anti-nucleocapsid IgG. Positive anti-spike IgG was detected in nearly half of the participants. Patients receiving fingolimod and anti-CD20s exhibited significantly lower levels of neutralizing Ab, anti-nucleocapsid IgG, and anti-spike IgG compared to those receiving IFN-β, glatiramer acetate, and dimethyl fumarate. Furthermore, those administered natalizumab demonstrated a reduced level of neutralizing Abs. Compared to those not receiving DMT and after accounting for potential confounders, individuals treated with fingolimod exhibited 96%, 93%, and 89% lower odds of developing positive neutralizing antibodies, anti-nucleocapsid IgG, and anti-spike IgG, respectively. Similarly, those administered anti-CD20s showed an 88% decrease in the likelihood of developing positive anti-nucleocapsid IgG. Notably, individuals who were administered dimethyl fumarate exhibited a favorable Ab response comparable to that of individuals receiving classical immunomodulators such as glatiramer acetate and IFN-β.
SARS-CoV-2 vaccine safety profile in PwMS: AEs and post-vaccination MS activity
In general, the present study mirrored the findings of previous studies on the safety of SARS-CoV-2 vaccines in PwMS [10, 11], with fever and injection site pain being the most common AEs. Additionally, consistent with previous studies, no patient experienced serious AEs [10, 11]. During the study, 4.8% of our patients experienced post-vaccination MS activity, including new onset (1.4%) or clinical relapse of the disease (3.3%). However, a recent meta-analysis, which included 23,088 doses of SARS-CoV-2 vaccines administered to 14,755 PwMS, reported a lower relapse proportion of 1.9% at an average time interval of 20 days post-vaccination [35]. The authors also indicated that the risk of relapse was not dependent on the type of SARS-CoV-2 vaccine administered [35]. The higher rate observed in our study could be ascribed to several reasons, such as the inclusion of patients with new-onset disease, the fact that the study was conducted at a single referral center, and the longer interval between vaccination and MS activity in our study (up to 6 weeks vs. 20 days). However, it is important to note that the estimated annual relapse rates in PwMS range between 0.3 and 1.7 per year [36], suggesting no “excess” risk of a neuroimmunological response induced by the vaccine [37]. Taken together, these findings indicate that the Sinopharm (BBIBP-CorV) SARS-CoV-2 inactivated virus vaccine is generally well tolerated by PwMS.
SARS-CoV-2 vaccine clinical efficacy in PwMS: breakthrough COVID-19 infection after vaccination
In our study, nearly 15% of vaccinated PwMS contracted a COVID-19 infection within six months post-vaccination. Consistently, a large study conducted among the general population reported a 10.3% infection rate between five to six months after vaccination, and this rate increased to 15.5% after more than six months [38]. However, the risk of contracting COVID-19 among vaccinated PwMS may differ from that among the general population due to several factors, including the MS treatment they are receiving. A prospective study among ocrelizumab-treated PwMS who received mRNA vaccines reported a low protection rate; 32 out of 54 patients (59.3%) developed a positive SARS-CoV-2 PCR test during the one-year follow-up of the study [39]. On the other hand, another large study among vaccinated PwMS revealed a considerably low rate of breakthrough COVID-19 infection; after a median follow-up of eight months, only 137 out of 19,641 vaccinated PwMS contracted the infection [40]. Therefore, while the risk of infection among the entire population of vaccinated PwMS may not be greater than that of the general population, patients on medications such as anti-CD20s or fingolimod may have a greater need for re-immunization.
