Abstract
Background
Invasive Aspergillosis (IA) is a life-threatening fungal disease with significant mortality rates. Timely diagnosis and treatment greatly enhance patient outcomes. This study aimed to explore the association between patient age and the development of IA, as well as the potential implications for risk stratification strategies.
Methods
We searched National Center for Biotechnology Information (NCBI) databases for publications until October 2023 containing age characteristics of patients with and without IA. A random-effects model with the application of inverse-variance weighting was used to pool reported estimates from each study, and meta-regression and subgroup analyses were utilized to assess sources of heterogeneity.
Results
A systematic review was conducted, resulting in the inclusion of 55 retrospective observational studies with a total of 13,983 patients. Meta-analysis revealed that, on average, patients with IA were approximately two and a half years older (95% Confidence Interval [CI] 1.84–3.31 years; I2 = 26.1%) than those without the disease (p < 0.0001). No significant moderators could explain the observed heterogeneity in age difference. However, subgroup analysis revealed that age differences were more pronounced within particular patient groups compared to others. For example, patients with and without IA who had primary severe lung infections exhibited a greater difference in mean age than other patient cohorts.
Conclusions
Further research, such as individual patient data meta-analysis, is necessary to better understand the potential relationship between increasing age and the likelihood of IA. Improved risk stratification strategies based on patient age could potentially enhance the early detection and treatment of IA, ultimately improving patient outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspergillus spp. are among the most common causes of fungal infections in humans and can cause a variety of diseases in immunocompromised individuals and those with chronic lung disorders [1]. Invasive aspergillosis (IA) is estimated to affect roughly 200,000 people every year, with an overall mortality rate of 50%, reaching 100% if misdiagnosed [2]. The timely diagnosis and treatment of IA might be challenging as the available fungal diagnostic tests lack sensitivity. Thus, accurate knowledge of risk factors that predispose individuals to IA is essential for pre-empting disease, initiating early treatment, and ultimately improving survival [3].
Several patient-related risk factors are well-known to be associated with IA. Prolonged neutropenia is a key risk factor and often plays a role in patients with hematologic malignancies and those receiving chemotherapy and/or bone marrow transplantation. Solid organ transplantation and the use of immunosuppressive therapy, as well as prolonged use of corticosteroids have been recognized as risk factors for developing IA [3]. Interestingly, in the last 20 years, IA has been described in patients lacking classical risk factors. Emerging at-risk groups now include non-neutropenic patients such as those being critically ill, those with severe viral pneumonias, and those who receive novel biological therapies targeting immune-signaling pathways [4]. The identification of specific host factors predisposing individuals to IA is essential for early recognition of the development of IA, and can also be used to design specific management strategies (e.g., antifungal prophylaxis).
Expanding our understanding of the link between aging and susceptibility to infectious diseases is imperative as the number of elderly adults (aged over 60 years old) grows every year and has estimated to reach 2.1 billion by 2050 (World Health Organization; updated 2021 Sep 10). Currently, it is well established that aging affects the immune system and lung function, making older individuals more susceptible to respiratory infections like influenza and COVID-19 [5]. However, it remains unclear whether clinicians need to consider patients’ age when managing patients at risk of IA. Multiple clinical studies reported age as an independent risk factor for IA, while others could not find any association between older age and risks for IA. To investigate this further, a systematic review and meta-analysis were performed with the primary aim of identifying whether individuals with IA are older or younger than those without infection. Our ultimate objective was to guide future research and improve clinical estimates on the possibility of IA.
Materials and methods
The systematic review and subsequent meta-analysis were conducted according to the PRISMA guidelines (Table S1). Prior to the completion of the systematic search by the first reviewer, the study was preregistered on the preregistration service of the Open Science Framework (OSF, Registration DOI: https://doi.org/10.17605/OSF.IO/HFMP7).
Systematic literature search and inclusion of relevant studies
The literature search was performed to retrieve available studies in which the age of patients who were infected with IA was reported separately to patients who did not acquire this infection. We used the PICO framework to specify the eligibility criteria for the studies [6]. The diagnosis of IA in patients with underlying conditions was used as a selection criterion. IA was defined as proven, probable, or putative, as classified by individual studies [7, 8]. No specific interventions were investigated. For the comparison, the age of patients who acquired IA was compared to the age of patients who were not diagnosed with the disease. The control group included either all patients without IA in a cohort or randomly selected from the pool of patients without the disease in case-control studies. The reported mean or median age in both groups was used as an outcome.
R programming software and the RISmed package were utilized to search for reports in the National Center for Biotechnology Information (NCBI) databases. The query was created using the keywords: invasive aspergillosis risk factors. Age was not included in the keywords for the reason of not missing studies where age was reported but was not identified as a major risk factor. The search was performed with the following combination of terms: invasive [All Fields] AND (“aspergillosis” [MeSH Terms] OR “aspergillosis” [All Fields]) AND (“risk factors” [MeSH Terms] OR (“risk” [All Fields] AND “factors” [All Fields]) OR “risk factors” [All Fields]). The resulting studies were filtered with the following constraints: written in English and published before 2023/10/01. The appearance of duplicates was assessed.
One reviewer (ES) and a team of three reviewers (AD, FS, TC) independently evaluated the titles and abstracts for relevance using the automated abstract screener available with the metagear [9]. Records were excluded if they were presented as single case reports, non-clinical laboratory research, reviews (including systematic reviews), studies focusing on various invasive fungal infections, preliminary results from trials, clinical guidelines, therapeutic drug monitoring, non-human studies, clinical investigations focusing only on pediatric patients, studies on chronic aspergillosis, studies describing solely clinical characteristics of IA, publications without abstracts. Selected relevant full-text records were further evaluated. Discrepancies were settled by consensus.
Data extraction
Two authors (ES, EW) conducted data extraction from included studies using spreadsheets, which were reviewed for accuracy and comprehensiveness. The following variables were recorded: year of publication, years of data collection, patient’s primary disease, administration of antifungal prophylaxis (even when administrated only to a portion of patients), study type (cohort or case-control), and the requirement for intensive care management. When age was expressed as a median, the authors of such studies were contacted to provide the mean age and standard deviation. If this was not provided, the median age and the first and third quartiles were recorded to estimate the approximate sample mean and standard deviation.
