Abstract
In this paper, we introduce a simple controllable experimental system that can exhibit rich dynamics. The setup comprises a sealed glass vial of ethanol with wicks immersed in it. The flame produced by such a lamp can show both steady combustion or oscillatory combustion (i.e. flickering) depending on the volume of fuel within and the number of wicks used in its construction. This tunability makes it a great model system to study the dynamics of flame oscillations and to explore emergent behaviour of coupled nonlinear oscillators in table-top experiments. In the present work, the behaviour of this system for different fuel volumes is explored and some typical phenomena reported in other experimental systems, viz. in-phase synchronization, anti-phase synchronization and amplitude death are demonstrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Experimental study of nonlinear oscillators are of great importance for both scientific inquiry and engineering applications. Nonlinear oscillators exhibit rich dynamics through bifurcations as a single system [1] and emergent phenomena like synchronization [2,3,4,5,6,7,8,9,10,11,12], chimera-states [13, 14], oscillation quenching [15,16,17], phase flip bifurcation [18] and quorum sensing [19, 20] when coupled together. Candle-flame oscillators have recently seen extensive use as a model experimental system to study emergent dynamics of coupled nonlinear oscillators [21,22,23,24,25,26,27,28,29]. A variety of emergent phenomena like in-phase and anti-phase synchronization [22, 23], amplitude death [24], phase flip bifurcation [25] and weak chimera states [26, 27] have been observed using these coupled flame oscillators. There is also wide interest in the mechanisms facilitating this inter-flame interaction among the oscillators [30,31,32]. A recent experimental work has demonstrated in-phase synchrony, anti-phase synchrony and amplitude death in dual diffusion flames from a pair of pipe burners with methane as fuel [33].
The prime aim of the present work is to provide one such alternate experimental system that is very simple to construct and is rich in dynamic behaviour. Our system is a lamp made using a glass vial and cotton twine with ethanol as its fuel. The ethanol lamp presented here has clear practical advantages over the other flame oscillator systems like the candle flame oscillators or pipe burners. Unlike candles there is no change in the height of the oscillator, hence a constant distance can be maintained between multiple flames. Ethanol flames produce very less soot, and hence are suitable to work with in closed lab spaces. Ethanol lamps have two parameters that can control the fuel flow, viz. number of wicks and volume of fuel in the vial, thus providing more control over the dynamics of an individual oscillator. In this paper we will introduce this simple experimental system, characterize its behaviour with different initial fuel volumes (which is unique to our system) and go on to demonstrate some typical phenomena that have been reported in other types of flame oscillators.
a Schematic representation (side view) of the ethanol lamp showing the glass vial with wicks dipped in ethanol. b Schematic representation of the cap (top view) with six symmetric holes. c Photograph of an ethanol lamp showing the cap sealed with parafilm (not visible) and then covered with aluminium foil
2 Construction and experimental setup
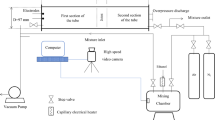
A schematic representation and a sample photograph of an ethanol lamp is shown in Fig. 1. The ethanol lamp is made from a small glass vial, that is sealed with a plastic cap. Holes are drilled into the cap (c.f. Fig. 1b) to allow for N wicks to be inserted into the vial. The vial is then filled with the desired amount of fuel and sealed with three alternating layers of parafilm and aluminium foil. The parafilm helps to seal the lamp and the aluminium foil aids in protecting the cap from the heat of the flame. In this work, we used a 20 mL glass vial (Height \(= 7.2\) cm, Diameter \(= 2.4\) cm) with six holes drilled into the cap in a hexagonal formation, i.e., the number of wicks can range between \(1\le N \le 6\). The wicks used were 10 cm long pieces of standard cotton twine of thickness \(\approx 3\) mm. The vial was sealed (with parafilm and aluminium foil) such that 1 cm of the wick was exposed to facilitate the combustion.
All experimental data of the flame dynamics were collected through direct imaging of the flames using a high speed camera (GoPro Hero 7 Black). The camera was placed at a distance of 26 cm from the lamp. Frames of 1280 \(\times \) 720 pixels were recorded at a frame rate of 240 frames per second. The videos thus obtained were analyzed using an in-house edge detection code developed on MATLAB (further detailed in the Supplementary Material [34]) that tracks the approximate area of the flame on each frame of the video.
