Abstract
Local fluctuations in the profile can vary significantly for nonstationarity signals produced, e.g., by physiological systems. To correctly identify distinctions between the states of such systems, these fluctuations should be processed thoroughly. In the current study, we apply extended detrended fluctuation analysis (EDFA) to simulated data with various signal distortions and to electrical activity signals in the brains of mice under normal conditions and after one day of sleep deprivation. We show that the latter states can be distinguished by several EDFA scaling exponents, but their performance is scale dependent. The maximum differences between the states quantified by conventional and extended fluctuation analysis may be associated with different scale ranges. We conclude that the statistical analysis of local fluctuations in the signal profile is useful for developing diagnostically significant markers of the system state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fluctuation analysis is widely used in many studies to describe the features of long-range power-law correlations in experimentally recorded time series. This range of scales is important in solving many diagnostic problems in physiology, and the ability of quantifying the features of the decay of correlations makes it possible to introduce informative markers of the system’s behavior [1,2,3,4]. The time-varying dynamics of natural systems and the rapid decrease in the correlation function for random processes limit the use of classical tools when processing physiological signals. Detrended fluctuation analysis (DFA) [5, 6], which involves the procedure of detrending, formally allows one to ignore the problems of non-stationarity, although even in this case, data preprocessing is important to obtain more reliable quantitative criteria for the state of the system [7, 8]. In particular, different variants of nonstationarity affect the estimated characteristics in different ways, and knowledge of the corresponding effects makes it possible to avoid misinterpretation of the results obtained [9]. A discussion about the effectiveness of the DFA method in comparison with other variants of fluctuation analysis was undertaken, e.g., in [10, 11].
Natural signals generally have a complex structure and time-varying dynamics. With regard to physiological systems, for example, the corresponding changes can be caused by processes of adaptation to changing functioning conditions, and the assumption of approximate stationarity for small fragments of experimental data may not be fulfilled with an increase in the duration of the analyzed processes. As a consequence, the analysis of experimentally recorded data should be carried out taking into account the corresponding changes. DFA analyzes the RMS fluctuations of the signal profile from the trend and does not take into account differences in these fluctuations for individual data segments. However, the latter can be significant when dealing with transients or intermittent behavior. Since the same values of the scaling exponent can be obtained both for relatively stationarity data sets and for highly nonstationari ones, an extended DFA (EDFA) was proposed [12, 13]. It offers an additional measure of the signal, namely, EDFA considers how the distribution of local fluctuations (for individual segments) changes when the segment size increases. This measure, introduced in addition to the scaling exponent of the conventional DFA, provides more complete information about the analyzed process. It may be less informative and not lead to a significant improvement in the diagnostic capabilities of fluctuation analysis [14]. In the case of changes in the time-varying dynamics for different physiological states, the introduced EDFA scaling exponent, on the contrary, can better reveal distinctions between the states of the system under study [15].
Regardless of the result, a more thorough analysis of fluctuations for different parts of the signal profile seems to be useful. It does not limit the capabilities of the conventional DFA (and does not change the algorithm for computing the scaling exponent), but only focuses on additional features of the processes under study associated with their complex structure. In this paper, we test the EDFA method with a more detailed analysis of signal profile fluctuations in the presence of nonstationarity, artifacts, and data loss. For this purpose, we consider 1/f-noise, which plays an important role in physiology, and many acquired datasets have similar spectral properties in the frequency domain.
Next, we apply this approach to the signals of electrical activity in the brain of mice (electrocorticograms, ECoG) under conditions of wakefulness and sleep deprivation. Sleep plays an important role in keeping the central nervous system healthy [16,17,18,19]. It affects attention, learning, long-term memory, decision making, etc. [20, 21]. Sleep deprivation for several days is crucial for brain health, but the short-term effects of staying up at night are less clear.
In the current study, we examine how one day of sleep deprivation is reflected in the correlation features of ECoG signals quantified using EDFA. We aim to show that the method’s performance is scale dependent, and the maximum differences between the states quantified by conventional and extended fluctuation analysis may be associated with different scale ranges. The paper is organized as follows. Section 2 shortly describes the DFA method with its extension, simulated datasets, experimental procedures and data. The results of statistical analysis of local fluctuations in the signal profile for simulated and experimental data with their discussion are given in Sect. 3. Section 4 summarizes the main findings of the given work.