SARS-CoV-2 vaccine-induced humoral immunogenicity in PwMS and the effect of DMTs
Cellular and humoral immune responses are of high importance following SARS-CoV-2 vaccination in PwMS on DMTs [8, 12, 13, 41]. Our findings regarding the humoral response following SARS-CoV-2 vaccination are consistent with those of previous studies [8, 11,12,13, 16, 42]. A retrospective monocentric study reported lower median anti-spike Ab titers in anti-CD20- and fingolimod-treated PwMS than in those receiving other DMTs [42]. Additionally, Capone et al. reported that the level of IgG against SARS-CoV-2 nucleoprotein was significantly lower in patients treated with ocrelizumab and fingolimod [11]. It is worth noting that while these studies conducted a “quantitative” evaluation of Abs (as opposed to our study, which considered the “serological positivity rate as a “binary” outcome), the overall trends of their results were in line with ours. Consistently, in a meta-analysis of 48 studies, Xi Wu et al. demonstrated that patients on anti-CD20s and S1P modulators had attenuated serologic responses, as reflected in the rate of serological positivity, compared to those without DMTs [8]. Our finding regarding a favorable Ab response in patients receiving dimethyl fumarate also agrees with that of Krajnc et al., who reported a 100% seroconversion rate in patients receiving this treatment [43]. Furthermore, Maniscalco et al. indicated that treatment with dimethyl fumarate (similar to IFN-β-1a) was associated with an increased humoral response to a booster dose of the Pfizer–BioNTech (BNT162b2) vaccine [44].
Apostolidis et al. showed that spike-specific and RBD-specific Abs were significantly reduced in patients on anti-CD2s, although they found an “adequate cellular” response to vaccination [12]. According to this study, anti-CD20-treated PwMS who lacked anti-RBD Abs differed in immune response coordination. This was characterized by a significant decrease in vaccine-induced circulating follicular helper T-cell responses, which occurred in parallel with increases in CD8+ T-cell responses [12]. Whether this cellular response can be considered “clinical protection” against COVID-19 should be further investigated [12]. Interestingly, a study by Smith et al. among 1,439 PwMS revealed no correlation between an increased risk of COVID-19 infection and any DMT type, although they reported that rituximab was associated with an increased risk of “severe” disease [45]. Other studies have also shown that virus-specific CD8+ T cells are crucial for the clearance of many viral infections, including COVID-19 [46, 47]. In brief, our findings align with the literature on other SARS-CoV-2 vaccines, indicating that fingolimod and anti-CD20s may independently predict a reduced humoral response. However, it is unclear whether this directly increases infection risk, given the significant role of the cellular immune system in preventing COVID-19. Consequently, future research should explore the influence of DMTs, particularly immunosuppressives, on cellular immunity in PwMS. This would aid in the development of a personalized vaccination program for PwMS.
Limitations and strengths
The generalizability of this study may be influenced by several practical limitations. First, we did not have baseline levels of Ab, which prevented us from using ‘seroconversion’ as a measure of humoral efficacy. Instead, we used ‘seropositivity’ as the outcome. However, to ensure a representative sample, we considered major confounding factors that could affect Ab levels by excluding patients with a definite or suspected history of COVID-19 before vaccination, as well as those who contracted COVID-19 between vaccination and Ab titration. Second, we did not evaluate cellular immune responses to vaccination. Finally, the number of PwMS in each medication category was limited. This might reduce the statistical power, and some outcomes that could be significant may not reach statistical significance [48]. Of note, the small sample sizes for certain treatments (e.g., natalizumab and teriflunomide) may limit the generalizability of our findings and the robustness of comparison of their immunogenicity.
Nonetheless, this study has multiple advantages. First, this study provides crucial insights into the safety and efficacy of the BBIBP-CorV vaccine in PwMS, a topic that is currently understudied, as most of the literature focuses on other vaccine platforms. Furthermore, as far as we are aware, there are limited studies examining the efficacy and safety of SARS-CoV-2 vaccines among Iranian PwMS and the wider Middle Eastern region, adding valuable diversity to the literature. Finally, by utilizing a prospective cohort design, we minimized selection and information bias [49]. We also made diligent efforts to mitigate any potential bias typically associated with prospective cohort studies, including confounding factors.
