Abstract
Background
Parkinson's disease (PD), while often associated with its distinctive motor symptoms, can also exert a notable impact on the cardiovascular system due to the development of severe autonomic dysfunction. One of the initial indicators of PD is the appearance of cardiovascular dysautonomia. As such, it is vital to monitor and manage cardiovascular health of individuals with PD, as it may have clinical implications in the development of commonly recognized motor and non-motor aspects of the disease. To study the association of history of cardiovascular disease (CVD) with occurrence and severity of PD, here, we lend data on the association of CVD history with the frequency and the occurrence of idiopathic PD (iPD) using data from the Luxembourg Parkinson’s study (iPD n = 676 patients and non-PD n = 874 controls).
Results
We report that patients with a history of CVD are at high risk of developing iPD (odds ratio; OR = 1.56, 95% confidence interval; CI 1.09–2.08). This risk is stronger in males and remains significant after adjustment with confounders (OR 1.55, 95% CI 1.05–2.30). This increased susceptibility to iPD is linked to the severity of iPD symptoms mainly the non-motor symptoms of daily living (MDS-UPDRS I) and motor complications (MDS-UPDRS IV) in the affected individuals.
Conclusion
Individuals with history of CVD have a high risk of developing severe forms of iPD. This observation suggests that careful monitoring and management of patients with a history of cardiac problems may reduce the burden of iPD.
Similar content being viewed by others
Background
With an increasing life expectancy, there is an increased prevalence of age-related disease burden. Among the diseases associated with age, neurodegenerative diseases such as Parkinson’s disease (PD) and cardiovascular diseases (CVD) remain major causes of death worldwide. The interplay between the nervous system and the cardiovascular system has been discussed for years [1] and recent papers rejuvenate the neurocardiology field [2,3,4]. PD is the second most common multifactorial age-related disorder that arises due to progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta in the midbrain. Dopaminergic neurons are responsible for the secretion of neurotransmitter dopamine, responsible for maintenance of motor activities. The loss of dopamine in the midbrain leads to impaired motor skills and appearance of motor symptoms in PD patients. This neuronal degeneration could be governed by several interlinked biological processes, such as protein aggregation, genetic mutations or mitochondrial dysfunction [5,6,7]. Mutations in genes like LRRK2 and SNCA are directly associated with increased PD risk by altering protein function [8]. Additionally, epigenetic mechanisms like DNA methylation have been shown to influence gene expression, potentially affecting dopamine production and PD progression [9]. The combination of genetic and epigenetic mechanisms contributes to an individual’s susceptibility to developing PD. As the disease progresses, other parts of the brain might also be affected leading to the development of non-motor symptoms. Due to the presence of several non-motor symptoms in early stages of PD, whether PD starts in the body first or the brain is still a matter of debate [1]. Our research aims to explore the body-first PD hypothesis highlighting the need to examine the brain and heart axis in the context of idiopathic PD (iPD) [2]. As part of the natural process of neurodegeneration, older individuals often exhibit symptoms resembling those of PD, such as tremors and bradykinesia, even though they might not receive a PD diagnosis [10]. These symptoms may arise from underlying vascular conditions such as cardiovascular abnormalities. Therefore, it becomes crucial to closely monitor these individuals, as cardiovascular issues may potentially contribute to the progression of PD [11]. Additionally, the functional and molecular links between PD and cardiovascular conditions needs to be thoroughly examined [2]. This association could involve several multifaceted mechanisms involving autonomic dysfunction and shared risk factors like aging, inflammation, comorbidities, medications, physical activity or genetic predispositions. Cardiovascular dysautonomia which includes orthostatic hypotension, hypertension and heart rate variability is observed as one of the early signs of PD [12]. Other cardiac complications in PD include cardiomyopathy, coronary heart disease, arrhythmias, sudden cardiac death and Levodopa-induced CVD [13, 14]. These cardiac defects additionally act as risk factors in developing sudden unexpected death in PD patients. In an American cross –sectional study, Zesiewicy et al. observed that elderly patients with PD experienced heart failure twice as often as those without PD [15]. Likewise, a Korean population-based study has shown that PD was associated with high risk of developing CVD [16]. Contrarily, an inverse relation between CVD and PD was established in a Danish cohort, where patients with myocardial infarction had 20 and 28% decreased risk of developing PD and secondary Parkinsonism, respectively [17]. These studies shed light on the need for diverse population based research on the relationship between CVD and PD [18]. Given the potential impact of cardiovascular factors on PD progression, investigating this association is crucial.