3 Results
3.1 Characterization with fuel volume
First, the behaviour of this system for varying amounts of initial fuel volume is characterized. To do this, we fix the number of wicks N to be 1 and the activity of the ethanol lamp was recorded for various initial ethanol volumes (V from 2 to 20 mL in steps of 2 mL). The video data are collected for 90 sec in each run but only the last 30 sec of data is analyzed, to capture asymptotically stable behaviour.
Figure 2 shows windows from the time series of flame activity recorded for different initial volumes of fuel in the lamp. Here, the x-axis is time t in seconds and the y-axis is \(y_1\) in arbitrary units which is proportional to the area of the flame. In panel (a), the initial volume of the fuel in the vial is 2 mL and a steady burning flame is seen. This can be inferred from the absence of any oscillation in the time series. In panel (b), the initial volume of fuel in the vial is 8 mL and oscillations clearly arise in the combustion. In panel (c), the volume of the fuel is 12 mL initially and we observe that the flame oscillations are robust and larger in amplitude. Panel (d) corresponds to flame activity with 20 mL of ethanol initially in the vial and large relaxation type oscillations are observed here. It is important to note that the transition from steady state to oscillation with increasing amplitude is reminiscent of activity near a Hopf-like bifurcation, with volume as the control parameter.
To characterize this change in behaviour with initial volume of fuel, in Fig. 3, we plot the frequency with the highest peak \(f_{peak}\), from the Fourier transform of the time series corresponding to each ethanol volume. When the time series has no oscillation, the highest peak on the Fourier transform will be near zero. This can be observed for lower volumes in our plot (c.f. Fig. 3). When the time series has prominent oscillations, the Fourier transform will reflect it and the maximal peak \(f_{peak}\) will correspond to the oscillation frequency of the flame. We see that for initial volumes more than 8 mL the ethanol lamp settles to oscillations around 10–12 Hz, which is completely in agreement with the previous works on flame oscillations in other systems [22].
The frequency corresponding to the highest peak \(f_{peak}\) from the Fast Fourier Transform (FFT) of each timeseries is plotted versus the volume of ethanol. A clear transition from steady combustion to oscillatory combustion is seen. Points corresponding to panels (a), (b), (c) and (d) from Fig. 2 are indicated on the plot
The standard deviation \(\sigma _y\) of each time series is plotted versus the corresponding initial fuel volume. The plot clearly depicts larger oscillation amplitudes for larger initial volumes. Points corresponding to panels (a), (b), (c) and (d) from Fig. 2 are indicated on the plot
In Fig. 4, we plot the dependence of the standard deviation \(\sigma _y\) of each time series data with the corresponding initial ethanol volume. Here, standard deviation of the time series data serves as a measure for the average amplitude of the flame oscillations as larger oscillations would lead to larger standard deviation values. We again see that for small initial volumes of fuel, \(\sigma _y \approx 0\). For initial volumes more than 8 mL, i.e., once the oscillations begin, there is a monotonous increase in the amplitude of oscillation as a function of fuel volume.
Our characterization of ethanol lamp with volume clearly shows the variation in behaviour possible with fuel volume as a control parameter. The number of wicks N serves as another parameter to control the rate of fuel flow, thereby controlling the flame dynamics. As explained in previous works on candle flame oscillations [22], if the fuel flow rate is high enough, there is a local dip in oxygen concentration around the flame causing a momentary decrease in flame size. Therefore, if the fuel rate is below a certain threshold, the dearth for oxygen is never created around the flame for it to shrink, leading to steady state dynamics. We believe that this system, albeit differently constructed is governed by the same constraints. In the next section, we study the emergent phenomena arising from the interaction of two ethanol lamps where the number of wicks has been fixed at \(N=2\) to allow for more fuel flow and robust oscillations.
3.2 Characterization with distance
Steady and oscillatory states were shown to exist for different initial fuel volumes in a single ethanol lamp. Now, interaction between two coupled ethanol lamps is explored. Number of wicks in each lamp is fixed at N = 2 and the initial fuel volume is fixed at V = 20 mL. The camera is placed at a distance of 26 cm from one of the two ethanol lamps. The position of the other lamp is varied such that distance between the lamps goes from 0 to 50 mm in steps of 5 mm. The same protocol is followed where data is recorded for 90 sec and the last 30 sec of the collected data is analyzed. Note here that the distance 0 mm denotes that the two glass vials are touching each other, but the flames themselves are separated by a finite distance.