Conclusions and further directions
This study added to the understanding of SARS-CoV-2 vaccine responses in PwMS, particularly in understudied populations and with under-represented vaccine platforms like the Sinopharm vaccine. In summary, the BBIBP-CorV inactivated virus vaccine appears to be well tolerated in PwMS, with promising clinical efficacy. However, a suboptimal humoral response to BBIBP-CorV vaccination was observed in PwMS on certain DMTs, including anti-CD20s and fingolimod. Notably, not only patients receiving classical immunomodulatory DMTs but also those treated with dimethyl fumarate exhibited a favorable antibody response. To optimize individual vaccination strategies for PwMS, future research should investigate the relationship between humoral and cellular responses and the frequency and severity of clinical COVID-19 infection across various DMTs while also considering the effects of varying doses and platforms of SARS-CoV-2 vaccines.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- Ab:
-
Antibody
- AE:
-
Adverse event
- CDC:
-
Centers for disease control and prevention
- CNS:
-
Central nervous system
- COVID-19:
-
Coronavirus disease 2019
- DMT:
-
Disease-modifying therapy
- EDSS:
-
Expanded disability status scale
- IFN:
-
Interferon
- IgG:
-
Immunoglobulin G
- IQR:
-
Interquartile range
- MS:
-
Multiple sclerosis
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- PPMS:
-
Primary progressive multiple sclerosis
- PwMS:
-
People with multiple sclerosis
- RBD:
-
Receptor binding domain
- RRMS:
-
Relapsing-remitting multiple sclerosis
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SD:
-
Standard deviation
- SPMS:
-
Secondary progressive multiple sclerosis
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
- S1P:
-
Sphingosine-1-phosphate receptor
References
Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23(1):683–747.
Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS. Multiple Scler J. 2020;26(14):1816–21.
Moghadasi AN, Mirmosayyeb O, Barzegar M, Sahraian MA, Ghajarzadeh M. The prevalence of COVID-19 infection in patients with multiple sclerosis (MS): a systematic review and meta-analysis. Neurol Sci. 2021;42(8):3093–9.
del Rio C, Malani PN. COVID-19 in 2022—The Beginning of the End or the End of the Beginning? JAMA. 2022.
Golshani M, Hrdý J. Multiple sclerosis patients and disease modifying therapies: impact on immune responses against COVID-19 and SARS-CoV-2 vaccination. Vaccines. 2022;10(2):279.
CDC. COVID-19 Vaccines for People who are Moderately or Severely Immunocompromised: Centers for Disease Control and Prevention. 2024 [updated October 18, 2023; cited 2024 February 22]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html#:~:text=CDC%20recommends%20the%202023%E2%80%932024,2024%20updated%20COVID%2D19%20vaccine
CDC. Stay Up to Date with COVID-19 Vaccines Centers for Disease Control and Prevention Centers for Disease Control and Prevention. 2024 [updated January 18, 2024; cited 2024 February 22]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html
Wu X, Wang L, Shen L, Tang K. Response of COVID-19 vaccination in multiple sclerosis patients following disease-modifying therapies: a meta-analysis. EBioMedicine. 2022;81:104102.
Czarnowska A, Tarasiuk J, Zajkowska O, Wnuk M, Marona M, Nowak K et al. Analysis of side effects following vaccination against COVID-19 among individuals with multiple sclerosis treated with DMTs in Poland. Front Neurol. 2022;13.
Sahraian MA, Ghadiri F, Azimi A, Moghadasi AN. Adverse events reported by Iranian patients with multiple sclerosis after the first dose of Sinopharm BBIBP-CorV. Vaccine. 2021;39(43):6347–50.
Capone F, Lucchini M, Ferraro E, Bianco A, Rossi M, Cicia A, et al. Immunogenicity and safety of mRNA COVID-19 vaccines in people with multiple sclerosis treated with different disease-modifying therapies. Neurotherapeutics. 2022;19(1):325–33.
Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990–2001.
Tallantyre EC, Vickaryous N, Anderson V, Asardag AN, Baker D, Bestwick J, et al. Covid-19 vaccine response in people with multiple sclerosis. Ann Neurol. 2022;91(1):89–100.
Blanquart F, Abad C, Ambroise J, Bernard M, Débarre F, Giannoli JM, et al. Temporal, age, and geographical variation in vaccine efficacy against infection by the Delta and Omicron variants in the community in France, December 2021 to March 2022. Int J Infect Dis. 2023;133:89–96.
Beard K, Sriwastava S. Insight in booster COVID-19 vaccine and disease modifying therapy in multiple sclerosis. J Neurol Sci. 2021;430.