The Luxembourg Parkinson’s (LuxPARK) study, that provides a comprehensive coverage of PD and other parkinsonian syndromes within Luxembourg and the Greater Region [19], offers a unique opportunity to explore the relationship between CVD history and the onset and severity iPD. Thus, we hypothesize that a history of CVD is associated with both the occurrence and severity of iPD. In the present study, we aim to examine the frequency of CVD in patients with iPD and assess relationship between the history of CVD and the onset and severity of iPD in the Luxembourg Parkinson’s (LuxPARK) study. Here, we consider both cardiac and cerebral health when evaluating iPD, as well as the potential sex-specific differences in iPD susceptibility.
Methods
Study design
This cross-sectional observational study performed using the clinical data from the LuxPARK study is reported following the STROBE guidelines [20]. The data in LuxPARK study were collected between 2015 and 2020. The protocol was performed according to the principles of the Declaration of Helsinki. All participants signed an informed consent and the study was approved by the national ethics committee (CNER Ref: 201407/13 and 20140713-SU3) and Data Protection Committee (CNPD Ref: 446/2017) [19]. The data was anonymized.
Study participants
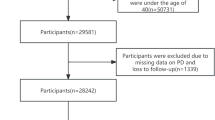
Participants from the LuxPARK study were divided into iPD (n = 676) or non-PD (n = 874) controls. For iPD classification and inclusion in the LuxPARK study, the patients met the United Kingdom Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria [21]. The patients who did not meet the above criteria were classified as atypical PD and were not included in the present study. Non-PD controls were participants recruited based on the exclusion criteria, such as, presence of a neurodegenerative disorder, active cancer, being under the age of 18, or being pregnant. An independent sample size calculation was performed for the LuxPARK study for baseline comparisons to allow finding significant differences between the two groups [19]. Briefly, sample size calculations were based on estimated PD prevalence and incidence in Luxembourg which suggested a sample size of 800 individuals per group to be sufficient to detect a moderate difference in prevalence between groups using a chi-square test with 80% power and a 5% two-sided significance level [19].
The number of iPD and non-PD controls recruited as a part of LuxPARK study during the study period determined the sample size for the present study. Baseline clinical visits (visit 1) for all participants (iPD and non-PD) took place between 2015 and 2020.
Clinical assessment and data collection
Clinical data of study participants were obtained at baseline. The clinical assessment was conducted via a health questionnaire performed by an experienced movement disorders specialist. This included demographic details such as age, sex, height, weight, and smoking history. Medical history included presence or absence of comorbidities at the time of baseline visit or in the past. For this study, the comorbidities studied were history of diabetes, CVD and hypertension. Further, detailed neurological examination for disease severity was performed using the Movement Disorder Society- Unified Parkinson’s Disease Rating Scale (MDS-UPDRS; [22]). Evaluation of general cognitive symptoms was achieved by Montreal Cognitive Assessment (MoCA; [23]). The effect on the autonomic symptoms was assessed using the Scales for Outcomes in Parkinson’s disease (SCOPA-AUT; [24]).
Statistical analysis
Statistical analyses were performed using R version 4.0.3. The missForest algorithm, utilizing random forests, was applied to impute missing values in both continuous and categorical variables. Following imputation, summary statistics were reviewed to confirm consistency with the original data distribution, and imputed values were subsequently assigned to their respective variables for further analysis. Continuous variables were rank normalized using ‘RankNorm’. Pearson’s Chi-square test was used to evaluate differences in frequencies between non-PD and iPD groups. R packages Hmisc, rms, lmtest and glmtoolbox were used to perform logistic regression analysis. The primary outcome was the un-adjusted (univariate) and adjusted (multivariate) relationship between CVD history and iPD occurrence. Common disease-associated risk factors such as age, sex, body mass index (BMI), history of diabetes, history of hypertension and smoking history were used as confounders for adjustment in the multivariate logistic regression analysis. The association between iPD, CVD history and autonomic symptoms was studied using the analysis of the SCOPA-AUT score. The score was log2 transformed and the difference between the groups was analyzed by Mann–Whitney Rank Sum test. The secondary outcome was the association of CVD history with iPD severity as determined by MDS-UPDRS scores and MoCA. Disease duration was also added as a confounder in the disease severity analysis. Lastly, for all of the analysis sex specific association was analyzed by dichotomizing the patients based on sex.
