Abstract
Background
Recent studies on the association between Helicobacter pylori (H. pylori) infection and obesity have reported conflicting results. Therefore, the purpose of our study was to investigate the association of obesity, abdominal obesity, and metabolic obesity phenotypes with H. pylori infection.
Methods
A cross-sectional study of 1568 participants aged 20 to 85 was conducted using the National Health and Nutrition Examination Survey (NHANES) cycle 1999–2000. Logistic regression models were employed to evaluate the association of general obesity as defined by body mass index (BMI), abdominal obesity as defined by waist circumference (WC) and waist-height ratio (WHtR), and metabolic obesity phenotypes with H. pylori seropositivity. Subgroup analyses stratified by age were conducted to explore age-specific differences in this association.
Results
After grouping individuals according to their WHtR, the prevalence rate of WHtR ≥ 0.5 in H. pylori-seropositive participants was significantly higher than that in H. pylori-seronegative participants (79.75 vs. 68.39, P < 0.001). The prevalence of H. pylori seropositivity in non-abdominal obesity and abdominal obesity defined by WHtR was 24.97% and 31.80%, respectively (P < 0.001). In the subgroup analysis, the adjusted association between abdominal obesity, as defined by the WHtR, and H. pylori seropositivity was significant in subjects aged < 50 years (OR = 2.23; 95% CI, 1.24–4.01; P = 0.01) but not in subjects aged ≥ 50 years (OR = 0.84; 95% CI, 0.35–1.99; P = 0.66). Subjects older than 50 years old had an OR (95% CI) for metabolically healthy obesity of 0.04 (0.01–0.35) compared with the control group. H. pylori seropositivity was consistently not associated with obesity as defined by BMI.
Conclusions
Abdominal obesity, as defined by the WHtR, was associated with H. pylori infection in subjects aged ≤ 50 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Helicobacter pylori (H. pylori) is a flagellated gram-negative bacterium [1, 2]. From January 2000 to June 2017, data from 73 countries showed that the prevalence of H. pylori infection was 44.3%, which was higher in developing countries than in developed countries (50.8% vs. 34.7%) [3]. It is well known that gastrointestinal diseases such as chronic gastritis, gastric cancer, and mucosa-associated lymphoid tissue lymphoma are linked to H. pylori [4]. According to current research, H. pylori infection is closely linked to various diseases outside the digestive tract [5], including diabetes mellitus (DM) and nonalcoholic fatty liver disease (NAFLD).
Obesity refers to the excessive accumulation of body fat. Obesity, a chronic metabolic disease, is the main component of the metabolic syndrome. Diseases caused by obesity include T2DM, hypertension, obstructive sleep apnea, and myocardial infarction. Over the past 50 years, the prevalence of obesity has increased globally and reached epidemic levels [6]. A meta-analysis found that the overall prevalence of global central obesity increased, whereas the prevalence of obesity in young and male subjects increased significantly according to temporal trends [7]. Therefore, as an important public health problem, obesity has been given attention in many countries [8]. Treatment of these diseases will increase the additional load on the healthcare system [9]. Current studies on the association between H. pylori infection and obesity have shown conflicting results. Some studies showed a positive correlation [10,11,12,13,14], while others found no correlation or even a negative correlation [15,16,17].
The body mass index (BMI) was commonly employed as a measure of obesity in previous articles. However, there are some limitations in the assessment of obesity by BMI. BMI does not provide any data on body composition. It is particularly insufficient for estimating body mass in physically active persons and in athletes who are often overweight, with a higher proportion of lean body mass but without any excess fat. Many epidemiological studies investigated the predictive value of BMI for cardiometabolic risk factors and cardiovascular events and consistently showed that BMI had a lower discriminatory power than waist circumference (WC) and waist-height ratio (WHtR) to distinguish individuals with high muscle mass from those with excess fat or abdominal obesity [18, 19]. Some scholars believe that the WHtR is a better parameter for cardiovascular risk, DM, and obesity [20,21,22,23]. An analysis of baseline data from a Chinese prospective cohort suggested WHtR to be the best indicator for dyslipidemia and hyperglycemia when compared with BMI and WC [24]. In recent years, a subtype of obesity that meets the diagnostic criteria for obesity without causing metabolic abnormalities such as T2DM or hyperlipidemia has been discovered. This obesity subtype is called metabolically healthy obesity (MHO). MHO is likely to progress to metabolically unhealthy obesity (MUO). In addition, there are metabolically healthy non-obese (MHN) and metabolically unhealthy non-obese (MUN) [25]. But there are few studies on the relationship between H. pylori infection and metabolic obesity phenotypes. Therefore, we assessed the cross-sectional association of general obesity, abdominal obesity, and metabolic obesity phenotypes with H. pylori seropositivity, utilizing data from the National Health and Nutrition Examination Survey (NHANES) conducted in the United States during 1999–2000.