Statistical analysis
Statistical analysis was performed using the RStudio software Version 1.4.1717. The following packages were utilized: dplyr, meta, metafor, dmetar, ggplot2, patchwork, flextable [10,11,12,13,14,15,16]. The dataset and the script of the analysis were uploaded at https://osf.io/hfmp7. The meta package was used to calculate and pool effects estimates. The effect size was calculated as a mean difference. To approximate means from the sample sizes, medians, and first and interquartile range, we used a method described by Luo et al. [17]. Approximate standard deviations were calculated from the same statistics using the method proposed by Shi et al. [18]. A random-effects model was applied to pool the results of each study with inverse-variance weighting. We used a restricted maximum-likelihood estimator for between-study variance tau2 [19]. Q-profile method for confidence interval of tau2 and tau [20]. The method proposed by Hartung and Knapp was applied to adjust test statistics and confidence intervals [21]. Outliers were defined as studies, in which the confidence interval did not overlap with the confidence interval of the pool effects analysis and were removed [22, 23]. Meta-analysis data was presented without outliers, effect size, prediction interval, and I2 heterogeneity before and after the removal of the outliers presented in Supplemental Table S2.
The dataset was further examined to evaluate the presence of possible influential studies that had a substantial impact on the overall result of the analysis. The baujat plots [24] did not detect any studies which would overly contribute to the heterogeneity (Fig. S1). The leave-one-out method was further utilized to estimate how omitting each study would affect the overall effect size and I2 heterogeneity (Fig. S2). This analysis showed that the exclusion of any of the studies from the analysis would not substantially alter heterogeneity and the effect size, confirming the absence of influential cases in the final dataset. For meta-regression and subgroup analyses, levels of moderator (study-level) variables were incorporated based on a pre-planned analysis of specific parameters of included studies [23]. Moderator variables were included in a random-effects model, which yielded a mixed-effects meta-regression model. The resulting value of I2 in this model reveals the amount of residual heterogeneity in the true effects, while R2 denotes the amount of heterogeneity that might be explained by the inclusion of a moderator in the model.
Data quality measures
Both publication bias and study quality bias were assessed via evaluation of small study bias [25, 26]. This was done by visually examining a funnel plot (Fig. S3) and implementing Egger’s regression test [25]. The evaluation did not indicate a small study bias as Eggers’ test did not show the presence of funnel plot asymmetry with p-value of 0.9727. In addition, a contour-enhanced funnel plot, which includes key areas of statistical significance (p = 0.1, p = 0.05, p = 0.01) (Fig. S4), was created to identify the risk of publication bias [27].
Newcastle-Ottawa quality assessment of studies
The quality and risk of bias in observational studies included in the meta-analysis was assessed using the Newcastle-Ottawa Scale tool [28]. The studies we evaluated based on three key components: the selection of study groups (involving representativeness and unbiased control selection), the comparability of groups (including the control for additional factors), and the rigor of outcome assessment (encompassing the ascertainment of diagnosis and the duration of follow-up). Notably, since none of the included studies were explicitly designed to examine age-related characteristics of patients, control for significant additional factors was lacking. Consequently, none of the studies qualified for a score in the comparability component, resulting in an overall assessment of either fair or poor quality according to the Agency for Healthcare Research and Quality (Table S3).
Results
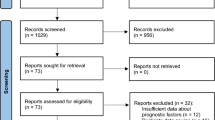
Of the 1067 studies screened, 65 were included in the meta-analysis (Fig. 1) [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93]. Studies were either a retrospective cohort (n = 46) or case-control (n = 19) design and were published before October, 2023. The main characteristics of selected studies are described in Table 1, while the quality assessment is presented in Table S3.
As a part of the characterization of studies, differences in the incidence of IA across different groups of patients and over time were evaluated using cohort studies (Table 1) involving 492,192 patients. This analysis showed that the incidence of IA did not significantly vary in patients with different underlying conditions as identified by using the Kruskal–Wallis test followed by the Dunn’s test indicating p > 0.05 (Fig. 2A). Incidence of IA was significantly higher in ICU patients compared to those who did not require admission to ICU (p = 0.0156, two-samples unpaired test Wilcoxon test) (Fig. 2B). Also, in our data set, the incidence of IA did not differ in the patient cohort that receives mold-active antifungal prophylaxis compared with those not receiving prophylaxis at all (p = 0.497, two-samples unpaired test Wilcoxon test) (Fig. 2C). Finally, we tested whether the incidence of IA has changed over time. The robust linear regression model indicated a possible relationship between disease incidence and the year when the data collection was concluded (Fig. 2D). The model’s result suggested a possible increase in incidence by 0.36% per year, but this increase was not statistically significant, as indicated by the p-value of 0.12.
Prior to performing the final meta-analysis, we carried out a sensitivity analysis, ten studies were indicated as outliers and removed [31, 37, 46, 47, 56, 70, 73, 81, 84, 89]. In total, 55 studies involving 13,983 patients (12,045 patients without and 1,938 with IA) were included in the final meta-analysis.
Evaluation of the difference in age of individuals with and without IA showed that patients who develop IA were generally older (MD = 2.58; 95% CI 1.84–3.31; p < 0.0001) (Fig. 3). This means that on average, people who develop IA are approximately two and a half years older than people who do not acquire this disease. The confidence interval suggests that in the universe of comparable studies, the patients with IA can be older by two to three years. Egger’s regression test did not reveal a small study bias (p = 0.97, Fig. S3). In addition, a low risk of publication bias was confirmed by examination of the contour-enhanced funnel plot that did not reveal an over-representation of effect sizes in the significant contours (Fig. S4). Also, there was no significant difference (p = 0.9685) between studies that reported age as mean (MD = 2.59; 95% CI 1.58–3.60) and studies that initially reported age as median (MD = 2.56; 95% CI 1.29–3.83). Finally, the mean age difference did not differ between cohort (MD = 2.35; 95% CI 1.60–3.10) and case-control (MD = 3.76; 95% CI 2.06–5.46) studies as indicated by the p-value of 0.1095.