Results
Clinical characteristics of the participants
1550 participants were included in the present study that included 874 non-PD controls and 676 iPD patients. The clinical characteristics of the participants in LuxPARK cohort showed that iPD patients have a severe clinical profile compared to non-PD controls. They were older (65.97 ± 10.39 years in iPD vs 59.21 ± 12.43 years in non-PD; p < 0.001), predominantly male (65% in iPD vs 51% in non-PD) and showed a higher proportion of CVD history (18.9% in iPD vs 9.3% in non-PD), hypertension (42.1% in iPD vs 34.3% in non-PD) and diabetes (9% in iPD vs 6.1% in non-PD). Patients with iPD as expected, displayed severe motor (MDS-UPDRS) and autonomic symptoms (SCOPA-AUT) and lower score on the MoCA, indicating poor cognitive function. Further, there was no significant difference between the smoking prevalence (p = 0.08) and the BMI between the two groups (p = 0.4). The proportions of non-PD and iPD patients with CVD history and co-morbidities are described in Table 1. Concerning the sex distribution and CVD history, within the iPD group, 128 patients had history of CVD, of which 102 (80%) were males and 26 (20%) were females. Meanwhile, within the non-PD controls 82 individuals had history of CVD, of which 29 (35%) were females and 53 (64%) were males, showing a higher incidence of CVD in men compared to women in both non-PD and iPD groups. Lastly, non-PD controls had significant other comorbidities such hypertension (n = 300; 60% males) and diabetes (n = 54; 64% males).
Association of CVD history with the occurrence of iPD
To study the association of CVD history with the occurrence of iPD we performed univariate and multivariate logistic regression analysis. In univariate logistic regression analysis, participants that had a history of CVD had a higher risk of iPD occurrence (odds ratio OR = 2.25, 95% confidence interval (CI) = 1.67–3.04, p-value < 0.001; Fig. 1A). This association remained significant after adjusting for the common disease associated confounding factors such as age, BMI, hypertension, diabetes and smoking (OR = 1.56, 95% CI 1.09–2.08, p = 0.013; Fig. 1B). As the overall incidence of CVD history in the cohort (non-PD + iPD) was higher in men (n = 155 out of 895; 17.3%) than in women (n = 55 out of 655; 8.3%; p < 0.001), we investigated the sex-specific association of CVD history with iPD occurrence. In the univariate analysis, CVD history was associated with iPD occurrence in both males and females (Fig. 2A and C). However, this association remained significant only in males after adjustment with confounders (OR = 1.55, 95% CI 1.05–2.30, p = 0.026; Fig. 2B and D). As autonomic dysfunction is a common risk factor for both iPD and CVD, we studied its association with both the diseases in the cohort and observed strong association between iPD (Fig. 3A), CVD (Fig. 3B), and autonomic symptoms at baseline in both males and females (Fig. 3C and D).
Sex-specific association of CVD history with the occurrence of iPD. A Univariate and B multivariate logistic regression to study the association between history of CVD and occurrence of iPD in males. C Univariate and D multivariate logistic regression to study the association between history of CVD and occurrence of iPD in females. Age, BMI, hypertension, diabetes and smoking were included as confounders in the multivariate models
Autonomic symptoms as assessed by the SCOPA-AUT score in (A) non-PD controls (n = 874) compared to iPD patients (n = 676), B participants who did not have history of CVD (n = 1337) compared to who had history of CVD (n = 213). C Sex specific association of SCOPA AUT with iPD D Sex specific association of SCOPA AUT with history of CVD. Statistical analysis was performed using the Mann–Whitney Rank Sum test
Association of CVD history with iPD severity
In an effort to gain a deeper understanding of how a patient's cardiac history relates to the severity of their iPD symptoms, we evaluated the extent of symptom severity using distinct assessment tools such as MDS-UPDRS scores and MoCA. In univariate logistic regression analysis, history of CVD in iPD patients was associated with MDS-UPDRS I (OR = 1.30, 95% CI 1.03–1.65, p = 0.02), MDS-UPDRS II (OR = 1.52, 95% CI 1.11–2.09, p = 0.008), MDS-UPDRS III (OR = 1.49, 95% CI 1.03–2.14, p = 0.02 and MoCA (OR = 0.57, 95% CI 0.44–0.73, p < 0.001) but not with MDS-UPDRS IV (OR = 1.11, 95% CI 0.88–1.39, p = 0.35; Fig. 4A). Upon adjustment with confounders, MDS-UPDRS I (OR = 1.38, 95% CI 1.07–1.78, p = 0.01) and MDS-UPDRS IV (OR = 1.43, 95% CI 1.09–1.86, p = 0.008) remained significantly associated with CVD history in iPD patients (Fig. 4B). Upon dichotomizing iPD patients into two groups based on sex, CVD history was significantly associated with disease severity scores (MDS-UPDRS I, II and IV) in males (Fig. 5A, B), with no such correlation observed in females (Fig. 5C, D).