Methods
Population
These data were obtained from the National Health and Nutrition Examination Survey (NHANES) cycle 1999–2000 because the participants in this cycle included data on H. pylori infection. The NHANES is a series of surveys led by the Center for Disease Control aimed at assessing the health and nutritional status of American adults and children since the 1960s. This was a complex, multi-stage probability sampling design using a nationally representative, non-institutionalized sample from the United States civilian population survey. The NHANES protocol was approved by the Institutional Review Board for Human Subjects of the United States Center for Disease Control and Prevention, and all participants provided written informed consent [26]. The data used for analysis in this study is publicly available on the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.
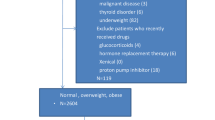
As is shown in Fig. 1, a total of 9965 subjects were selected. After excluding individuals who were pregnant and those without anthropometric measurements or serum biochemical parameters. Our study sample comprised 1568 U.S. citizens.
Helicobacter pylori seropositivity
H. pylori-specific immunoglobulin G (IgG) was determined using the Wampole Laboratories H. pylori IgG enzyme-linked immunosorbent assay (ELISA), which was designed to detect and qualitatively determine H. pylori IgG antibodies in human serum. Participants were divided into H. pylori seropositive (optical density (OD) ≥ 1.1) and seronegative (OD < 0.9) groups, using the standard ELISA cut-off value. To avoid misleading statistical results, ambiguous values (0.9–1.1) were excluded from the analysis [27, 28].
Obesity and metabolic phenotypes
The exposure variables were BMI, WC, and WHtR. Anthropometric measurements were performed using standardized procedures. In brief, a stadiometer was used for height measurement and an electronic digital scale to measure body weight. WC was measured at the high point of the iliac crest at minimal respiration. BMI was calculated by dividing body weight (kg) by the square of height (kg/m2). BMI was calculated to the nearest 0.1 kg/m2 and categorized according to the recommended cut points as follows: normal and underweight (< 25.0 kg/m2), overweight (25.0–30.0 kg/m2), and obesity (≥ 30 kg/m2) [29]. Abdominal obesity was defined as WC and WHtR [30, 31]. Abdominal obesity was defined by WC according to the thresholds for the US population included in the 2005 revision of the National Cholesterol Education Program’s (NCEP) Adult Treatment Panel III (ATP III): WC < 102 cm was considered normal for men, and WC ≥ 102 cm was considered abdominal obesity. WC < 88 cm was considered normal for women, and WC ≥ 88 cm was considered abdominal obesity [31]. WHtR was defined as the ratio of waist (cm) and height (cm), and based on previous studies, a widely used cut-off point was used to classify normal (WHtR < 0.5) or abdominal obesity (WHtR ≥ 0.5) [21, 22, 32, 33]. The complete measurement techniques for these variables were easily accessed at www.cdc.gov/nchs/nhanes/.
Metabolically healthy or unhealthy status was defined in accordance with the criteria outlined in the NCEP/ATP III as the following risk factors [34]: (1) systolic blood pressure ≥ 130 mm Hg and/or a diastolic blood pressure ≥ 85 mm Hg, or prescription medication for hypertension; (2) triglyceride ≥ 150 mg/dL (1.7 mmol/L); (3) fasting plasma glucose ≥ 100 mg/dL (5.6 mmol/L) or anti-diabetic treatment; and (4) high-density lipoprotein (HDL) cholesterol < 40 mg/dL (1.0 mmol/L) in men and < 50 mg/dL (1.3 mmol/L) in women. We constituted four groups of patients according to metabolic obesity phenotypes: the MHN phenotype was defined as the absence of all metabolic risk factors and a BMI < 30 kg/m2; the MHO phenotype was defined as the absence of all metabolic risk factors and a BMI ≥ 30 kg/m2; the MUN phenotype was defined as the presence of one or more metabolic risk factors and a BMI < 30 kg/m2; and the MUO phenotype was defined as the presence of one or more metabolic risk factors and a BMI ≥ 30 kg/m2 [35].