Our meta-analysis revealed the Q-value of 73.03 with 54 degrees of freedom and a corresponding p-value of 0.0432. This allows us to reject the hypothesis that all studies included in this meta-analysis share a common effect size. The I2 statistics is 26.1% (95% CI 0.0% − 47.4%), indicating that the variance in observed effects suggests variance in true effects rather than sampling error. The 95% prediction interval, ranging from 0.96 to 4.19 years, suggests that the true mean difference in age varies across populations of patients. This prediction interval indicates that while in certain populations, patients with IA are moderately older than those without this disease, in other populations, the difference in age is trivial.
Therefore, to explore the causes of heterogeneity we implemented meta-regression analyses. The following moderator variables were tested: the year when studies began or ended, the duration of studies, the incidence of IA, data collection before 2014, study design, ICU admission, and antifungal prophylaxis. This test revealed that the variability in the observed effect sizes cannot be accounted for by the included moderators as p > 0.05 (Table S4).
To assess another possible source of heterogeneity we performed a group analysis comparing the age differences in patients with severe pulmonary infections, hematologic patients and all other types of patients (including critically ill individuals, and those with organ disorders and transplantation) unified in one group (Fig. 4). Interestingly, the subgroup analysis revealed that patients with respiratory tract infections were approximately three years older (MD = 3.37) with a 95% CI of 2.46 to 4.27 years. The mean age difference for those with hematologic conditions was lower (MD = 1.95; 95% CI 0.41–3.49). Similarly, patients with other underlying conditions showed a mean age difference of 1.58 years (95% CI 0.09–3.06). The test for subgroup differences indicated that there is a significant difference between these patient classifications (Q = 5.94, df = 2, p = 0.0513) and that the mean age difference is likely more substantial in patients with severe lung infections compared to other patient groups.
Discussion
To our knowledge, this is the first systematic review and meta-analysis study assessing the age of patients with various underlying conditions who developed IA. We found that patients with IA are older than those who do not develop this disease. In total, 13,983 patients from 55 studies were included in the final meta-analysis. On average, patients with IA were found to be about two and a half years older (95% CI 1.84–3.31 years, p < 0.0001) than individuals without IA. Therefore, the results of this research suggest that age should be considered while assessing potential risks of developing IA, but additional studies are needed to establish whether age is an independent risk factor and to determine the age at which susceptibility to IA increases.
Our finding is consistent with the previous meta-analyses that evaluated risk factors for IA in a single patient population. In kidney transplantation studies, based on data from six studies, recipient age prior to transplantation was roughly six years (95% CI 3.91–8.01 years) higher for individuals with IA compared to those without IA, where the mean age of patients with IA was over 55 years of age in almost all included studies [94]. Similarly, according to meta-analysis of eight studies, COVID-19 patients diagnosed with IA were typically seven and a half years older (95% CI 2.02–13.03 years) than those who only had COVID-19 [95]. Our study extends the observation in these two cohorts and suggests that such age difference might be true across various populations of patients who are susceptible to Aspergillus infections. However, the results of our analysis were heterogenous, suggesting that age difference varies from one patient population to another. In accordance, a narrative review from 2012 reported advanced age as a patient risk factor for IA associated with some but not all underlying conditions including allogeneic hematopoietic stem cells, lung or heart-lung, heart, or small bowel transplantation, leukemia, multiple myeloma, non-Hodgkin’s lymphoma, and burns [3].
Our investigation suggests that the incidence of IA is likely higher in older individuals who already have severe respiratory infections. This could be attributed to the increased possibility of dysregulated immune responses in older individuals. Severe respiratory infections can lead to exacerbated inflammation, resulting in cell infiltration, tissue damage, and hypoxia [96]. As aging is associated with increased basal levels of lung inflammation [97, 98], respiratory infections can be particularly detrimental to aged lungs. Consequently, by causing exaggerated inflammation, primary severe infections create conditions favorable for fungal infection and establishment of the disease [96].
Interestingly, in a previously published study amongst patients with lymphoproliferative diseases and receiving an autologous hematopoietic stem cell transplantation, older age was not observed as a risk factor [99]. However, in another study including patients who underwent bone marrow transplantation, older age was found to be a significant risk factor for the development of late (more than 40 days after transplantation) but not early IA (less than 40 days after transplantation). There, patients, older than 40 were 5-fold times more likely to develop this disease in contrast to younger individuals that were younger than 18 [100]. In our meta-analysis, we grouped all patients with hematologic malignancies together and observed that the age difference between those with IA and those without IA ranged from trivial to moderately older, with a 95% prediction interval of 0.59 to 3.31 years. Such heterogeneity might be explained by patient stratification strategies that were more common in the past. Because elderly people were more predisposed to adverse effects of immunosuppression, younger individuals were more likely to undergo immunosuppressive procedures in preparation and post stem cell transplantation and thus, they were more likely to develop IA in contrast to older hematologic patients. Therefore, the underrepresentation of elderly individuals in the “hematology” subgroup would explain such heterogeneity of age difference.
The age difference within the group comprising critically ill patients, individuals with organ disorders, and patients undergoing organ transplantation showed considerable heterogeneity. Notably, the wide prediction interval of -2.24 to 5.40 years indicates that, in these cohorts, while some individuals with IA were slightly younger than their counterparts, others could be substantially older. To gain a deeper understanding of these variations and their clinical implications, future research should investigate the specific factors or conditions that contribute to such heterogeneity.
Overall, our findings suggest that older age may put patients at additional risk of developing IA. This has an important clinical significance because advanced age might also be associated with an increased mortality rate if the disease is established. For example, older age was an independent risk factor for mortality among patients with IA who are critically ill [101] or suffer from acute myeloid leukemia [30]. An 11-year follow-up report study from Taiwan has found that in-hospital mortality from IA increased with age being the highest for the 80 + age group [102]. However, this requires further investigation. In another study, where only ICU patients with IA were included, mortality was not different for individuals older or younger than 75 years old [103].