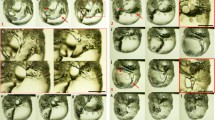
In-phase synchronization of the flame oscillations are depicted for a distance D = 0 mm. Here the initial volume of ethanol in both lamps is fixed at 20 mL and the number of wicks N = 2. a Frames at three consecutive time points depicting the in-phase evolution of the flame oscillations. b Time series of the flame area measures \(y_1\) and \(y_2\) showing robust in-phase synchrony. c Phase portrait of \(y_1\) vs \(y_2\) overlaid over a dotted line of slope 1. The alignment between the phase portrait and the unit slope line indicates robust in-phase synchrony
Figure 5 depicts robust in-phase oscillations of the ethanol lamps. This is shown when distance D between the two lamps is 0 mm. Panel (a) of the figure depicts three consecutive snapshots from the video recording. This shows the two flames evolving together, i.e., in phase with each other. Panel (b) is the corresponding time series showing the observables \(y_1\) and \(y_2\) of the two oscillators synchronized in-phase with each other. In Panel (c) phase portrait of \(y_1\) and \(y_2\) is plotted alongside a dashed line of slope 1. The strong alignment suggests reliable in-phase evolution over a long time period. Figure 6 depicts anti-phase synchrony between the two flame oscillations at a distance of 15 mm. Once again from the snapshots, time series plots and the phase portrait it is clear that two oscillators are synchronized with each other but are out of phase (or anti-phase).
Anti-phase synchronization of the flame oscillations are depicted for a distance D = 15 mm. Here the volume of ethanol in both lamps is fixed at 20 mL and the number of wicks N = 2. a Frames at three consecutive time points depicting the anti-phase evolution of the flame oscillations. b Time series of the flame area measures \(y_1\) and \(y_2\) showing robust anti-phase synchrony. c Phase portrait of \(y_1\) vs \(y_2\) overlaid over a dotted line of slope -1
Cross-correlation coefficient CCC of \(y_1\) and \(y_2\) plotted for various values of distance. Here, initial volume of fuel in the lamps is fixed at 20 mL and the number of wicks N = 2. (i) denotes the positive CCC corresponding to in-phase oscillations depicted in Fig. 5. (ii) denotes the negative CCC corresponding to the anti-phase oscillations depicted in Fig. 6
We observe that change in distance leads to change in the nature of synchrony among the coupled oscillators. To characterize this change we use the cross-correlation coefficient (CCC) to measure the information transfer between the two systems. This measure CCC is defined as

where \(\sigma _{y_1}\) and \(\sigma _{y_2}\) are the standard deviations of the two time series and cov(\(y_1\),\(y_2\)) is the covariance between the two observables \(y_1\) and \(y_2\). This measure takes a value between − 1 and + 1. CCC = + 1 implies complete in-phase synchrony of the two time series and CCC = − 1 implies complete out-of-phase synchrony. CCC \(\approx \) 0 when the two timeseries are uncorrelated. In Fig. 7, correlation coefficient is plotted as a function of distance for two ethanol lamps with two wicks and 20 mL of fuel in them. For small distances, the CCC is close to + 1 denoting strong in-phase synchronization between the oscillators. For intermediate distances the CCC becomes negative denoting anti-phase synchronization. It is interesting to note that the anti-phase synchrony is not as robust as the in-phase synchrony for the given set of parameter values. For higher values of distance the CCC rises and settles around zero, i.e., the oscillators are rendered autonomous.
The transition from in-phase to anti-phase synchronization may go through a region of quenched oscillations. This phenomenon, where two oscillators when coupled quench each others’ activity has been reported previously in a variety of coupled flame oscillators [24, 25, 33]. To demonstrate this phenomenon of amplitude death in ethanol lamps, we show the time series of \(y_1\) and \(y_2\) (refer Fig. 8) at a distance of 10 mm (Initial ethanol volume V = 14 mL and number of wicks N = 2). The two oscillators quench each others’ oscillations and stay at very low activity for a long time window (roughly 5 sec or 50 oscillator cycles). This state of quenched oscillations is very sensitive to environmental fluctuations, yet it does occur often at distances between the in-phase and anti-phase synchronous states.