Variables
We collected data on age, gender, household size, race, educational level, height, waist circumference, weight, body mass index, waist-height ratio, smoking behavior, triglyceride, total cholesterol, high-density lipoprotein, fasting glucose, glycated hemoglobin A1c (HbA1c), and C-reactive protein. For covariates, age, gender, race, educational level, household size, WHtR, DM, hypertension, and smoking behavior were used as categorical variables. BMI, WC, systolic blood pressure, diastolic blood pressure, triglyceride, total cholesterol, high-density lipoprotein, fasting glucose, HbA1c, homeostasis model assessment of insulin resistance (HOMA-IR) value, and serum C reactive protein were used as continuous variables [27, 28, 36]. HOMA-IR was calculated using the following formula: fasting insulin [µU/mL] × fasting glucose [mmol/L] / 22.5.
All blood pressure data (systolic blood pressure and diastolic blood pressure) were measured by professionals certified for blood pressure measurements in the Mobile Examination Center (MEC). The blood pressure measurements were taken after the participants remained at rest for 5 minutes and measured three times in a row. Participants were considered to have hypertension for any of the following reasons: if they responded “yes” to the question “Have you ever been told by a doctor or other health professional that you have hypertension, also called high blood pressure?”; if they self-reported antihypertensive drug use; or if they had a high biological measurement value (systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg). The participant was considered DM if they met at least one of the following criteria: (1) self-reported doctor-diagnosed DM; (2) plasma HbA1c level ≥ 6.5%; (3) fasting plasma glucose level ≥ 126 mg/dL [37]. A previous diagnosis of DM was obtained from self-reported medical conditions [38].
The participants were categorized as nonsmokers, former smokers, or current smokers on the basis of their responses to those questions. Nonsmokers were defined as those who reported at baseline that they had smoked < 100 cigarettes in their lives. Former smokers were defined as those who had previously smoked > 100 cigarettes in their lifetime but were not currently smoking. Current smokers were defined as those who had previously smoked > 100 cigarettes in their lifetime and were currently smoking.
Statistical analysis
To compare the baseline characteristics of the study sample, we assessed continuous variables as mean ± standard deviation (SD) and categorical variables as numbers or percentages (n, %). We evaluated the differences between the two groups using the Student’s t-test and the chi-square test. After adjusting for covariates, logistic regression analysis was used to examine the association between anthropometric indices and H. pylori seropositivity in the crude analysis and ORs (95% CIs). All statistical analyses were performed using appropriate NHANES sampling weights.
Results
Characteristics of included subjects
Our study sample comprised 1568 subjects, of whom 889 were H. pylori seronegative and 679 were H. pylori seropositive. The overall prevalence of H. pylori infection was 43.30% (679/1568). The subjects with higher rates of H. pylori seropositivity included those who were younger than 50 years old, had lower levels of education, had four or more household members living with them, had lower levels of high-density lipoprotein, had higher levels of fasting glucose, WHtR, and HbA1c, and were current smokers (all P < 0.05). In the baseline survey, we did not find any significant difference in BMI or WC between the two groups (Table 1).
The prevalence of H. Pylori seropositivity after grouping according to BMI, WC and WHtR
Figure 2 indicates the prevalence of H. pylori seropositivity after grouping individuals. The blue bars indicate that the prevalence of H. pylori seropositivity in the normal and underweight (< 25.0 kg/m2), overweight (25.0–30.0 kg/m2), and obese (≥ 30 kg/m2) subjects was 26.28%, 33.82%, and 29.00%, respectively (p = 0.262). The red bars indicate that the prevalence of H. pylori seropositivity in non-abdominal obesity and abdominal obesity defined by WC was 31.86% and 27.08% in males (p = 0.204) and 26.18% and 31.02% in females (p = 0.259), respectively. The black bars indicate that the prevalence of H. pylori seropositivity in non-abdominal obesity and abdominal obesity defined by WHtR was 24.97% and 31.80%, respectively (p < 0.001).