The main strength of this study is its large sample size of participants: the control group consisted of 12,045 patients, while the IA group included 1,938 individuals. This contrasts with a typical clinical study of patients with IA that on average includes only 250 and 30 participants in each group respectively. Other strengths include the pre-registered protocol basis (study design and analysis plan created prior to analysis), and the application of a random-effects model to account for substantial heterogeneity among included studies. Another strength is the inclusion of data from patients with a wide range of underlying conditions and from different countries that allow us to synthesize a data set representative of real-world populations affected by IA. Our present study also had several limitations: lack of correction for important confounders such as chronic conditions, gender, or environmental factors. In addition, some studies did not distinguish between probable, proven, or putative IA diagnosis and thus could introduce false positive results. Selection bias may exist because we included only papers with full reports in English.
Another limitation of the study includes a limited direct utility for decision-making parties. Although we suggest a potential association between older age and increased frequency of IA, we were unable to provide specific age constraints. Future cross-sectional studies should aim to identify age thresholds at which susceptibility to IA is heightened [104, 105]. Additionally, to perform regression analysis on IA incidence and age while accounting for confounding variables, individual patient data would be necessary.
Conclusion
To summarize, our systematic review and meta-analysis found that patients with IA are older than those without the disease. This finding warrants further research into determining whether older age is an independent risk factor for the disease. Our study extends previous observations and suggests that the age difference may be true across various patient populations susceptible to IA. Advanced age may also be associated with increased mortality rates among patients with IA, which underscores the importance of optimized risk stratification strategies. While our study had several limitations, it represents a significant step toward understanding the relationship between age and IA development.
Availability of data and materials
The datasets generated and analysed during the current study are available in the Open Science Framework repository, [https://osf.io/69e8u/?view_only=c700416f336c41cbbee8627c0492ac77].
References
Arastehfar A, Carvalho A, Houbraken J, Lombardi L, Garcia-Rubio R, Jenks JD, et al. Aspergillus Fumigatus and aspergillosis: from basics to clinics. Studies in Mycology. CBS-KNAW Fungal Biodiversity Centre. 2021. https://doi.org/10.1016/j.simyco.2021.100115.
Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012. https://doi.org/10.1126/scitranslmed.3004404.
Herbrecht R, Bories P, Moulin JC, Ledoux MP, Letscher-Bru V. Risk stratification for invasive aspergillosis in immunocompromised patients. Ann N Y Acad Sci. 2012. https://doi.org/10.1111/j.1749-6632.2012.06829.x.
Latgé JP, Chamilos G. Aspergillus Fumigatus and aspergillosis in 2019. Clin Microbiol Rev. 2020;33. https://doi.org/10.1128/CMR.00140-18.
Schneider JL, Rowe JH, Garcia-de-Alba C, Kim CF, Sharpe AH, Haigis MC. The aging lung: physiology, disease, and immunity. Cell. 2021;184:1990–2019. https://doi.org/10.1016/j.cell.2021.03.005.
Mattos CT, de Ruellas AC. O Evidence-based Orthod. 2015. https://doi.org/10.1590/2176-9451.20.1.017..
Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, the Mycoses Study Group Education and Research Consortium. Revision and update of the Consensus definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and. Clin Infect Dis. 2020;71:1367–76. https://doi.org/10.1093/cid/ciz1008.
Blot SI, Taccone FS, Van Den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, et al. A clinical algorithm to diagnose Invasive Pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. https://doi.org/10.1164/rccm.201111-1978OC.
Lajeunesse MJ. Facilitating systematic reviews, data extraction and meta-analysis with the metagear package for R. Methods Ecol Evol. 2016;7:323–30. https://doi.org/10.1111/2041-210X.12472.
Viechtbauer W. Conducting Meta-analyses in R with the metafor Package. J Stat Softw. 2010;36:1–48. https://doi.org/10.18637/jss.v036.i03.
Lin Pedersen Thomas. patchwork: The Composer of Plots. 2021.
Harrer M, Cuijpers P, Furukawa T, Ebert DD. dmetar: Companion R Package For The Guide Doing Meta-Analysis in R. 2019. Available: http://dmetar.protectlab.org/..
Wickham H, François R, Henry L, Müller K. dplyr: A grammar of data manipulation. R package version 1.0.6. 2021.
Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York; 2016. Available: https://ggplot2.tidyverse.org..
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60. https://doi.org/10.1136/ebmental-2019-300117.
Gohel David. flextable: Functions for Tabular Reporting. R package version 0.6.9. https://CRAN.R-project.org/package=flextable.. 2021.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805. https://doi.org/10.1177/0962280216669183.
Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11:641–54. https://doi.org/10.1002/jrsm.1429.
Viechtbauer W. Bias and Efficiency of Meta-Analytic Variance Estimators in the Random-Effects Model. J Educ Behav Stat. 2005;30(3):261–93. https://doi.org/10.3102/10769986030003261.
Viechtbauer W. Confidence intervals for the amount of heterogeneity in meta-analysis. Stat Med. 2007;26:37–52. https://doi.org/10.1002/sim.2514.
Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–710. https://doi.org/10.1002/sim.1482.
Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1:112–25. https://doi.org/10.1002/jrsm.11.
Harrer M, Cuijpers P, Ebert AFT. Doing Meta-analysis with R: a Hands-On Guide. 1st ed. Boca Raton: Chapman & Hall/CRC; 2021.
Baujat B, Mahé C, Pignon JP, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. 2002;21:2641–52. https://doi.org/10.1002/sim.1221.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. https://doi.org/10.1136/bmj.315.7109.629.
Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–412. https://doi.org/10.1001/jama.273.5.408.
Palmer TM, Peters JL, Sutton AJ, Moreno SG. Contour-enhanced funnel plots for meta-analysis. Stata J. 2008. Available: http://www.stata.com/support/faqs/stat/meta.html..
Wells GA, Wells G, Shea B, Shea B, O’Connell D, Peterson J et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available: https://api.semanticscholar.org/CorpusID:79550924..
