Abstract
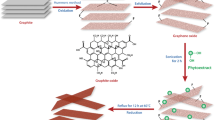
This study presents a facile synthesis technique to produce few-layer graphene oxide from Cinnamomum camphora (Camphor L.). Camphor upon carbonization and chemical oxidation leads to the formation of few-layer graphene oxide sheets of around ten layers with a lateral size of 4.14 nm and stacking height of 3.10 nm. AFM and SEM analysis results reveal the wrinkled morphology of the graphene oxide sheets formed. The sharp G band and the relative intensity of the defect to the graphitic band in the Raman spectrum indicate the formation of nanocrystalline graphene oxide sheets with fewer defects. The FTIR spectrum and the deconvoluted C 1s XPS spectrum of graphene oxide synthesized reveal the presence of predominant sp2 hybridized carbon along with carbon bound to various oxygen functionalities. In brief, the formation of high-quality few-layer graphene oxide from an abundant, low-cost, and readily available botanical precursor is herein reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Camphor trees (Cinnamomum camphora) are widely distributed across the provinces of Southern and Eastern Asia and are easily and at vast found in China. The huge agronomy of camphor trees is located in Yangtze River valley, including the south zone of China. This broad-leaved tree, which is the primary source of camphor, belongs to the Lauraceae family and is grown in many countries as an ornamental plant [1]. Deep-rooted in its economic and cultural aspects, this tree is a unique heritage of East Asia, particularly China and Japan. There are vast plantation areas in China with different varieties of these trees. Camphor oil, used in preparing various products like cream, ointments, and lotions, is derived from the roots, branches, and barks of the Cinnamomum camphora trees through the distillation process. This species has been habitually utilized for medicine, pesticide, timber, ornamental use, pesticide, and a non-negotiable part of the habitants’ cultural rituals. [2]. Apart from this, this plant species’ pharmaceutical properties attract global attention as it can be used for the medication of muscular stresses, rheumatic disorders, etc., in addition to exceptional antibacterial properties [1, 2].
Multiple studies have been reported on the synthesis of nanoparticles from Cinnamomum camphora. Mohamed et al. (2020) have reported the synthesis of silver nanoparticles (Ag-NPs) from Cinnamomum camphora for the first time from its extract. Ag-NPs were green synthesized using a simple and eco-friendly method involving callus extract of C. camphora as stabilizer and reductant [3]. A highly efficient green synthesis of carbon nanoparticles (CNPs) has been proposed by Oza et al. [4], where graphitic shell condensed carbon nanotubes (CNTs) and carbon dots (CDs) with Camphor (Cinnamomum camphora) is verified. Similarly, Yang et al. [5] have conveyed single-pot bio-production of palladium by an easy process consuming the broth of Cinnamomum camphora leaf. It was observed that by varying the concentration of palladium ions, the size of the particles could be easily controlled from size 3.2 to 6.0 nm.
Investigating the possibility of the synthesis of various carbon nanoparticles like graphene, CNTs, and fullerene, etc., from biomaterials is an attractive research area, considering its easy availability, least cost of production, and ease of synthesis procedures. For example, Andrianiaina et al. [6] have reported the synthesis of graphene via bioprocess using the natural extract obtained from artemisia species. Due to the presence of chemical complexes having antioxidant properties, the sample was testified as an active chelating agent. This single layer of carbon has acquired tremendous research interest for established researchers, attributable to their 2D arrangement [7]. Studies demonstrated that a perfect graphene layer is profoundly arranged and shows remarkable practices, including high Young’s modulus of 1.0 TPa, extraordinary surface areas of 2630 m2 g–1, high thermal conductivity in the range 5000 W m–1 K–1 as well as robust chemical toughness [8].
In this context, we herein report the synthesis of few-layer graphene oxide prepared from the botanical hydrocarbon Cinnamomum camphora (Camphor), an ecologically benevolent source of nanocarbon, taking into consideration its abundancy, ease of availability and biological source.
MATERIALS AND METHODS
Camphor was burnt, and the soot was collected. Two grams of camphor soot was added to 2 g of sodium nitrate (NaNO3) and subsequently treated with 50 mL of concentrated sulphuric acid (H2SO4) in a reaction vessel, arranged in an ice bath. After 15 min, about 6 g of potassium permanganate (KMnO4) was added in drops with continuous stirring. The mixture was then let to cool down for half an hour. The mixture was then stirred continuously for two days. Subsequently, distilled water, warm water, and 40 mL of hydrogen peroxide were added, and the mixture was kept undisturbed for 12 h. The precipitate formed was separated from the solution by repeatedly washing with water and acetone via centrifugation. The sample was then sonicated in water for 20 min and evaporated to obtain graphene oxide (CS1).