Association of CVD history with iPD severity. A Univariate and B multivariate logistic regression to study the association between history of CVD and disease severity scores in iPD patients. Age, sex, BMI, hypertension, diabetes, smoking and disease duration were included as confounders in the multivariate models
Sex-specific association of CVD history with iPD severity. A Univariate and B multivariate logistic regression to study the association between history of CVD and disease severity scores in male iPD patients. C Univariate and D multivariate logistic regression model to study the association between history of CVD and disease severity scores in female iPD patients. Age, BMI, hypertension, diabetes, smoking and disease duration were included as confounders in the multivariate models
Discussion
Our findings from the LuxPARK cohort support the established clinical profile of iPD patients compared to non-PD controls. As expected, the iPD group displayed a male predominance, aligning with well-documented characteristics of the disease. This sex disparity in PD patients could be attributed to hormonal differences, as estrogen, a female sex hormone, has been shown to possess neuroprotective qualities [25]. Additionally, high exposure to occupational toxins and X chromosome linked genetic factors could contribute to more prevalence in males [26]. Further, in our study, we observed that patients with iPD have a higher frequency of underlying CVD and cardiovascular co-morbidities such as hypertension and diabetes, compared to non-PD controls, supporting the existence of functional links between cardiovascular, cerebrovascular and neurological health in iPD patients. Furthermore, using logistic regression models we show a significant association between the history of cardiac problems and the occurrence of iPD. With males being at a greater risk for CVD [27], our investigation further reveals that males with CVD history are exposed to a greater risk of iPD development than females with CVD history. The autonomic nervous system is mainly involved in controlling the heart rate. In the present study, we observed increased autonomic dysfunction in patients with iPD compared to the non-PD controls. Finally, to understand how prior cardiac defects could influence iPD severity, we further studied its association with CVD history. Our analysis of disease severity scores in iPD patients revealed a significant association of CVD history with MDS-UPDRS I and MDS- UPDRS IV (Unified Parkinson's Disease Rating Scale Part IV) scores.
While the primary pathogenesis of PD has long been associated with neurodegenerative processes, emerging evidence suggests that vascular factors play a significant role in the development and progression of PD [11]. Our study supports the body first hypothesis of PD development and shows that a history of CVD might be a risk factor for development of severe forms of PD. Similar to our findings, in a previous study, patients with iPD had frequent autonomic dysfunction, with an involvement of parasympathetic nervous system and frequent involvement of sympathetic cardiovascular dysfunction [28]. Our results suggest that underlying cardiovascular problems could be a risk factor for developing iPD, especially in males. This could be attributed to common disease-associated risk factors between CVD and PD, chronic inflammation because of the underlying cardiac problems or due to CVD induced vascular damage leading to reduced blood flow to the brain causing the neuronal death [29, 30]. Therefore, CVD history as a risk and sex-specific differences must be taken into account when assessing patients with suspected iPD and developing preventive measures.
Understanding the progression of iPD can provide valuable insights into the management and care of patients. Our findings suggest that the presence of CVD is linked to early non-motor symptoms (MDS-UPDRS I) and secondary motor complications (MDS-UPDRS IV) in iPD patients. These results align with emerging evidence that non-motor symptoms, including cardiac autonomic dysfunction, observed in early stages of PD, can significantly impact overall clinical representation [31]. Early signs of autonomic dysfunction, like constipation or urinary problems, could precede motor symptoms, potentially indicating involvement of the autonomic nervous system early in the disease process. Additionally, problems with blood pressure regulation causing dizziness could hinder mobility, potentially accelerating disease progression [32]. As such, identifying and managing these non-motor symptoms, particularly in iPD patients with CVD, becomes crucial for comprehensive care. Additionally, the secondary motor complications such as dystonia and dyskinesia may be induced due to several reasons, including drug-induced side effects (for example, levodopa exposure) [33]. Further, levodopa has been shown to initiate additional adverse effects, notably, cardiovascular defects [13]. Thus, the association between CVD with MDS-UPDRS IV could potentially indicate the deleterious effects of levodopa, leading to aggravation of cardiovascular symptoms in these patients, thus causing the development of drug-induced motor complications. Therefore, vigilant monitoring of individuals with pre-existing CVD who are receiving levodopa or similar medications for iPD is necessary. Next, our observation that MDS-UPDRS I, MDS-UPDRS II and MDS-UPDRS IV scores were significantly associated with CVD history in males highlights the sex-specific effects associated with iPD and CVD. While the absence of statistical significance in the female iPD patients might be in part due to low number of females in the study cohort, the gender-specific association suggests that CVD history may exert different influences on disease severity in males compared to females. Our results raise intriguing questions about the potential hormonal, genetic, or environmental factors that might underlie these disparities.