2 Methods and experiments
2.1 Extended DFA
DFA was developed in the works [5, 6], the authors of which focused on the correlation analysis of signals from living systems and proposed a variant of replacing the decreasing correlation function with an increasing function, which makes it possible to better analyze the region of long-range correlations. Algorithmically, DFA is a variant of root mean square random walk analysis that includes a detrending procedure for the signal profile. The transition to the profile (random walk) of the signal x(i), \(i=1,\ldots ,N\)
and its subsequent segmentation into parts of equal length n are carried out in all versions of the fluctuation analysis. A feature of DFA is the approximation and removal of a local trend \(y_n(k)\) within each part.
The estimation of the standard deviations of the detrended profile depending on the segment length is used to obtain an increasing dependence F(n) over a wide range of n
In the presence of scaling, it exhibits a power-law behavior
characterized by the exponent \(\alpha \). The latter can take the same value in different ranges of scales or differ depending on n. In many research tasks, the region of long-range correlations (large n) is analyzed, but shorter-range correlations can also be of diagnostic value. For this reason, along with the global exponent, estimates of local scaling exponents are useful.
Many natural systems are characterized by complex structure of the recorded experimental data. It is associated both with the presence of a large number of independent rhythmic components, demonstrating differences in time-varying behavior (a simpler case), and with their cooperative dynamics, accompanied by the appearance of combinational frequencies, which are also able to demonstrate complex behavior in time (more complex case). Such a structure can produce strong variability in the detrended signal profile for different segments that occurs, e.g., when the signal includes a transient process or possesses an intermittent behavior (alternation of segments with different properties). In this case, the role of some segments becomes decisive, while others will make a small contribution to the estimation of F(n). There are other examples of signals, for which a small part of the segments plays a dominant role. From the point of view of the conventional DFA algorithm, the different role of individual segments is not taken into account, and averaged root-mean-square fluctuations are estimated. Nevertheless, taking into account the degree of heterogeneity of the analyzed process is important and can provide useful diagnostic information about structural changes in signals. In [12, 13], an extended DFA was proposed, the idea of which is a more careful analysis of the distribution of local fluctuations of a detrended profile. In particular, the behavior of the standard deviations of local fluctuations \(F_{loc}\) (calculated within one segment) often has a power-law dependence of the form
and it is described by the scaling exponent \(\beta \), which generally does not coincide with \(\alpha \). In the presence of strongly pronounced tails of the distribution of local fluctuations (an increase in the probability of \(F_{loc}\) values that are large in absolute value), estimates of moments or cumulants of a higher order can be useful, for example,
2.2 Simulated data
In our study, 1/f noise was chosen as an example of simulated data. This process has statistics characteristic of many physiological processes in the low-frequency region. To study the influence of various factors on the results of fluctuation analysis, the following procedures were performed with this signal:
-
1.
Adding a low frequency trend. The cases of linear, quadratic and cubic trend added separately, sequentially and simultaneously were considered.
-
2.
Adding artifacts. Individual “outliers” or short segments of the process with values increased by several times were chosen as artifacts.
-
3.
Intermittent behavior, in which white noise was added instead of part of the 1/f noise fragments.
-
4.
Removal of a part of signal fragments with randomly selected durations and locations (the case of data loss).
2.3 Experimental procedures and data
Experimental procedures were performed on ten male mice in accordance with the standard Guide for the Care and Use of Laboratory Animals and protocols approved by the local commission on bioethics of the Saratov State University. Two-channel ECoG (Pinnacle Technology, Taiwan) was acquired by means of silver electrodes with a tip diameter of 2–3 \(\mu \)m. The location of the electrodes was chosen at a depth of 150 \(\mu \)m in coordinates (L: 2.5 mm, D: 2 mm) from Bregma on either side of the midline. The head plate was installed and small burrs were drilled under inhalation anesthesia with isoflurane. ECoG wire electrodes were inserted into the holes and fixed with dental acrylic. Experiments were started 10 days later (recovery after this procedure).