Levesque E, Ait-Ammar N, Dudau D, Clavieras N, Feray C, Foulet F, et al. Invasive pulmonary aspergillosis in cirrhotic patients: analysis of a 10-year clinical experience. Ann Intensive Care. 2019;9:9. https://doi.org/10.1186/s13613-019-0502-2.
Michallet M, Sobh M, Morisset S, Kraghel S, Nicolini FE, Thomas X, et al. Risk factors for invasive aspergillosis in acute myeloid leukemia patients prophylactically treated with posaconazole. Med Mycol. 2011;49:681–7. https://doi.org/10.3109/13693786.2011.557668.
Napolioni V, Pariano M, Borghi M, Oikonomou V, Galosi C, De Luca A, et al. Genetic polymorphisms affecting IDO1 or IDO2 activity differently associate with aspergillosis in humans. Front Immunol. 2019;10:10. https://doi.org/10.3389/fimmu.2019.00890.
Mikulska M, Raiola AM, Bruno B, Furfaro E, Van Lint MT, Bregante S, et al. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transpl. 2009;44:361–70. https://doi.org/10.1038/bmt.2009.39.
White PL, Parr C, Barnes RA. Predicting Invasive Aspergillosis in Hematology Patients by Combining Clinical and Genetic Risk Factors with Early Diagnostic Biomarkers. J Clin Microbiol. 2017;56(1):e01122-17. https://doi.org/10.1128/JCM.01122-17.
Michallet M, Bénet T, Sobh M, Kraghel S, El Hamri M, Cannas G, et al. Invasive aspergillosis: an important risk factor on the short- and long-term survival of acute myeloid leukemia (AML) patients. Eur J Clin Microbiol Infect Dis. 2012;31:991–7. https://doi.org/10.1007/s10096-011-1397-5.
Lahmer T, Brandl A, Rasch S, Baires GB, Schmid RM, Huber W, et al. Prevalence and outcome of invasive pulmonary aspergillosis in critically ill patients with liver cirrhosis: an observational study. Sci Rep. 2019;9:9. https://doi.org/10.1038/s41598-019-48183-4.
Waldeck F, Boroli F, Suh N, Wendel Garcia PD, Flury D, Notter J, et al. Influenza-associated aspergillosis in critically-ill patients—a retrospective bicentric cohort study. Eur J Clin Microbiol Infect Dis. 2020;39:1915–23. https://doi.org/10.1007/s10096-020-03923-7.
Chen L, Han X, Li Y, Zhang C, Xing X. Invasive pulmonary aspergillosis in immunocompetent patients hospitalised with influenza A-related pneumonia: a multicenter retrospective study. BMC Pulm Med. 2020;20:20. https://doi.org/10.1186/s12890-020-01257-w.
Luong ML, Chaparro C, Stephenson A, Rotstein C, Singer LG, Waters V, et al. Pretransplant aspergillus colonization of cystic fibrosis patients and the incidence of post-lung transplant invasive aspergillosis. Transplantation. 2014;97:351–7. https://doi.org/10.1097/01.TP.0000437434.42851.d4.
Chauvet P, Mallat J, Arumadura C, Vangrunderbeek N, Dupre C, Pauquet P, et al. Risk factors for Invasive Pulmonary aspergillosis in critically ill patients with Coronavirus Disease 2019-Induced Acute Respiratory Distress Syndrome. Crit Care Explor. 2020;2:e0244. https://doi.org/10.1097/cce.0000000000000244.
Kurosaki F, Bando M, Nakayama M, Mato N, Nakaya T, Yamasawa H, et al. Clinical features of pulmonary aspergillosis associated with interstitial pneumonia. Intern Med. 2014;53:1299–306. https://doi.org/10.2169/internalmedicine.53.1578.
Dellière S, Dudoignon E, Fodil S, Voicu S, Collet M, Oillic PA, et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. 2021;27:790e1-7790. https://doi.org/10.1016/j.cmi.2020.12.005.
Bellelli V, Siccardi G, Conte L, Celani L, Congeduti E, Borrazzo C, et al. Preliminary attempt to predict risk of invasive pulmonary aspergillosis in patients with influenza: decision trees may help? Antibiotics. 2020;9:1–8. https://doi.org/10.3390/antibiotics9100644.
Tejerina EE, Abril E, Padilla R, Rodríguez Ruíz C, Ballen A, Frutos-Vivar F, et al. Invasive aspergillosis in critically ill patients: an autopsy study. Mycoses. 2019;62:673–9. https://doi.org/10.1111/myc.12927.
Razazi K, Arrestier R, Haudebourg AF, Benelli B, Carteaux G, Decousser JW, et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit Care. 2020;24. https://doi.org/10.1186/s13054-020-03417-0.
Heylen L, Maertens J, Naesens M, Wijngaerden E, Van, Lagrou K, Bammens B et al. Invasive Aspergillosis after Kidney Transplantation: Case-Control Study. 2015. Available: http://cid.oxfordjournals.org/..
Seok H, Huh K, Young Cho S, Kang C-I, Ryeon Chung D, Seong Huh W et al. Risk factors for development and mortality of invasive pulmonary aspergillosis in kidney transplantation recipients. https://doi.org/10.1007/s10096-020-03871-2/Published..
Kaya S, Gençalioǧlu E, Sönmez M, Köksal I. The importance of risk factors for the prediction of patients with invasive pulmonary aspergillosis. Rev Assoc Med Bras. 2017;63:764–70. https://doi.org/10.1590/1806-9282.63.09.764.
Bitterman R, Hardak E, Raines M, Stern A, Zuckerman T, Ofran Y et al. Baseline chest computed tomography for early diagnosis of Invasive Pulmonary aspergillosis in Hemato-oncological Patients-a prospective cohort study. https://doi.org/10.1093/cid/ciz194/5373534..
Chen J, Yang Q, Huang J, Li L. Risk factors for invasive pulmonary aspergillosis and hospital mortality in acute-on-chronic liver failure patients: a retrospective-cohort study. Int J Med Sci. 2013;10:1625–31. https://doi.org/10.7150/ijms.6824.