Etemadifar M, Sedaghat N, Nouri H, Lotfi N, Chitsaz A, Khorvash R, et al. SARS-CoV-2 serology among people with multiple sclerosis on disease-modifying therapies after BBIBP-CorV (sinopharm) inactivated virus vaccination: same story, different vaccine. Multiple Scler Relat Disorders. 2022;57:103417.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
World Medical Association Declaration. Of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4.
Sheikhi F, Yousefian N, Tehranipoor P, Kowsari Z. Estimation of the basic reproduction number of alpha and Delta variants of COVID-19 pandemic in Iran. PLoS ONE. 2022;17(5):e0265489.
Zali A, Khodadoost M, Gholamzadeh S, Janbazi S, Piri H, Taraghikhah N, et al. Mortality among hospitalized COVID-19 patients during surges of SARS-CoV-2 alpha (B.1.1.7) and delta (B.1.617.2) variants. Sci Rep. 2022;12(1):18918.
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–73.
McCombe PA. The Short and Long-Term effects of pregnancy on multiple sclerosis and experimental autoimmune encephalomyelitis. J Clin Med. 2018;7(12).
Hatcher SE, Waubant E, Nourbakhsh B, Crabtree-Hartman E, Graves JS. Rebound syndrome in patients with multiple sclerosis after Cessation of Fingolimod Treatment. JAMA Neurol. 2016;73(7):790–4.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15.
Wu Y, Kang L, Guo Z, Liu J, Liu M, Liang W. Incubation period of COVID-19 caused by Unique SARS-CoV-2 strains: a systematic review and Meta-analysis. JAMA Netw Open. 2022;5(8):e2228008.
Butler M, Tamborska A, Wood GK, Ellul M, Thomas RH, Galea I, et al. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J Neurol Neurosurg Psychiatry. 2021;92(11):1144–51.
WHO. Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification. 2018.
NIH. Clinical Spectrum of SARS-CoV-2 Infection: National Institutes of Health. 2021 [updated October 19, 2021; cited 2022 June 6]. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
CDC. Selected Adverse Events Reported after COVID-19 Vaccination. Centers for Disease Control and Prevention; [cited 2022 August 3]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html#:~:text=Anaphylaxis%20after%20COVID%2D19%20vaccination,and%20immediately%20treat%20the%20reaction
Avasarala J. Redefining Acute relapses in multiple sclerosis: implications for phase 3 clinical trials and treatment algorithms. Innov Clin Neurosci. 2017;14(3–4):38–40.
CDC, Science Brief. SARS-CoV-2 infection-induced and vaccine-induced immunity. CDC COVID-19 Science Briefs. Atlanta (GA): National Center for, immunization, respiratory diseases. Division of Viral Diseases, Centers for Disease Control and Prevention (US); 2020.
Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50.
Frenck RW Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–50.
Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11.
Stefanou MI, Palaiodimou L, Theodorou A, Christodoulou MV, Tzartos JS, Tzanetakos D, et al. Safety of COVID-19 vaccines in multiple sclerosis: a systematic review and meta-analysis. Mult Scler. 2023;29(4–5):585–94.
Inusah S, Sormani MP, Cofield SS, Aban IB, Musani SK, Srinivasasainagendra V, et al. Assessing changes in relapse rates in multiple sclerosis. Multiple Scler J. 2010;16(12):1414–21.
Epstein S, Xia Z, Lee AJ, Dahl M, Edwards K, Levit E, et al. Vaccination against SARS-CoV-2 in neuroinflammatory disease: early safety/tolerability data. Multiple Scler Relat Disorders. 2022;57:103433.
Israel A, Merzon E, Schäffer AA, Shenhar Y, Green I, Golan-Cohen A et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study. BMJ. 2021;375.
Novak F, Bajwa HM, Coia JE, Nilsson AC, Nielsen C, Holm DK, et al. Low protection from breakthrough SARS-CoV-2 infection and mild disease course in ocrelizumab-treated patients with multiple sclerosis after three mRNA vaccine doses. Journal of Neurology, Neurosurgery & Psychiatry; 2023.