Sleep deprivation was performed from 20:00 to 08:00 by analogy with work [22] by bringing new objects and sounds into the room with mice [23]. Visual monitoring was carried out to confirm that the animals were learning the objects. ECoG with a sampling rate of 2 kHz was acquired in awake mice one day before and immediately after sleep deprivation (each recording was four hours long). At the stage of preprocessing, a Butterworth band-pass filter with cutoff frequencies of 1 Hz and 100 Hz and a notch filter 50 Hz were applied. Artifacts were removed using the method [24].
3 Results and discussion
3.1 Simulated data
Initially, the stability of the extended DFA method was studied for various factors that complicate the analysis of the signal structure, including non-stationarity, the presence of artifacts, and data loss. Similar investigations have previously been conducted with conventional DFA. In the case of the extended algorithm, the effect of nonstationarity on the results of computing the \(\beta \) exponent was discussed earlier, but the moments (or cumulants) of the distribution of local fluctuations of a higher order were not taken into account. The performed testing on the example of 1/f noise confirmed the stability of the results of computing the scaling exponent in the presence of different variants of the polynomial trend added to the test signal both separately and simultaneously (Table 1). In particular, estimated dependences of the EDFA method in the absence of nonstationarity and with added trends have no significant distinctions. Differences in the estimates of scaling exponents in this test did not exceed 3–5%, which is an acceptable result when analyzing signals of relatively short duration. Thus, both for the conventional DFA method and for its extended version, the results are quite resistant to the presence of a trend. Nevertheless, this variant of non-stationarity is not particularly difficult and can be eliminated at the pre-processing stage (to avoid possible problems for signals of a more complex structure). Similar testing was carried out for 1/f noise with added artifacts (data fragments with a very different range of values) and other types of non-stationarity, including switching between different behavior (for example, adding small parts of white noise to 1/f noise). It also showed that the computed characteristics correspond to the expected ones within a small error (in our estimates, also not exceeding 5%). The same conclusion was made for the case of data loss (exclusion from the signal of randomly selected segments of different durations). This result is similar to the results of the study [25], which found significantly less sensitivity to data loss for correlated processes in contrast to anticorrelated ones.
3.2 Experimental data
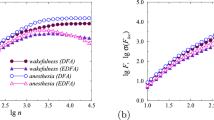
Despite the results for simulated datasets, in relation to the experimental data, pre-processing was carried out and included filtering and elimination of artifacts. Even in the case of their not very significant influence on the estimates of scaling exponents, it was advisable to minimize errors when selecting diagnostically significant markers of the state of the organism. Before performing statistical analysis for the entire group of laboratory animals, estimations were performed for individual mice to identify possible differences in ECoG signals caused by sleep deprivation and establish the range of scales where these differences are most pronounced. The effects of sleep deprivation have previously been noted in slow-wave dynamics, but they also appear in other frequency ranges. Using different EDFA scaling exponents, it is possible to better identify distinctions in the signals. For example, Fig. 1 shows the dependences computed using the EDFA method for the ECoG signals of one mouse (the results are averaged over two recording channels). According to Fig. 1a, the conventional DFA makes it possible to reveal differences in the slopes of the \(\lg ~F\) vs \(\lg ~n\) dependences, and these differences are more noticeable in the initial parts of the dependences. At the same time, estimates of the local slopes of the dependences used to estimate the \(\beta \) and \(\mu \) exponents show more pronounced differences in the region of long-range correlations (Fig. 1b and c).
To quantitatively describe the differences in states, we used the Student’s t test and estimated t within a sliding window with a value 0.6 along the \(\lg n\) axis. For each mouse, the results were averaged over the channels, and statistical differences were assessed between the states before and after sleep deprivation for all 10 animals. The results are shown in Fig. 2 with the critical value \(t_c=2.28\) (dashed line) and allow us to note several circumstances.