López-Medrano F, Fernández-Ruiz M, Silva JT, Carver PL, van Delden C, Merino E, et al. Multinational case-control study of risk factors for the development of late invasive pulmonary aspergillosis following kidney transplantation. Clin Microbiol Infect. 2018;24:192–8. https://doi.org/10.1016/j.cmi.2017.06.016.
Rodriguez-Goncer I, Thomas S, Foden P, Richardson MD, Ashworth A, Barker J, et al. Invasive pulmonary aspergillosis is associated with adverse clinical outcomes in critically ill patients receiving veno-venous extracorporeal membrane oxygenation. Eur J Clin Microbiol Infect Dis. 2018;37:1251–7. https://doi.org/10.1007/s10096-018-3241-7.
Herrera S, Gohir W, Foroutan F, Aguilar C, Juvet S, Martinu T, et al. Cytokine profile in lung transplant recipients with aspergillus spp colonization. Transpl Infect Disease. 2019;21:e13060. https://doi.org/10.1111/tid.13060.
Nagao M, Fujimoto Y, Yamamoto M, Matsumura Y, Kaido T, Takakura S, et al. Epidemiology of invasive fungal infections after liver transplantation and the risk factors of late-onset invasive aspergillosis. J Infect Chemother. 2016;22:84–9. https://doi.org/10.1016/j.jiac.2015.11.005.
Wauters J, Baar I, Meersseman P, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med. 2012;38(11):1761–8. https://doi.org/10.1007/s00134-012-2673-2.
Garnacho-Montero J, Amaya-Villar R, Ortiz-Leyba C, León C, Alvarez-Lerma F, Nolla-Salas J, et al. Isolation of aspergillus spp. from the respiratory tract in critically ill patients: risk factors, clinical presentation and outcome. Crit Care. 2005;9. https://doi.org/10.1186/cc3488.
Sharma A, Mishra T, Kumar N, Soubani AO. Influenza-associated aspergillosis: nationwide trends, predictors and outcomes from 2005 to 2014. Chest. 2020;158:1857–66. https://doi.org/10.1016/j.chest.2020.06.010.
Huang L, Zhang N, Huang X, Xiong S, Feng Y, Zhang Y, et al. Invasive pulmonary aspergillosis in patients with influenza infection: a retrospective study and review of the literature. Clin Respiratory J. 2019;13:202–11. https://doi.org/10.1111/crj.12995.
Robin C, Cordonnier C, Sitbon K, Raus N, Lortholary O, Maury S, et al. Mainly post-transplant factors are Associated with Invasive aspergillosis after allogeneic stem cell transplantation: a study from the Surveillance Des Aspergilloses invasives en France and Société Francophone De Greffe De Moelle Et de Thérapie Cellulaire. Biol Blood Marrow Transplant. 2019;25:354–61. https://doi.org/10.1016/j.bbmt.2018.09.028.
Zhang X, Yang M, Hu J, Zhao H, Li L. Epidemiology of invasive pulmonary aspergillosis in patients with liver failure: clinical presentation, risk factors, and outcomes. J Int Med Res. 2018;46:819–27. https://doi.org/10.1177/0300060517729907.
Cook JC, Cook A, Tran RH, Chang PP, Rodgers JE. A case-control study of the risk factors for developing aspergillosis following cardiac transplant. Clin Transpl. 2018;32. https://doi.org/10.1111/ctr.13367.
Xu H, Li L, Huang WJ, Wang LX, Li WF, Yuan WF. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: a case control study from China. Clin Microbiol Infect. 2012;18:403–8. https://doi.org/10.1111/j.1469-0691.2011.03503.x.
Zou P, Wang C, Zheng S, Guo F, Yang L, Zhang Y, et al. Invasive pulmonary aspergillosis in adults with avian influenza a (H7N9) pneumonia in China: a retrospective study. J Infect Dis. 2020;221:193-S197. https://doi.org/10.1093/INFDIS/JIZ682.
Muñoz P, Rodríguez C, Bouza E, Palomo J, Yañez JF, Domínguez MJ, et al. Risk factors of invasive aspergillosis after heart transplantation: protective role of oral itraconazole Prophylaxis. Am J Transplant. 2004;4:636–43. https://doi.org/10.1111/j.1600-6143.2004.00390.x.
Fukuda T, Boeckh M, Guthrie KA, Mattson DK, Owens S, Wald A, et al. Invasive aspergillosis before allogeneic hematopoietic stem cell transplantation: 10-year experience at a single transplant center. Biol Blood Marrow Transplant. 2004;10:494–503. https://doi.org/10.1016/j.bbmt.2004.02.006.
López-Medrano F, Silva JT, Fernández-Ruiz M, Carver PL, van Delden C, Merino E, et al. Risk factors Associated with Early Invasive Pulmonary aspergillosis in kidney transplant recipients: results from a multinational matched case–control study. Am J Transplant. 2016;16:2148–57. https://doi.org/10.1111/ajt.13735.
Hahn T, Cummings KM, Michalek AM, Lipman BJ, Segal BH, McCarthy PL. Efficacy of high-efficiency Particulate Air Filtration in preventing aspergillosis in immunocompromised patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2002;23:525–31. https://doi.org/10.1086/502101.
Thursky K, Byrnes G, Grigg A, Szer J, Slavin M. Risk factors for post-engraftment invasive aspergillosis in allogeneic stem cell transplantation. Bone Marrow Transpl. 2004;34:115–21. https://doi.org/10.1038/sj.bmt.1704543.
Gustot T, Maillart E, Bocci M, Surin R, Trépo E, Degré D, et al. Invasive aspergillosis in patients with severe alcoholic hepatitis. J Hepatol. 2014;60:267–74. https://doi.org/10.1016/j.jhep.2013.09.011.
Mühlemann K, Wenger C, Zenhäusern R, Täuber MG. Risk factors for invasive aspergillosis in neutropenic patients with hematologic malignancies. Leukemia. 2005;19:545–50. https://doi.org/10.1038/sj.leu.2403674.