While our study provides valuable insights, it is important to note that there are limitations in our work. Firstly, the study was based on patient reported questionnaires, where the patients were asked for ‘History of Present Illness’ including ‘Cardiovascular disease’, ‘Hypertension’ or ‘None of the above’. Additionally, the duration of these pre-existing co-morbidities was not evaluated in the study. As such, it would be meaningful to perform a detailed assessment of the types of CVD to further study the implications of different types of cardiac defects in patients with iPD. Secondly, our study is observational in nature, and therefore, causality cannot be established. The relationship between CVD and iPD severity may be bidirectional, with PD potentially contributing to the development or aggravation of CVD. However, this possibility requires further prospective evaluation to delineate the cause and effect relationship between vascular health and neurodegenerative processes in PD.
Despite these limitations, our study further strengthens our knowledge regarding the association between CVD and iPD. We have demonstrated that the presence of CVD is significantly associated with iPD occurrence and with increased disease severity, particularly in terms of early non-motor symptoms and secondary motor complications in iPD patients. Moreover, we have highlighted sex-specific differences in this association, with males showing a significant link between CVD history and higher MDS-UPDRS I, MDS-UPDRS II, and MDS-UPDRS IV scores. Our findings highlight the importance of holistic patient care for individuals with iPD, taking into account both the neurological and cardiovascular aspects of their health. The shared risk factors between PD and CVD and the bidirectional relationship between the two conditions highlight the need of comprehensive management strategies targeting both neurological and cardiovascular health. To closely monitor patients, easy to perform tests like the Head-up tilt test to examine the heart rate variability could be measured during the clinical visits [34]. Additionally, at-home blood pressure diaries for patients before the clinical follow-up could help personalizing the treatment of these patients.
Conclusions
Our study contributes insights into the complex relationship between iPD and CVD, paving the way for improved care and tailored treatment strategies for affected individuals. Further research is needed to elucidate the underlying mechanisms driving these associations and to explore potential interventions that can alleviate the impact of CVD on iPD progression, especially in sex-specific contexts.
Availability of data and materials
Patient data used in the preparation of this manuscript were obtained from the National Centre of Excellence in Research on Parkinson’s Disease (NCER-PD). NCER-PD datasets are not publicly available, as they are linked to the Luxembourg Parkinson’s Study and its internal regulations. The NCER-PD Consortium is willing to share its available data. Its access policy was devised based on the study ethics documents, including the informed consent form, as approved by the national ethics committee. Requests to access datasets should be directed to the Data and Sample Access Committee via email: request.ncer-pd@uni.lu.
References
Sherman DG, Hart RG, Shi F. Heart-brain interactions: neurocardiology or cardioneurology comes of age. Mayo Clin Proc. 1987;62(12):1158–60.
Acharya S, Salgado-Somoza A, Stefanizzi FM, Lumley AI, Zhang L, Glaab E, May P, Devaux Y. Non-coding RNAs in the brain-heart axis: the case of Parkinson’s Disease. Int J Mol Sci. 2020;21(18):6513.
Devaux Y. MicroRNAs as biomarkers in the brain-heart axis? Eur Heart J Acute Cardiovasc Care. 2022;11(8):617–9.
Acharya S, Lumley AI, Devaux Y. Targeting regulatory RNAs at the heart of Parkinson’s disease. Eur Heart J. 2023. https://doi.org/10.1093/eurheartj/ehad010.
Deng H, Wang P, Jankovic J. The genetics of Parkinson disease. Ageing Res Rev. 2018;42:72–85.
Stefanis L. alpha-synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(2): a009399.
Surmeier DJ. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 2018;285(19):3657–68.
van der Brug MP, Singleton A, Gasser T, Lewis PA. Parkinson’s disease: from human genetics to clinical trials. Sci Transl Med. 2015;7(305):205ps220.
Song H, Chen J, Huang J, Sun P, Liu Y, Xu L, Wei C, Mu X, Lu X, Wang W, et al. Epigenetic modification in Parkinson’s disease. Front Cell Dev Biol. 2023;11:1123621.
Algotsson C, Rosso A, Elmstahl S, Siennicki-Lantz A. Prevalence and functional impact of parkinsonian signs in older adults from the good aging in Skane study. Parkinsonism Relat Disord. 2023;111:105416.
Louis ED, Luchsinger JA. History of vascular disease and mild parkinsonian signs in community-dwelling elderly individuals. Arch Neurol. 2006;63(5):717–22.
Kim JS, Lee SH, Oh YS, Park JW, An JY, Park SK, Han SR, Lee KS. Cardiovascular autonomic dysfunction in mild and advanced Parkinson’s disease. J Mov Disord. 2016;9(2):97–103.