Schiavetti I, Cordioli C, Stromillo ML, Teresa Ferrò M, Laroni A, Cocco E, et al. Breakthrough SARS-CoV-2 infections in MS patients on disease-modifying therapies. Multiple Scler J. 2022;28(13):2106–11.
König M, Torgauten HM, Tran TT, Holmøy T, Vaage JT, Lund-Johansen F, et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak Immune Response after COVID-19 vaccination. JAMA Neurol. 2022;79(3):307–9.
Mariottini A, Bertozzi A, Marchi L, Di Cristinzi M, Mechi C, Barilaro A, et al. Effect of disease-modifying treatments on antibody-mediated response to anti-COVID19 vaccination in people with multiple sclerosis. J Neurol. 2022;269(6):2840–7.
Krajnc N, Hegen H, Traxler G, Leutmezer F, Di Pauli F, Kornek B, et al. Humoral immune response to SARS-CoV-2 third vaccination in patients with multiple sclerosis and healthy controls: a prospective multicenter study. Mult Scler Relat Disord. 2022;65:104009.
Maniscalco GT, Liotti A, Ferrara AL, Prestipino E, Salvatore S, Di Battista ME, et al. Humoral efficacy of the third SARS-CoV-2 vaccine dose in multiple sclerosis subjects undergoing different disease-modifying therapies. Multiple Scler Relat Disorders. 2022;68:104371.
Smith TE, Madhavan M, Gratch D, Patel A, Saha V, Sammarco C, et al. Risk of COVID-19 infection and severe disease in MS patients on different disease-modifying therapies. Multiple Scler Relat Disorders. 2022;60:103735.
Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–93.
Graça D, Brglez V, Allouche J, Zorzi K, Fernandez C, Teisseyre M, et al. Both Humoral and Cellular Immune responses to SARS-CoV-2 are essential to prevent infection: a prospective study in a Working Vaccinated Population from Southern France. J Clin Immunol. 2023;43(8):1724–39.
Nayak BK. Understanding the relevance of sample size calculation. Indian J Ophthalmol. 2010;58(6):469–70.
Ignatius T, Shelly L. Clinical epidemiology workshop 5—Sources of bias in cohort studies. Hong Kong Med J. 2012;18(2):137.
Acknowledgements
We appreciate the participation of all neurologists and people with multiple sclerosis who made this research possible.
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.J., Z.E., M.A., K.A., F.N., A.A., M.M., B.P., MH.H.; Data curation: M.J., M.AL.; Formal analysis: M.J., M.AL.; Funding acquisition: N/A; Investigation: M.J., Z.E., K.A.; Methodology: M.J., MH.H.; Project administration: MH.H.; Resources: MH.H.; Software: M.AL.; Supervision: M.J., Z.E., MH.H.; Validation; M.J., MH.H.; Visualization: M.AL.; Writing - original draft: M.J., M.AL., Z.E., M.A., K.A., F.N., A.A., M.M., B.P.; Writing - review & editing: M.J., MH.H. All authors approved the final version to be published and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted at Imam Khomeini Hospital Complex and approved by the ethics committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.IKHC.REC.1400.322). All individuals provided written informed consent for participation and publication, according to the Declaration of Helsinki.
Consent for publication
All individuals provided written informed consent for participation and publication, according to the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12883_2024_3793_MOESM1_ESM.pdf
Supplementary Material 1: The supplementary material for this article includes Table S1 (STROBE Statement), Table S2 (Individual characteristics of participants with post-vaccination MS activity), Table S3 (Individual characteristics of participants with post-vaccination COVID-19 infection), and Table S4 (The unadjusted impact of variables on SARS-CoV-2 neutralizing Ab, anti-nucleocapsid IgG, anti-spike IgG).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jameie, M., Azizmohammad Looha, M., Ebadi, Z. et al. Immunogenicity, clinical efficacy, and safety of the sinopharm (BBIBP-CorV) SARS-CoV-2 vaccine among people with multiple sclerosis receiving disease-modifying therapies: a prospective cohort study. BMC Neurol 24, 291 (2024). https://doi.org/10.1186/s12883-024-03793-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-024-03793-y