Schwarzinger M, Sagaon-Teyssier L, Cabaret O, Bretagne S, Cordonnier C. Performance of serum biomarkers for the early detection of invasive aspergillosis in febrile, neutropenic patients: a multi-state model. PLoS ONE. 2013;8:e65776. https://doi.org/10.1371/journal.pone.0065776.
Kontoyiannis DP, Chamilos G, Lewis RE, Giralt S, Cortes J, Raad II, et al. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer. 2007;110:1303–6. https://doi.org/10.1002/cncr.22909.
Kim SH, Hong JY, Bae S, Lee H, Wi YM, Ko JH, et al. Risk factors for Coronavirus Disease 2019 (COVID-19)-Associated Pulmonary aspergillosis in critically ill patients: a Nationwide, Multicenter, Retrospective Cohort Study. J Korean Med Sci. 2022;37. https://doi.org/10.3346/jkms.2022.37.e134..
Gu Y, Ye X, Liu Y, Wang Y, Shen K, Zhong J, et al. A risk-predictive model for invasive pulmonary aspergillosis in patients with acute exacerbation of chronic obstructive pulmonary disease. Respir Res. 2021;22:22. https://doi.org/10.1186/s12931-021-01771-3.
Er B, Er AG, Gülmez D, Şahin TK, Halaçlı B, Durhan G, et al. A screening study for COVID-19-associated pulmonary aspergillosis in critically ill patients during the third wave of the pandemic. Mycoses. 2022;65:724–32. https://doi.org/10.1111/myc.13466.
Le Pavec J, Pradère P, Gigandon A, Dauriat G, Dureault A, Aguilar C, et al. Risk of Lung Allograft Dysfunction Associated with aspergillus infection. Transpl Direct. 2021;7:E675. https://doi.org/10.1097/TXD.0000000000001128.
Calderón-Parra J, Mills-Sanchez P, Moreno-Torres V, Tejado-Bravo S, Romero-Sánchez I, Balandin-Moreno B, et al. COVID-19-associated pulmonary aspergillosis (CAPA): risk factors and development of a predictive score for critically ill COVID-19 patients. Mycoses. 2022;65:541–50. https://doi.org/10.1111/myc.13434.
Xu Y, Shao M, Liu N, Tang J, Gu Q, Dong D. Invasive pulmonary aspergillosis is a frequent complication in patients with severe fever with thrombocytopenia syndrome: a retrospective study. Int J Infect Dis. 2021;105:646–52. https://doi.org/10.1016/j.ijid.2021.02.088.
de Almeida JN, Doi AM, Watanabe MJL, Maluf MM, Calderon CL, Silva M, et al. COVID-19-associated aspergillosis in a Brazilian referral centre: diagnosis, risk factors and outcomes. Mycoses. 2022;65:449–57. https://doi.org/10.1111/myc.13433.
Lahmer T, Kriescher S, Herner A, Rothe K, Spinner CD, Schneider J, et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: results from the prospective AspCOVID-19 study. PLoS One. 2021;16: e0238825. https://doi.org/10.1371/journal.pone.0238825.
Song L, Zhao Y, Wang G, Zou W, Sai L. Investigation of predictors for invasive pulmonary aspergillosis in patients with severe fever with thrombocytopenia syndrome. Sci Rep. 2023;13:13. https://doi.org/10.1038/s41598-023-28851-2.
Waldeck F, Boroli F, Zingg S, Walti LN, Wendel-Garcia PD, Conen A, et al. Higher risk for influenza-associated pulmonary aspergillosis (IAPA) in asthmatic patients: a Swiss multicenter cohort study on IAPA in critically ill influenza patients. Influenza Other Respir Viruses. 2023;17. https://doi.org/10.1111/irv.13059.
Dai Y, Pu Q, Hu N, Zhu J, Han Y, Shi P, et al. The dose–response relationship between smoking and the risk factor for invasive pulmonary aspergillosis in patients with severe fever with thrombocytopenia syndrome. Front Microbiol. 2023;14:14. https://doi.org/10.3389/fmicb.2023.1209705.
van Grootveld R, van der Beek MT, Janssen NAF, Ergün M, van Dijk K, Bethlehem C, et al. Incidence, risk factors and pre-emptive screening for COVID-19 associated pulmonary aspergillosis in an era of immunomodulant therapy. J Crit Care. 2023;76:154272. https://doi.org/10.1016/j.jcrc.2023.154272.
Chao CM, Lai CC, Ou HF, Ho CH, Chan KS, Yang CC, et al. The impacts of aspergillosis on outcome, burden and risks for mortality in influenza patients with critical illness. J Fungi. 2021;7. https://doi.org/10.3390/jof7110922.
Prattes J, Wauters J, Giacobbe DR, Salmanton-García J, Maertens J, Bourgeois M, et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients—a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect. 2022;28:580–7. https://doi.org/10.1016/j.cmi.2021.08.014.
Hurt W, Youngs J, Ball J, Edgeworth J, Hopkins P, Jenkins DR, et al. COVID-19-associated pulmonary aspergillosis in mechanically ventilated patients: a prospective, multicentre UK study. Thorax. BMJ Publishing Group; 2023. https://doi.org/10.1136/thorax-2023-220002.
Janssen NAF, Nyga R, Vanderbeke L, Jacobs C, Ergün M, Buil JB, et al. Multinational observational cohort study of covid-19-associated pulmonary aspergillosis1. Emerg Infect Dis. 2021;27:2892–8. https://doi.org/10.3201/eid2711.211174.
Bentvelsen RG, Van Arkel ALE, Rijpstra TA, Kant MKM, Brugge SVDS, Van Der, Loth DW, et al. Regional Impact of COVID-19-Associated pulmonary aspergillosis (CAPA) during the First Wave. J Fungi. 2022;8. https://doi.org/10.3390/jof8020096.
Katada Y, Nakagawa S, Nagao M, Yoshida Y, Matsuda Y, Yamamoto Y, et al. Risk factors of breakthrough aspergillosis in lung transplant recipients receiving itraconazole prophylaxis. J Infect Chemother. 2022;28:54–60. https://doi.org/10.1016/j.jiac.2021.09.020.