4 Discussion and conclusions
Thus, we have introduced ‘ethanol lamp’, a novel flame oscillator that can exhibit both steady combustion and oscillatory combustion. It is easy to deploy and has two control parameters viz. volume of the fuel and number of wicks, that can be varied to control its dynamics. The behaviour of a single ethanol lamp as a function of fuel volume was characterized. Various emergent behaviour possible with two coupled ethanol lamps were demonstrated.
The controllability of oscillations by two independent parameters, namely the initial fuel volume and the number of wicks, make this system an optimal work horse for studying dynamical phenomena of flames. These oscillators have the additional advantage of maintaining a fixed height during the entire experimental run, when compared to similar oscillators made up of candles. They have the potential to show a plethora of dynamics, out of which only a subset were explored and presented in this work. For example, one can try to explore aging transitions [35] by coupling a set of such systems in the oscillatory regime to another set which is in the fixed point regime. Controlling the distance between individual oscillators can be used to achieve synchronization in a network of such oscillators. These coupled networks can potentially exhibit Kuramoto transitions to synchrony [36], chimera states [13, 14, 26, 27] and other exquisite emergent phenomena.
Oscillating flames are a challenging system to control and study, because of the inherent ease of the flames to be disturbed by minuscule air drafts. Even though this system is able to circumvent some of the problems that are observed in similar oscillators made up of candles, it is still extremely sensitive to disturbances in air and the surroundings. In addition, due to the complex nonlinear nature of this system, the dynamics have multiple stable dynamics for the same system parameters. To mitigate some of these challenges, one can try to perform experiments in a hermetically sealed enclosure, as long as the container is large enough to maintain a relatively stable concentration of oxygen and carbon dioxide. Such an enclosure would further reduce any stray air-drafts that end up changing the oscillator’s state, especially in parameter regions where it exhibits multiple stable configurations. Maintaining a stricter control over the ambient humidity and temperature may also be helpful in overcoming these challenges. For the purpose of this work, the experiments were deliberately chosen to exhibit the best examples of the variety of interesting dynamics that this system can have. Nevertheless, this system can be used in both the experimental study of flame dynamics and emergent behaviour of coupled nonlinear oscillators.
References
S.H. Strogatz, Nonlinear dynamics and chaos with student solutions manual: with applications to physics, biology, chemistry, and engineering (CRC press, 2018)
A. Pikovsky et al., Synchronization: a universal concept in nonlinear sciences, vol. 12 (Cambridge university press, 2003)
J.M. Cruz, M. Rivera, P. Parmananda, Experimental observation of different types of chaotic synchronization in an electrochemical cell. Phys. Rev. E 75(3), 035201 (2007)
J.M. Cruz, M. Rivera, P. Parmananda, Chaotic synchronization under unidirectional coupling: numerics and experiments. J. Phys. Chem. A 113(32), 9051–9056 (2009)
D.K. Verma et al., Synchronization in autonomous mercury beating heart systems. J. Phys. Chem. A 118(26), 4647–4651 (2014)
D.K. Verma et al., Kuramoto transition in an ensemble of mercury beating heart systems. Chaos: Interdiscip. J. Nonlinear Sci. 25(6), 064609 (2015)
T. Singla et al., Synchronization using environmental coupling in mercury beating heart oscillators. Chaos: Interdiscip. J. Nonlinear Sci. 26(6), 063103 (2016)
P. Kumar, P. Parmananda, Control, synchronization, and enhanced reliability of aperiodic oscillations in the Mercury Beating Heart system. Chaos: Interdiscip. J. Nonlinear Sci. 28(4), 045105 (2018)
Jyoti Sharma et al., Rotational synchronization of camphor ribbons. Phys. Rev. E 99(1), 012204 (2019)
J. Sharma et al., Rotational synchronization of camphor ribbons in different geometries. Phys. Rev. E 101(5), 052202 (2020)