H. Pylori seropositivity and general obesity
We used logistic regression analysis to assess the association between H. pylori seropositivity and BMI stratification, adjusted for confounding factors, and determined the odds ratios for being overweight or obese based on H. pylori infection status. Among all subjects, the OR (95% CI) for being overweight in Model 1 was 1.43 (0.99–2.07), compared with normal and underweight, but the statistical significance disappeared after adjustment for additional covariates in Models 2, 3, and 4. The OR (95% CI) for being overweight among subjects younger than 50 years was significant only in Model 1, at 1.67 (1.04–2.66); being overweight or obese was not associated with H. pylori seropositivity among subjects older than 50 years. In summary, no association was observed between being overweight or obese and H. pylori seropositivity after adjusting for covariates (Table 2).
H. Pylori seropositivity and abdominal obesity
Among all subjects, the OR (95% CI) for subjects with a WHtR ≥ 0.5 in Model 1 was 1.82 (1.37–2.41), compared with a WHtR of < 0.5, but the statistical significance disappeared after adjustment for additional covariates in Models 2, 3, and 4. Abdominal obesity defined by the WHtR was not associated with H. pylori seropositivity among subjects older than 50 years; these findings did not change after adjusting for covariates in models 2, 3, and 4 (Table 3). Among subjects aged ≤ 50 years, the OR (95% CI) for subjects with WHtR ≥ 0.5 in Model 1 was 1.87 (1.35–2.58) compared with the control group. This finding was still statistically significant in Models 2, 3, and 4, with ORs (95% CI) of 1.47 (0.99–2.18), 1.67 (1.16–2.41), and 2.23 (1.24–4.01), respectively. However, we did not observe an association between H. pylori seropositivity and abdominal obesity as defined by WC (Table 4).
H. Pylori seropositivity and metabolic obesity phenotypes
We analyzed the data to investigate the relationships between metabolic obesity phenotypes and H. pylori seropositivity. The OR (95% CI) for MUN was significant only in Model 1 among all subjects, at 1.79 (1.12–2.86). In Model 1, subjects older than 50 years had an OR (95% CI) of 0.09 (0.01–0.69) of the MHO phenotype, compared with the control group; after adjustment for major covariates, their ORs (95% CI) were 0.05 (0.00–2.87), 0.04 (0.01–0.35), and 0.04 (0.01–0.35) in Models 2, 3, and 4, respectively (Table 5). However, among subjects younger than 50 years, none of the models revealed any statistical significance related to H. pylori seropositivity.
Discussion
The purpose of our study was to investigate the association of obesity, abdominal obesity, and metabolic obesity phenotypes with H. pylori infection using the NHANES cycle 1999–2000. This study is the first to use NHANES data to prove that abdominal obesity, as defined by WHtR, is related to H. pylori seropositivity in subjects younger than 50 years old after adjusting for certain covariates compared to the control group. However, we did not find an association between H. pylori seropositivity and obesity in MUO individuals and overweight/obese individuals defined by BMI. We will explain these results later. Recently, studies on the relationship between H. pylori infection and obesity have been conducted. A large-scale cross-sectional study involving 76,915 participants in West China found that the prevalence of H. pylori infection in subjects with abdominal obesity (42.20%) was significantly higher than that in subjects with normal WC (39.10%) [10]. A cohort study in Israel involving 235,107 individuals showed that H. pylori positivity was positively associated with an increased BMI, or overweight/obesity [11]. Chen et al. conducted a study on 2604 subjects in Taiwan and discovered that subjects with H. pylori infection and those aged less than 50 years may have an increased risk of obesity (BMI ≥ 30) compared to those without this type of infection [12]. Similarly, a cross-sectional study showed that the prevalence of H. pylori infection in obese individuals was higher than that in individuals with a low BMI (BMI < 25 kg/m2) [13]. A recent meta-analysis showed that the risk of H. pylori infection was positively correlated with the prevalence of obesity [14]. According to the existing evidence, the relationship between H. pylori infection and obesity may involve several mechanisms. First, H. pylori may affect gut hormones involved in food intake and energy expenditure, such as ghrelin, obestatin, and leptin. Some studies have reported that serum leptin levels are significantly reduced in patients with H. pylori infection [39, 40]. Low levels of leptin delay satiety while eating, causing excessive nutrient intake and obesity [41]. Second, insulin resistance is a significant risk factor for metabolic disorders. H. pylori may contribute to metabolic disorders and obesity by producing pro-inflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-1, IL-6, and IL-8, which increase inflammatory responses and promote insulin resistance [42, 43]. The third factor is that the intestinal immune function of obese people is impaired, and the ability of monocytes to transform into macrophages is reduced in these patients. Meanwhile, the cytotoxic activity of natural killer cells in obese individuals is lower than that in healthy individuals with a BMI [44]. These two points may indicate that the immune environment of obese individuals promotes H. pylori survival [45, 46].