Gunaydin ZY, Ozer FF, Karagoz A, Bektas O, Karatas MB, Vural A, Bayramoglu A, Celik A, Yaman M. Evaluation of cardiovascular risk in patients with Parkinson disease under levodopa treatment. J Geriatr Cardiol. 2016;13(1):75–80.
Scorza FA, Fiorini AC, Scorza CA, Finsterer J. Cardiac abnormalities in Parkinson’s disease and Parkinsonism. J Clin Neurosci. 2018;53:1–5.
Zesiewicz TA, Strom JA, Borenstein AR, Hauser RA, Cimino CR, Fontanet HL, Cintron GB, Staffetti JF, Dunne PB, Sullivan KL. Heart failure in Parkinson’s disease: analysis of the United States medicare current beneficiary survey. Parkinsonism Relat Disord. 2004;10(7):417–20.
Park JH, Kim DH, Park YG, Kwon DY, Choi M, Jung JH, Han K. Association of Parkinson disease with risk of cardiovascular disease and all-cause mortality: a nationwide population-based cohort study. Circulation. 2020;141(14):1205–7.
Sundboll J, Szepligeti SK, Szentkuti P, Adelborg K, Horvath-Puho E, Pedersen L, Henderson VW, Sorensen HT. Risk of Parkinson disease and secondary parkinsonism in myocardial infarction survivors. J Am Heart Assoc. 2022;11(5): e022768.
Chua SKK, Saffari SE, Lee SJY, Tan EK. Association between Parkinson’s disease and coronary artery disease: a systematic review and meta-analysis. J Parkinsons Dis. 2022;12(6):1737–48.
Hipp G, Vaillant M, Diederich NJ, Roomp K, Satagopam VP, Banda P, Sandt E, Mommaerts K, Schmitz SK, Longhino L, et al. The Luxembourg Parkinson’s study: a comprehensive approach for stratification and early diagnosis. Front Aging Neurosci. 2018;10:326.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–4.
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, et al. Movement disorder society-sponsored revision of the Unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70.
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9.
Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord. 2004;19(11):1306–12.
Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72(5):381–405.
Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry. 2004;75(4):637–9.
Siokatas G, Papatheodorou I, Daiou A, Lazou A, Hatzistergos KE, Kararigas G. Sex-related effects on cardiac development and disease. J Cardiovasc Dev Dis. 2022;9(3):90.
Schmidt C, Herting B, Prieur S, Junghanns S, Schweitzer K, Reichmann H, Berg D, Ziemssen T. Autonomic dysfunction in patients with progressive supranuclear palsy. Mov Disord. 2008;23(14):2083–9.
Derejko M, Slawek J, Wieczorek D, Brockhuis B, Dubaniewicz M, Lass P. Regional cerebral blood flow in Parkinson’s disease as an indicator of cognitive impairment. Nucl Med Commun. 2006;27(12):945–51.
Grosu L, Grosu AI, Crisan D, Zlibut A, Perju-Dumbrava L. Parkinson’s disease and cardiovascular involvement: edifying insights (review). Biomed Rep. 2023;18(3):25.
Ziemssen T, Reichmann H. Cardiovascular autonomic dysfunction in Parkinson’s disease. J Neurol Sci. 2010;289(1–2):74–80.
Chen Z, Li G, Liu J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol Dis. 2020;134:104700.
Calabresi P, Standaert DG. Dystonia and levodopa-induced dyskinesias in Parkinson’s disease: is there a connection? Neurobiol Dis. 2019;132:104579.
Jaipurkar R, Mohan L, Tomar R. Autonomic cardiovascular regulation in Parkinson’s disease by head-up tilt test—A cross-sectional study. Natl J Physiol Pharmacy Pharmacol. 2018;8(9):1287.