V. Manaoj Aravind, K. Murali, S. Sinha, in Synchronized Hopping Induced by Interplay of Coupling and Noise. Nonlinear Dynamics and Control (Springer, 2020), pp. 325–334
M. Aravind, S. Sinha, P. Parmananda, Competitive interplay of repulsive coupling and cross-correlated noises in bistable systems. Chaos: Interdiscip. J. Nonlinear Sci. 31(6), 061106 (2021)
D.M. Abrams, S.H. Strogatz, Chimera states for coupled oscillators. Phys. Rev. Lett. 93(17), 174102 (2004)
J. Sharma et al., Chimeralike states in a minimal network of active camphor ribbons. Phys. Rev. E 103(1), 012214 (2021)
M. Dasgupta, M. Rivera, P. Parmananda, Suppression and generation of rhythms in conjugately coupled nonlinear systems. Chaos: Interdiscip. J. Nonlinear Sci. 20(2), 023126 (2010)
T. Mandal et al., Conjugate feedback induced suppression and generation of oscillations in the Chua circuit: Experiments and simulations. Chaos: Interdiscip. J. Nonlinear Sci. 23(1), 013130 (2013)
R. Phogat et al., Cessation of oscillations in a chemo-mechanical oscillator. Eur. Phys. J. B 91(6), 1–7 (2018)
J.M. Cruz et al., Phase-flip transition in coupled electrochemical cells. Phys. Rev. E 81(4), 046213 (2010)
H. Singh, P. Parmananda, Quorum sensing via static coupling demonstrated by Chua’s circuits. Phys. Rev. E 88(4), 040903 (2013)
A. Biswas et al., Oscillatory activity regulation in an ensemble of autonomous mercury beating heart oscillators. Phys. Rev. E 99(3), 032223 (2019)
T. Ishida, S. Harada, Oscillation of the light of flames. Kagaku to Kyoiku 47, 716–716 (1999)
H. Kitahata et al., Oscillation and synchronization in the combustion of candles. J. Phys. Chem. A 113(29), 8164–8168 (2009)
D.M. Forrester, Arrays of coupled chemical oscillators. Sci. Rep. 5(1), 1–7 (2015)
K. Okamoto et al., Synchronization in flickering of three-coupled candle flames. Sci. Rep. 6(1), 1–10 (2016)
K. Manoj, S.A. Pawar, R.I. Sujith, Experimental evidence of amplitude death and phase-flip bifurcation between in-phase and anti-phase synchronization. Sci. Rep. 8(1), 1–7 (2018)
K. Manoj et al., Synchronization route to weak chimera in four candleflame oscillators. Phys. Rev. E 100(6), 062204 (2019)
K. Manoj, S.A. Pawar, R.I. Sujith, Experimental investigation on the susceptibility of minimal networks to a change in topology and number of oscillators. Phys. Rev. E 103(2), 022207 (2021)
T. Chen et al., Frequency and phase characteristics of candle flame oscillation. Sci. Rep. 9(1), 1–13 (2019)
A. Gergely et al., Flickering candle flames and their collective behavior. Sci. Rep. 10(1), 1–13 (2020)
Y. Nagamine et al., Mechanism of candle flame oscillation: Detection of descending flow above the candle flame. J. Phys. Soc. Jpn. 86(7), 074003 (2017)
S. Dange et al., Role of buoyancy-driven vortices in inducing different modes of coupled behaviour in candle-flame oscillators. AIP Adv. 9(1), 015119 (2019)
T. Yang, X. Xia, P. Zhang, Vortex-dynamical interpretation of antiphase and in-phase flickering of dual buoyant diffusion flames. Phys. Rev. Fluids 4(5), 053202 (2019)
N. Fujisawa, K. Imaizumi, T. Yamagata, Synchronization of dual diffusion flame in co-flow. Exp. Thermal Fluid Sci. 110, 109924 (2020)
See supplemental material at (link) for details about the MATLAB algorithm used to calculate the flame area
H. Daido, K. Nakanishi, Aging transition and universal scaling in oscillator networks. Phys. Rev. Lett. 93(10), 104101 (2004)
F.A. Rodrigues et al., The Kuramoto model in complex networks. Phys. Rep. 610, 1–98 (2016)
Acknowledgements
We (P.P., M.A. and I.T.) are grateful for the support provided by DST [Grant no. EMR/2016/000275]. I.T. would also like to thank CSIR (India) for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data Availability Statement
This manuscript has data included as electronic supplementary material.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1 (mp4 790 KB)
Supplementary file 2 (mp4 775 KB)
Supplementary file 3 (mp4 1999 KB)
Rights and permissions
About this article
Cite this article
Aravind, M., Tiwari, I., Vasani, V. et al. Ethanol lamp: a simple, tunable flame oscillator and its coupled dynamics. Eur. Phys. J. Spec. Top. 231, 179–184 (2022). https://doi.org/10.1140/epjs/s11734-021-00414-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjs/s11734-021-00414-4