Abdominal obesity, as defined by WHtR, was associated with H. pylori infection in subjects younger than 50 years old compared with the control group in our study. As mentioned above, a meta-analysis has shown that the prevalence of central obesity among young subjects has increased significantly [7]. Our study found that the correlation between H. pylori seropositivity and obesity was most significant among young subjects. Therefore, we believe that this may be related to the fact that most subjects were infected with H. pylori when they were young. The inflammatory responses generated by H. pylori may be more active during this period and may have a greater impact on insulin resistance [12]. In older subjects, more diseases lead to systemic inflammatory responses, which can attenuate the effects of H. pylori infection on obesity [47,48,49]. In addition, we explored the relationship between metabolic obesity phenotypes and H. pylori, and the results showed that MHO ≥ 50 years subjects seem protected against H. pylori infection seropositivity (OR(95% CI): 0.04 (0.01, 0.35), P = 0.01). It suggests that the comorbidities present in obesity could be related to the H. pylori infection in middle-aged and elderly subjects. However, some studies did not find any clinical correlation between H. pylori infection and obesity. A study aimed at exploring the association between H. pylori infection and obesity or weight gain in a Chinese population did not observe a correlation between H. pylori infection and obesity after adjusting for confounders (RR: 0.831, 95% CI: 0.577–1.197, P = 0.321) [15]. A cross-sectional analysis of two separate populations of older adults (n = 13,044) from the Netherlands and Germany was conducted. Meta-analysis of cross-sectional data revealed no association between anti-H. pylori IgG titer and BMI, nor between H. pylori positivity and BMI. Mendelian randomization showed no causal relation between H. pylori genetic risk score and BMI or obesity, nor between BMI or obesity genetic risk scores and H. pylori positivity [16]. Interestingly, a retrospective study compared the prevalence of H. pylori colonization between obese and healthy-weight children. The results showed that H. pylori colonization was 10% in obese children compared with 21% in healthy-weight children (RR = 2.1, 95% CI, 1.1–4.0). Multivariate analysis showed that H. pylori colonization was associated with a 50% reduction in the odds of obesity (adjusted OR = 0.5, 95% CI, 0.2–1.0) [17]. Even some studies have found that eradicating H. pylori infection increases the incidence of obesity [50,51,52,53]. In the above studies, different methods were used to detect H. pylori. Therefore, we believe that these inconsistent findings may be related to the following reasons: firstly, different detection methods for H. pylori infection will affect the research results. Because of its advantages of being non-invasive, rapid, and low-cost, the stool antigen test (SAT) is recommended for the preliminary diagnosis of H. pylori infection; the sensitivity and specificity of the test were 95.5% and 97.6%, respectively [54]. A study found that the sensitivity and specificity of the urea breath test (UBT) were 96% and 93%, respectively, which was similar to the accuracy of the SAT using ELISA [55]. The H. pylori antibody test in urine only detects exposure status, with a specificity of 91% [56]. In our study, serological testing was used to diagnose H. pylori infection because it is non-invasive and fast. However, the diagnostic accuracy of serological tests is poor. One study demonstrated sensitivity ranging from 76 to 84% and specificities from 79 to 90% [57]. Different detection methods have different sensitivity and specificity, which causes the difference in the diagnosis rate of H. pylori, thus affecting the result. In addition, it cannot distinguish an active infection from a previous infection because antibodies continue to exist in the blood after eradication [58]. Second, infections with different H. pylori strains may have produced inconsistent results. For example, one study found that the prevalence of H. pylori infection and CagA strains differed among Americans of various races [59]. Lastly, this study used different measures to define obesity, and the findings suggest that H. pylori is associated with obesity as defined by WHtR but not with general obesity as defined by BMI or abdominal obesity as defined by WC, which may be related to the following reasons: WHtR is a more accurate body fact anthropometric index to define obesity. A study of 4052 participants was conducted to investigate the association between four anthropometric measures and the prevalence of diabetes. The research shows that the WHtR was more closely related to diabetes than BMI and WHR among study participants [60]. A meta-analysis showed that WHtR was a better predictor than WC and BMI for diabetes, dyslipidemia, hypertension, and cardiovascular disease risk in both sexes in populations of various nationalities and ethnic groups, and WHR should be considered as a screening tool [23]. This also led to a positive correlation between abdominal obesity defined by WHR and H. pylori infection seropositivity in subjects less than 50 years old in this study.