Statistical analysis of differences in states before and after sleep deprivation using Student’s t test and three scaling exponents of EDFA, namely, the scaling exponent of conventional DFA (\(\alpha \)) and two exponents of its extension (\(\beta \) and \(\gamma \)) computed according to Eqs. (4) and (6). The local values of the scaling exponents are computed within the \(\lg ~n\) window with a length of 0.6 and averaged for each mouse. Student’s t test was applied to compare local values of each exponent for the whole group (10 mice) in two states (before and after sleep deprivation)
-
1.
Significant differences between states can be diagnosed using all scaling exponents, both the conventional DFA and the extended approach. For all exponents, it is possible to choose a range of scales in which the values of t go beyond the black line related to significant differences with \(p < 0.05\).
-
2.
The maximum differences between the states (according to the value t) were found in the range of \(\lg n\sim 2.3\) using the conventional DFA. For the extended method, higher values of t in comparison with the conventional method were obtained in the range of long-range correlations. For this reason, if we focus only on slow-wave dynamics, then we can conclude that the extended fluctuation analysis has advantages.
-
3.
The results obtained are an interesting example of how the efficiency of using different scaling exponents can vary depending on the range of scales. We can conclude that careful consideration of local fluctuations in the signal profile can be useful in identifying diagnostically significant markers of the state of the physiological system.
4 Conclusion
This paper considers applications of extended fluctuation analysis to simulated and experimental data. The main emphasis is on a more thorough statistical analysis of local fluctuations in the signal profile. The considered example of 1/f noise shows the stability of the method under signal distortions (trend, intermittency, artifacts, data loss). Further, the effects of one-day sleep deprivation on the electrical activity of the mouse brain were studied. It is shown that the EDFA method makes it possible to diagnose changes in the ECoG signals, and various scaling exponents can be used for this purpose. An interesting result of the study is the different efficiency of the conventional and extended methods depending on the range of scales. Thus, the conventional method revealed more pronounced changes in the region of smaller scales compared to the extended approach. This result allows us to conclude that statistical analysis of local fluctuations in the signal profile is useful for a more complete description of structural changes in physiological processes when solving diagnostic problems.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
M. Kobayashi, R. Musha, 1/f fluctuation of heartbeat period. IEEE Trans. Biomed. Engin. 29, 456 (1982)
B. Pilgram, D.T. Kaplan, Nonstationarity and 1/f noise characteristics in heart rate. Am. J. Physiol. 276, R1 (1999)
G. Rangarajan, M. Ding (eds.), Processes with long-range correlations: theory and applications (Springer, Berlin, Heidelberg, 2003)
T. Penzel, J.W. Kantelhardt, L. Grote, J.H. Peter, A. Bunde, Comparison of detrended fluctuation analysis and spectral analysis for heart rate variability in sleep and sleep apnea. IEEE Trans. Biomed. Eng. 50, 1143 (2003)
C.-K. Peng, S.V. Buldyrev, S. Havlin, M. Simons, H.E. Stanley, A.L. Goldberger, Mosaic organization of DNA nucleotides. Phys. Rev. E 49, 1685 (1994)
C.-K. Peng, S. Havlin, H.E. Stanley, A.L. Goldberger, Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 5, 82 (1995)
K. Hu, P.C. Ivanov, Z. Chen, P. Carpena, H.E. Stanley, Effect of trends on detrended fluctuation analysis. Phys. Rev. E 64, 011114 (2001)
Z. Chen, P.C. Ivanov, K. Hu, H.E. Stanley, Effect of nonstationarities on detrended fluctuation analysis. Phys. Rev. E 65, 041107 (2002)