Acknowledgements
We would like to thank all participants of the Luxembourg Parkinson’s Study for their important support of our research. We would like to thank Dr. Taina Marques for support with data procurement from the Luxembourg Parkinson’s study, and Dr. Eduardo Rosales Jubal for reviewing the statistical methods used in the paper. Furthermore, we acknowledge the joint effort of the National Centre of Excellence in Research on Parkinson’s Disease (NCER-PD) Consortium members from the partner institutions Luxembourg Centre for Systems Biomedicine, Luxembourg Institute of Health, Centre Hospitalier de Luxembourg, and Laboratoire National de Santé generally contributing to the Luxembourg Parkinson’s Study as listed below: Geeta Acharya, Gloria Aguayo, Myriam Alexandre, Muhammad Ali, Wim Ammerlann, Giuseppe Arena, Rudi Balling, Michele Bassis, Katy Beaumont, Regina Becker, Camille Bellora, Guy Berchem, Daniela Berg, Alexandre Bisdorff, Ibrahim Boussaad, Kathrin Brockmann, Jessica Calmes, Lorieza Castillo, Gessica Contesotto, Nico Diederich, Rene Dondelinger, Daniela Esteves, Guy Fagherazzi, Jean-Yves Ferrand, Manon Gantenbein, Thomas Gasser, Piotr Gawron, Soumyabrata Ghosh, Marijus Giraitis, Enrico Glaab, Elisa Gómez de Lope, Jérôme Graas, Mariella Graziano, Valentin Groues, Anne Grünewald, Wei Gu, Gaël Hammot, Anne-Marie Hanff, Linda Hansen, Michael Heneka, Estelle Henry, Sylvia Herbrink, Sascha Herzinger, Michael Heymann, Michele Hu, Alexander Hundt, Nadine Jacoby, Jacek Jaroslaw Lebioda, Yohan Jaroz, Sonja Jónsdóttir, Quentin Klopfenstein, Jochen Klucken, Rejko Krüger, Pauline Lambert, Zied Landoulsi, Roseline Lentz, Inga Liepelt, Robert Liszka, Laura Longhino, Victoria Lorentz, Paula Cristina Lupu, Tainá M. Marques, Clare Mackay, Walter Maetzler, Katrin Marcus, Guilherme Marques, Patricia Martins Conde, Patrick May, Deborah Mcintyre, Chouaib Mediouni, Francoise Meisch, Myriam Menster, Maura Minelli, Michel Mittelbronn, Brit Mollenhauer, Friedrich Mühlschlegel, Romain Nati, Ulf Nehrbass, Sarah Nickels, Beatrice Nicolai, Jean-Paul Nicolay, Fozia Noor, Marek Ostaszewski, Clarissa P. C. Gomes, Sinthuja Pachchek, Claire Pauly, Laure Pauly, Lukas Pavelka, Magali Perquin, Rosalina Ramos Lima, Armin Rauschenberger, Rajesh Rawal, Dheeraj Reddy Bobbili, Kirsten Roomp, Eduardo Rosales, Isabel Rosety, Estelle Sandt, Stefano Sapienza, Venkata Satagopam, Margaux Schmitt, Sabine Schmitz, Reinhard Schneider, Jens Schwamborn, Amir Sharify, Ekaterina Soboleva, Kate Sokolowska, Hermann Thien, Elodie Thiry, Rebecca Ting JIIN Loo, Christophe Trefois, Johanna Trouet, Olena Tsurkalenko, Michel Vaillant, Mesele Valenti, Gilles van Cutsem, Carlos Vega, Liliana Vilas Boas, Maharshi Vyas, Richard Wade-Martins, Paul Wilmes, Evi Wollscheid-Lengeling, Gelani Zelimkhanov.
Consortium name
Muhammad Ali3, Nancy E. Ramia3, Giuseppe Arena3, Rudi Balling3, Michele Bassis3, Regina Becker3, Ibrahim Boussaad3, Piotr Gawron3, Soumyabrata Ghosh3, Enrico Glaab3, Elisa Gómez De Lope3, Valentin Groues3, Anne Grünewald3, Wei Gu3, Michael Heneka3, Sascha Herzinger3, Jacek Jaroslaw Lebioda3, Yohan Jaroz3, Quentin Klopfenstein3, Zied Landoulsi3, Tainá M. Marques3, Patricia Martins Conde3, Patrick May3, Francoise Meisch3, Sarah Nickels3, Marek Ostaszewski3, Clarissa P. C. Gomes3, Sinthuja Pachchek3, Armin Rauschenberger3, Rajesh Rawal3, Dheeraj Reddy Bobbili3, Kirsten Roomp3, Isabel Rosety3, Stefano Sapienza3, Venkata Satagopam3, Sabine Schmitz3, Reinhard Schneider3, Jens Schwamborn3, Ekaterina Soboleva3, Rebecca Ting Jiin Loo3, Christophe Trefois3, Carlos Vega3, Maharshi Vyas3, Paul Wilmes3, Evi Wollscheid-Lengeling3, Jochen Klucken3,4,5, Rejko Krüger3,4,5, Claire Pauly3,5, Lukas Pavelka3,5, Linda Hansen3,5, Gilles Van Cutsem3,5, Geeta Acharya4, Gloria Aguayo4, Myriam Alexandre4, Wim Ammerlann4, Katy Beaumont4, Camille Bellora4, Jessica Calmes4, Lorieza Castillo4, Gessica Contesotto4, Daniela Esteves4, Guy Fagherazzi4, Jean-Yves Ferrand4, Marijus Giraitis4,5, Jérôme Graas4, Gaël Hammot4, Anne-Marie Hanff4,21, Estelle Henry4, Michael