The strength of our study is that our data came from a nationally representative sample, and we also used different anthropological measures, so our results can provide strong evidence to determine the relationship between obesity and H. pylori infection. There are some limitations to our study. First, because this was a cross-sectional study, we could not ascertain the causal relationship between obesity and H. pylori infection. Second, serological testing cannot indicate that the patient is currently infected, which may reduce the rate of H. pylori infection. Finally, the relationship between the different H. pylori strains and obesity could not be explored.
In conclusion, we report that abdominal obesity, as defined by the WHtR, is associated with H. pylori infection in subjects aged ≤ 50 years. Whereas MUO and overweight/obese individuals (defined by BMI) were not associated with H. pylori infection.
Data availability
The datasets analyzed in this study are available in the NHANES repository (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
References
Sharndama HC, Mba IE. Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms[J].Brazilian journal of microbiology: [publication of the Brazilian society for Microbiology],2022, 53 (1): 33–50.
Alexander SM, Retnakumar RJ, Chouhan D et al. Helicobacter pylori in Human stomach: the inconsistencies in Clinical outcomes and the probable Causes[J].Front Microbiol,2021, 12: 713955.
Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection[J]. Aliment Pharmacol Ther. 2018;47(7):868–76.
Makola D, Peura DA, Crowe SE. Helicobacter pylori infection and related gastrointestinal diseases[J].Journal of clinical gastroenterology,2007, 41 (6): 548–58.
Santos MLC, De Brito BB, Da Silva FF, et al. Helicobacter pylori infection: beyond gastric manifestations[J]. World J Gastroenterol. 2020;26(28):4076–93.
Blüher M. Obesity: global epidemiology and pathogenesis[J]. Nat Rev Endocrinol. 2019;15(5):288–98.
Wong MCS, Huang J, Wang J et al. Global, regional and time-trend prevalence of central obesity: a systematic review and meta-analysis of 13.2 million subjects[J]. Eur J Epidemiol 2020, 35 (7): 673–83.
Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 Years[J]. N Engl J Med. 2017;377(1):13–27.
Tsur AM, Twig G. The actual burden of obesity-accounting for multimorbidity[J]. Lancet Diabetes Endocrinol. 2022;10(4):233–4.
Wang QWKQY. Central obesity is associated with helicobacter pylori infection: a large-scale cross-sectional retrospective study in West China[J],2020.
Suki M, Leibovici Weissman Y, Boltin D, et al. Helicobacter pylori infection is positively associated with an increased BMI, irrespective of socioeconomic status and other confounders: a cohort study[J]. Eur J Gastroenterol Hepatol. 2018;30(2):143–8.
Chen LW, Kuo SF, Chen CH et al. A community-based study on the association between Helicobacter pylori Infection and obesity[J].Sci Rep,2018, 8 (1): 10746.
Nasif WA, Hasan Mukhtar M, El-Moursy Ali AS, et al. Body mass index is associated with Helicobacter pylori infection and increased oxidative DNA damage in an obese population[J]. J Int Med Res. 2022;50(2):3000605221076975.
Baradaran A, Dehghanbanadaki H, Naderpour S, et al. The association between Helicobacter pylori and obesity: a systematic review and meta-analysis of case-control studies[J]. Clin Diabetes Endocrinol. 2021;7(1):15.
Xu MY, Liu L, Yuan BS, et al. Association of obesity with Helicobacter pylori infection: a retrospective study[J]. World J Gastroenterol. 2017;23(15):2750–6.
Den Hollander WJ, Broer L, Schurmann C et al. Helicobacter pylori colonization and obesity - a mendelian randomization study[J].Sci Rep,2017, 7 (1): 14467.
Vo HD, Goli S, Gill R et al. Inverse correlation between Helicobacter pylori colonization and obesity in a cohort of inner city children[J].Helicobacter,2015, 20 (1): 64–8.
Carlsson AC, Risérus U, Engström G et al. Novel and established anthropometric measures and the prediction of incident cardiovascular disease: a cohort study[J].International journal of obesity (2005),2013, 37 (12): 1579–85.