A.N. Pavlov, O.N. Pavlova, O.V. Semyachkina-Glushkovskaya, J. Kurths, Extended detrended fluctuation analysis: effects of nonstationarity and application to sleep data. Eur. Phys. J. Plus 136, 10 (2021)
R.M. Bryce, K.B. Sprague, Revisiting detrended fluctuation analysis. Sci. Reports 2, 315 (2012)
Y.H. Shao, G.F. Gu, Z.Q. Jiang, W.X. Zhou, D. Sornette, Comparing the performance of FA, DFA and DMA using different synthetic long- range correlated time series. Sci. Reports 2, 835 (2012)
A.N. Pavlov, A.S. Abdurashitov, A.A. Koronovskii Jr., O.N. Pavlova, O.V. Semyachkina-Glushkovskaya, J. Kurths, Detrended fluctuation analysis of cerebrovascular responses to abrupt changes in peripheral arterial pressure in rats. Commun. Nonlinear Sci. Numer. Simulat. 85, 105232 (2020)
A.N. Pavlov, A.I. Dubrovsky, A.A. Koronovskii Jr., O.N. Pavlova, O.V. Semyachkina-Glushkovskaya, J. Kurths, Extended detrended fluctuation analysis of electroencephalograms signals during sleep and the opening of the blood-brain barrier. Chaos 30, 073138 (2020)
G.A. Guyo, A.N. Pavlov, O.V. Semyachkina-Glushkovskaya, Anesthesia effects in rats electrocorticograms characterized using detrended fluctuation analysis and its extension. Eur. Phys. J. Spec, Top. (2023) (in press)
A.N. Pavlov, A.I. Dubrovsky, A.A. Koronovskii Jr., O.N. Pavlova, O.V. Semyachkina-Glushkovskaya, J. Kurths, Extended detrended fluctuation analysis of sound-induced changes in brain electrical activity. Chaos, Solitons Fractals 139, 109989 (2020)
L. Xie, H. Kang, Q. Xu, M.J. Chen, Y. Liao, M. Thiyagarajan, J. O’Donnell, D.J. Christensen, C. Nicholson, J.J. Iliff, T. Takano, R. Deane, M. Nedergaard, Sleep drives metabolite clearance from the adult brain. Science 342, 373 (2013)
C.M. Depner, E.R. Stothard, K.P. Wright Jr., Metabolic consequences of sleep and circadian disorders. Curr. Diabetes Rep. 14, 507 (2014)
N.E. Fultz, G. Bonmassar, K. Setsompop, R.A. Stickgold, B.R. Rosen, J.R. Polimeni, L.D. Lewis, Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366, 628 (2019)
T. Penzel, M. Möller, H.F. Becker, L. Knaack, J.H. Peter, Effect of sleep position and sleep stage on the collapsibility of the upper airways in patients with sleep apnea. Sleep 24, 90 (2001)
C. Duclos, M.P. Beauregard, C. Bottari, M.C. Ouellet, N. Gosselin, The impact of poor sleep on cognition and activities of daily living after traumatic brain injury: a review. Aust. Occup. Ther. J. 62, 2 (2015)
E.J. van Someren, C. Cirelli, D.J. Dijk, E. van Cauter, S. Schwartz, M.W. Chee, Disrupted sleep: from molecules to cognition. J. Neurosci. 35, 13889 (2015)
T.M. Achariyar, B. Li, W. Peng, P.B. Verghese, Y. Shi, E. McConnell, A. Benraiss, T. Kasper, W. Song, T. Takano, D.M. Holtzman, M. Nedergaard, R. Deane, Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol. Neurodegener. 11, 74 (2016)
J. Zhang, Y. Zhu, G. Zhan, P. Fenik, L. Panossian, M.M. Wang, S. Reid, D. Lai, J.G. Davis, J.A. Baur, S. Veasey, Extended wakefulness: compromised metabolics in and degeneration of locus ceruleus neurons. J. Neurosci. 34, 4418 (2014)
N.P. Castellanos, V.A. Makarov, Recovering EEG brain signals: artifact suppression with wavelet enhanced independent component analysis. J. Neurosci. Methods 158, 300 (2006)
Q.D.Y. Ma, R.P. Bartsch, P. Bernaola-Galván, M. Yoneyama, P.C. Ivanov, Effects of extreme data loss on detrended fluctuation analysis. Phys. Rev. E 81, 031101 (2010)
Acknowledgements
This work was supported by the Russian Science Foundation (Agreement 23-75-30001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pavlov, A.N., Semyachkina-Glushkovskaya, O.V. Statistical analysis of local fluctuations in the signal profile: application to electrocorticograms. Eur. Phys. J. Spec. Top. 233, 471–477 (2024). https://doi.org/10.1140/epjs/s11734-023-01047-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjs/s11734-023-01047-5