Lee R, Cho SY, Lee DG, Ahn H, Choi H, Choi SM, et al. Risk factors and clinical impact of COVID-19-associated pulmonary aspergillosis: multicenter retrospective cohort study. Korean J Intern Med. 2022;37:851–63. https://doi.org/10.3904/kjim.2022.069.
Apostolopoulou A, Clancy CJ, Skeel A, Nguyen MH. Invasive pulmonary aspergillosis complicating Noninfluenza respiratory viral infections in solid organ transplant recipients. Open Forum Infect Dis. 2021;8. https://doi.org/10.1093/ofid/ofab478.
Feys S, Lagrou K, Lauwers HM, Haenen K, Jacobs C, Brusselmans M, et al. High burden of COVID-19-associated pulmonary aspergillosis (CAPA) in severely immunocompromised patients requiring mechanical ventilation. Clin Infect Dis. 2023. https://doi.org/10.1093/cid/ciad546.
Dubler S, Etringer M, Weigand MA, Brenner T, Zimmermann S, Schnitzler P, et al. Impact of Invasive Pulmonary aspergillosis in critically Ill Surgical patients with or without solid organ transplantation. J Clin Med. 2023;12:3282. https://doi.org/10.3390/jcm12093282.
Pérez-Jacoiste Asín MA, López-Medrano F, Fernández-Ruiz M, Silva JT, San Juan R, Kontoyiannis DP, et al. Risk factors for the development of invasive aspergillosis after kidney transplantation: systematic review and meta-analysis. Am J Transplant. 2021;21:703–16. https://doi.org/10.1111/ajt.16248.
Chong WH, Saha BK, Neu KP. Comparing the clinical characteristics and outcomes of COVID-19-associate pulmonary aspergillosis (CAPA): a systematic review and meta-analysis. Infection. 2022;50:43–56. https://doi.org/10.1007/s15010-021-01701-x.
Fabián S, Elaine B, D BG, C CP, Adilia W. Pathogenesis of respiratory viral and fungal coinfections. Clin Microbiol Rev. 2021;35:e00094-00021. https://doi.org/10.1128/CMR.00094-21.
Lei Y, Guerra Martinez C, Torres-Odio S, Bell SL, Birdwell CE, Bryant JD, et al. Elevated type I interferon responses potentiate metabolic dysfunction, inflammation, and accelerated aging in mtDNA mutator mice. Sci Adv. 2023;7:eabe7548. https://doi.org/10.1126/sciadv.abe7548.
D’Souza SS, Zhang Y, Bailey JT, Fung ITH, Kuentzel ML, Chittur SV, et al. Type I Interferon signaling controls the accumulation and transcriptomes of monocytes in the aged lung. Aging Cell. 2021;20:20. https://doi.org/10.1111/acel.13470.
Gil L, Kozlowska-Skrzypczak M, Mol A, Poplawski D, Styczynski J, Komarnicki M. Increased risk for invasive aspergillosis in patients with lymphoproliferative diseases after autologous hematopoietic SCT. Bone Marrow Transpl. 2009;43:121–6. https://doi.org/10.1038/bmt.2008.303.
Wald A, Leisenring W, Van Burik JA, Bowden RA. Epidemiology of aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–66. https://doi.org/10.1086/516480.
Taccone FS, Van den Abeele AM, Bulpa P, Misset B, Meersseman W, Cardoso T, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:1–15. https://doi.org/10.1186/s13054-014-0722-7.
Sun KS, Tsai CF, Chen SCC, Huang WC. Clinical outcome and prognostic factors associated with invasive pulmonary aspergillosis: an 11-year follow-up report from Taiwan. PLoS ONE. 2017;12. https://doi.org/10.1371/journal.pone.0186422..
Matthaiou DK, Dimopoulos G, Taccone FS, Bulpa P, Van Den Abeele AM, Misset B, et al. Elderly versus nonelderly patients with invasive aspergillosis in the ICU: a comparison and risk factor analysis for mortality from the AspICU cohort. Med Mycol. 2018;56:668–78. https://doi.org/10.1093/mmy/myx117.
Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–61. https://doi.org/10.1016/S1473-3099(10)70251-6.
Thorlacius-Ussing L, Sandholdt H, Larsen AR, Petersen A, Benfield T. Age-dependent increase in incidence of Staphylococcus aureus Bacteremia, Denmark, 2008–2015. Emerg Infect Dis. 2019;25:875–82. https://doi.org/10.3201/eid2505.181733.
Acknowledgements
The authors would like to express their gratitude to all corresponding authors of the original research studies who responded to the data request.
Funding
ES and AW were supported by the Medical Research Council Centre for Medical Mycology at the University of Exeter [MR/N006364/2, www.mrc.ac.uk]. AdSD is supported by the NIHR Newcastle Biomedical Research Centre (BRC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
ES, AW, LW were responsible for study concept and design, ES, FS, AD, TC, EW performed systematic review and synthesis of data, analysis and interpretation of data, FS drafted Introduction section of the manuscript, ES wrote the rest of the manuscript, ES, FS, AD, TC, EW, LW, AW edited and criticaly revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplemental Table 1. PRISMA checklist that provides locations where each item of systematic review and meta-analysis is reported. Supplemental Table 2. Meta-analysis results before and after outliers (determined by sensitivity analysis) were removed. Supplemental Table 3. Newcastle - Ottawa assessment of non-randomized studies included in meta-analysis. Supplemental Table 4. Meta-regression analysis of age difference in years. Supplemental Figure 1. Baujat plot illustrating contribution of individual studies to the overall heterogeneity. Supplemental Figure 2. Leave-One-Out meta-analysis illustrating forest plots, where pooled effects were recalculated with one study omitted each time. Supplemental Figure 3. Funnel plot depicting the small-study effect. Supplemental Figure 4. Contour-enhanced funnel plot with colors representing the significance level of each individual studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shekhova, E., Salazar, F., Da Silva Dantas, A. et al. Age difference of patients with and without invasive aspergillosis: a systematic review and meta-analysis. BMC Infect Dis 24, 220 (2024). https://doi.org/10.1186/s12879-024-09109-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09109-2