Heymann4, Alexander Hundt4, Sonja Jónsdóttir4, Pauline Lambert4, Victoria Lorentz4, Paula Cristina Lupu4, Guilherme Marques4, Deborah Mcintyre4, Chouaib Mediouni4, Myriam Menster4, Maura Minelli4, Ulf Nehrbass4, Fozia Noor4, Magali Perquin4, Rosalina Ramos Lima4, Eduardo Rosales4, Estelle Sandt4, Margaux Schmitt4, Amir Sharify4, Kate Sokolowska4, Hermann Thien4, Johanna Trouet4, Olena Tsurkalenko4, Michel Vaillant4, Mesele Valenti4, Guy Berchem5, Nico Diederich5, Liliana Vilas Boas5, Gelani Zelimkhanov5, Laura Longhino5, Romain Nati5, Beatrice Nicolai5, Elodie Thiry5, Friedrich Mühlschlegel6, Alexandre Bisdorff7, Rene Dondelinger7, Sylvia Herbrink8, Roseline Lentz9, Michele Hu10, Richard Wade-Martins11, Clare Mackay12, Daniela Berg13, Kathrin Brockmann13, Thomas Gasser13, Inga Liepelt13, Brit Mollenhauer14, Katrin Marcus15, Robert Liszka16, Walter Maetzler17, Mariella Graziano18, Nadine Jacoby19, Jean-Paul Nicolay20, Laure Pauly3,21, Michel Mittelbronn3,6,21,22,23
3Luxembourg Centre for Systems Biomedicine, University of Luxembourg, Esch-sur-Alzette, Luxembourg
4Luxembourg Institute of Health, Strassen, Luxembourg
5Centre Hospitalier de Luxembourg, Strassen, Luxembourg
6Laboratoire National de Santé, Dudelange, Luxembourg
7Centre Hospitalier Emile Mayrisch, Esch-sur-Alzette, Luxembourg
8Centre Hospitalier du Nord, Ettelbrück, Luxembourg
9Parkinson Luxembourg Association, Leudelange, Luxembourg
10Oxford Parkinson’s Disease Centre, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, UK
11Oxford Parkinson’s Disease Centre, Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, UK
12Oxford Centre for Human Brain Activity, Wellcome Centre for Integrative Neuroimaging, Department of Psychiatry, University of Oxford, Oxford, UK
13Center of Neurology and Hertie Institute for Clinical Brain Research, Department of Neurodegenerative Diseases, University Hospital Tübingen, Tübingen, Germany
14Paracelsus-Elena-Klinik, Kassel, Germany
15Ruhr-University of Bochum, Bochum, Germany
16Westpfalz-Klinikum GmbH, Kaiserslautern, Germany
17Department of Neurology, University Medical Center Schleswig–Holstein, Kiel, Germany
18Association of Physiotherapists in Parkinson's Disease Europe, Esch-sur-Alzette, Luxembourg
19Private practice, Ettelbruck, Luxembourg
20Private practice, Luxembourg, Luxembourg
21Faculty of Science, Technology and Medicine, University of Luxembourg, Esch-sur-Alzette, Luxembourg
22Luxembourg Center of Neuropathology, Dudelange, Luxembourg
23Department of Life Sciences and Medicine, University of Luxembourg, Esch-sur-Alzette, Luxembourg
Funding
This work has been funded by grants from the Fonds National de la Recherche (FNR) of Luxembourg to S.A. (grant #AFR14566210) and to Y.D. (C14/BM/8225223, C17/BM/11613033, COVID-19/2020-1/14719577/miRCOVID), from the Ministry of Higher Education and Research of Luxembourg, and from the Heart Foundation—Daniel Wagner.
Author information
Authors and Affiliations
Consortia
Contributions
Y.D. conceptualized the research. S.A., A.L. and Y.D. designed the analysis. S.A. performed the statistical analysis. S.A. wrote the first draft of the manuscript. A.L. and Y.D. reviewed and corrected the manuscript. Y.D. approved the final version of the manuscript. The executive committee of NCER-PD reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Luxembourg Comité National d’Ethique de Recherche (CNER Ref: 201407/13 and 20140713-SU3) and Commission nationale pour la protection des données (CNPD Ref: 446/2017). The patients/participants provided their written informed consent to participate in this study. All experiments were performed in accordance with relevant guidelines and regulations (Declaration of Helsinki).
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Acharya, S., Lumley, A.I., Devaux, Y. et al. Cardiovascular history and risk of idiopathic Parkinson’s disease: a cross-sectional observational study. BMC Neurosci 25, 33 (2024). https://doi.org/10.1186/s12868-024-00875-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12868-024-00875-y