Schneider HJ, Glaesmer H, Klotsche J, et al. Accuracy of anthropometric indicators of obesity to predict cardiovascular risk[J]. J Clin Endocrinol Metab. 2007;92(2):589–94.
Swainson MG, Batterham AM, Tsakirides C et al. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables[J].PLoS One,2017, 12 (5): e0177175.
Yoo EG. Waist-to-height ratio as a screening tool for obesity and cardiometabolic risk[J]. Korean J Pediatr. 2016;59(11):425–31.
Browning LM, Hsieh SD. Ashwell M.A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value[J].Nutrition research reviews,2010, 23 (2): 247–69.
Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis[J]. Obes Rev 2012, 13 (3): 275–86.
Liu J, Tse LA, Liu Z et al. Predictive values of anthropometric measurements for cardiometabolic risk factors and Cardiovascular diseases among 44 048 Chinese[J]. J Am Heart Association 2019, 8 (16): e010870.
Mayoral LP, Andrade GM, Mayoral EP et al. Obesity subtypes, related biomarkers & heterogeneity[J]. Indian J Med Res 2020, 151 (1): 11–21.
Curtin LR, Mohadjer LK, Dohrmann SM et al. The National Health and Nutrition Examination Survey: Sample Design, 1999–2006[J].Vital and health statistics Series 2, Data evaluation and methods research,2012, (155): 1–39.
Meier HCS, Miller FW, Dinse GE et al. Helicobacter pylori seropositivity is associated with antinuclear antibodies in US adults, NHANES 1999–2000[J].Epidemiology and infection,2020, 148: e20.
Huang J, Liu Z, Ma J et al. The Association between Helicobacter pylori Seropositivity and Bone Mineral density in Adults[J].Mediators of inflammation,2022, 2022: 2364666.
Obesity. preventing and managing the global epidemic. Report of a WHO consultation[J].World Health Organization technical report series,2000, 894: i-xii, 1-253.
Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. Consensus Statement Int Diabetes Federation[J] Diabet Med. 2006;23(5):469–80.
Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III)[J].Jama,2001, 285 (19): 2486–97.
Rangel-Baltazar E, Rodríguez-Ramírez S, Cuevas-Nasu L et al. Short stature modifies the Waist-to-height ratio cut-off points as an Indicator of Cardiovascular Risk in Mexican Adult women and Men[J].Archives of medical research,2023, 54 (5): 102839.
Ashwell M, Gibson S. Waist to height ratio is a simple and effective obesity screening tool for cardiovascular risk factors: analysis of data from the British National Diet and Nutrition Survey of adults aged 19–64 years[J].Obes facts,2009, 2 (2): 97–103.
Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report[J].Circulation,2002, 106 (25): 3143 – 421.
Hinnouho GM, Czernichow S, Dugravot A et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study[J].European heart journal,2015, 36 (9): 551–9.
Huang JW, Xie C, Niu Z et al. The relation between Helicobacter pylori immunoglobulin G seropositivity and leukocyte telomere length in US adults from NHANES 1999–2000[J].Helicobacter,2020, 25 (6): e12760.
2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018[J].Diabetes Care,2018, 41 (Suppl 1): S13-s27.
Menke A, Casagrande S, Geiss L et al. Prevalence of and trends in diabetes among adults in the United States, 1988–2012[J].Jama,2015, 314 (10): 1021–9.
Mantero P, Matus GS, Corti RE, et al. Helicobacter pylori and corpus gastric pathology are associated with lower serum ghrelin[J]. World J Gastroenterol. 2018;24(3):397–407.
Ibrahim AS, Eldeeb MM, Salama OA et al. Possible association of ghrelin/obestatin balance with cardiometabolic risk in obese subjects with Helicobacter pylori[J].Endocrine regulations,2018, 52 (2): 101–9.
Obradovic M, Sudar-Milovanovic E, Soskic S, et al. Leptin and obesity: role and clinical Implication[J]. Front Endocrinol (Lausanne). 2021;12:585887.
Gunji T, Matsuhashi N, Sato H et al. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population[J].Helicobacter,2009, 14 (5): 144–50.
Siregar GA, Halim S, Sitepu VR. Serum TNF-a, IL-8, VEGF levels in Helicobacter pylori infection and their association with degree of gastritis[J].Acta Medica Indonesiana,2015, 47 (2): 120–6.
Martí A, Marcos A, Martínez JA. Obesity and immune function relationships[J]. Obes Rev. 2001;2(2):131–40.
Arslan E, Atilgan H. Yavaşoğlu I.The prevalence of Helicobacter pylori in obese subjects[J].European journal of internal medicine,2009, 20 (7): 695–7.
Moulin CM, Marguti I, Peron JP et al. Impact of adiposity on immunological parameters[J].Arquivos brasileiros de endocrinologia e metabologia,2009, 53 (2): 183–9.
Crabtree JE. Gastric mucosal inflammatory responses to Helicobacter pylori[J].Aliment Pharmacol Ther,1996, 10 Suppl 1: 29–37.
Polyzos SA, Kountouras J, Zavos C et al. The association between Helicobacter pylori infection and insulin resistance: a systematic review[J].Helicobacter,2011, 16 (2): 79–88.
Chen LW, Chien CY, Yang KJ et al. Helicobacter pylori infection increases insulin resistance and metabolic syndrome in residents younger than 50 Years Old: A Community-based Study[J].PLoS One,2015, 10 (5): e0128671.
Choi JS, Ko KO, Lim JW et al. The Association between Helicobacter pylori Infection and body weight among Children[J].Pediatric gastroenterology, hepatology & nutrition,2016, 19 (2): 110–5.
Kamada T, Hata J, Kusunoki H, et al. Eradication of Helicobacter pylori increases the incidence of hyperlipidaemia and obesity in peptic ulcer patients[J]. Dig Liver Dis. 2005;37(1):39–43.
Abdullahi M, Annibale B, Capoccia D et al. The eradication of Helicobacter pylori is affected by body mass index (BMI)[J].Obesity surgery,2008, 18 (11): 1450–4.
Azuma T, Suto H, Ito Y et al. Eradication of Helicobacter pylori infection induces an increase in body mass index[J].Aliment Pharmacol Ther,2002, 16 Suppl 2: 240–4.
Godbole G, Mégraud F, Bessède ER. Diagnosis of Helicobacter pylori infection[J].Helicobacter,2020, 25 Suppl 1: e12735.
Ferwana M, Abdulmajeed I, Alhajiahmed A, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis[J]. World J Gastroenterol. 2015;21(4):1305–14.
Mabe K, Kikuchi S, Okuda M et al. Diagnostic accuracy of urine Helicobacter pylori antibody test in junior and senior high school students in Japan[J].Helicobacter,2017, 22 (1).
Kayali S, Aloe R, Bonaguri C et al. Non-invasive tests for the diagnosis of helicobacter pylori: state of the art[J].Acta bio-medica: Atenei Parmensis,2018, 89 (8-s): 58–64.
Pichon M, Pichard B, Barrioz T et al. Diagnostic accuracy of a noninvasive test for detection of Helicobacter pylori and Resistance to Clarithromycin in Stool by the Amplidiag H. pylori + ClariR real-time PCR Assay[J].Journal of clinical microbiology,2020, 58 (4).
Ioannou GN, Weiss NS, Kearney DJ. Is Helicobacter pylori seropositivity related to body mass index in the United States?[J]. Aliment Pharmacol Ther. 2005;21(6):765–72.
Zhang FL, Ren JX, Zhang P et al. Strong Association of Waist Circumference (WC), body Mass Index (BMI), Waist-to-height ratio (WHtR), and Waist-to-hip ratio (WHR) with diabetes: a Population-based cross-sectional study in Jilin Province, China[J].Journal of diabetes research,2021, 2021: 8812431.
Acknowledgements
Not applicable.
Funding
This study was supported by the Health Industry Research Project of Gansu Province (GSWSKY2020-07), the Natural Science Foundation of Gansu Province (22JR4ZA102), and the Research Funds of the First Hospital of Lanzhou University(ldyyyn2019-74).
Author information
Authors and Affiliations
Contributions
Formal analysis & Data curation: Danni Chen. Shiling Wang. Supervision: Wei Yang, Hong Lu. Qian Ren. Validation: Shiling Wang. Writing—original draft: Danni Chen. Writing—review & editing: Qian Ren All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work does not include any studies performed on humans or animals.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, D., Wang, S., Yang, W. et al. Obesity, abdominal obesity, metabolic obesity phenotypes, and Helicobacter pylori infection: results from NHANES 1999–2000. BMC Infect Dis 24, 676 (2024). https://doi.org/10.1186/s12879-024-09409-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09409-7