RESULTS AND DISCUSSION
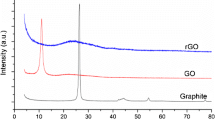
X-ray diffraction pattern of the synthesized sample (CS1) is presented in Fig. 1. The sample shows a distinct, highly intense peaks at ~25.4° and a broad peak around 44.3° owing to (002) and (110) planes of graphite oxide, with partial reduction [9–13]. The XRD pattern of CS, when compared to that of pure graphite, exhibits a broadened (002) peak. This is because of the effect of disorder in the sp2 hybridized carbon. The sp2 sites in the carbon possess both σ and π states. The σ-bonded amorphous carbon can be distinguished from the π-bonded carbon by the medium-range ordering. However, when the π states form pairs of aligned/six-fold aromatic rings/ graphitic clusters, π-bonding gets maximized. The existence of a strong and broadened π band in CS1 confirms that CS1 is composed of nanocrystalline graphitic carbon with minor defects. The disorder in the sample might arise due to lattice imperfection in the stacked graphene oxide sheets [11, 14, 15]. It is also noticed that the lateral size (La) of CS1 is about 4.14 nm, while its stacking height (Lc) is found to be 3.10 nm, with an interlayer spacing of 0.34 nm, indicating the formation of about ten layers of graphene oxide.
Atomic force microscopic analysis. The AFM analysis of the synthesized graphene oxide (CS1) is presented in Fig. 2. The cross-section height profile analysis clarifies that CS1 consists of less than ten layers. Furthermore, the height profile affirms that the graphene oxide layers formed are either wrinkled or folded. The topography shows periodicity, confirming the development of a sheet-like structure. The forward scan fit displays that the dimension of the synthesized sample CS1 is in the nanometre range, considering its height.
Scanning electron microscopic analysis. SEM image of CS1 displays sheets of graphene oxide, agglomerated in clusters, as presented in Fig. 3. Upon Hummers’ treatment, the laminar structural formation is observed in the topography. The graphene oxide sheets are randomly distributed throughout the sample, forming islands.
Constituent elements present in CS1 were analyzed and quantified using EDS analysis. The analysis exposes the predominant presence of carbon with an infinitesimal amount of oxygen. With the Hummers’ treatment, oxygen groups are incorporated in the carbon backbone of the sample. The occurrence of other elements, namely, Mn, S, and K is from the chemicals used for the Hummers’ synthesis method.
Raman spectral analysis. Raman spectroscopy provides valuable information on the defects, crystallite size, and structure of different carbon nanomaterials and is considered to be the most important characterization tool in the structural studies of graphitic materials [3, 16–20]. By providing various Raman fingerprints for single, bi, and few-layer graphitic sheets, this investigation technique grabs the research interest for its unique and accurate data analysis properties [21–26]. In Fig. 4, the peak noticed at ~1602 cm–1 is due to the G band, revealing the presence of high-frequency E2g first-order mode of graphitic structure. The defect band D1 is observed at ~1372 cm–1. In addition to G and D1 peaks, the deconvoluted first-order Raman spectrum of CS1 exhibits D2, D3, and D4 peaks, arising due to disorder in the graphitic lattice, presence of amorphous carbon, and the existence of mixed sp2–sp3 hybridized carbon in the sample, respectively. The 2D band is found to be very weak, indicating the stacking of layers in the graphene oxide sheets. The relative intensity ratio of the defect to the graphitic band is calculated to be 0.98 for CS1, which is comparable to other reported results [27–30].
XPS analysis. The C 1s XPS spectrum of graphene oxide, depicted in Fig. 5, shows four significant components that represent carbon atoms in different states [31]. The four fitted components, located at 284.5, 285.3, 286.4, and 288.7 eV are assigned to the presence of sp2 carbon, sp3 carbon, C in C–O bond (bound to O either as epoxy or hydroxyl) and C in C=O bond (of alcohol, phenol, or ether), respectively. The presence of C–O and C=O components reveal the oxidized state of the CS1 sample synthesized [32–37]. The inset of Fig. 5 displays the O 1s XPS spectrum, further confirming the occurrence of diverse bonding structures of O with the graphitic carbon backbone. The X-ray photoelectron spectrometry results are in accordance with Raman and XRD results and consistent with the EDS elemental analysis.
FT-IR analysis. FT-IR spectroscopic analysis (Fig. 6) was carried out to investigate the occurrence of oxygen functional groups located at both the basal plane and the edges of the synthesized graphene oxide. The FTIR spectrum reveals that the Hummers’ treatment has led to strong infrared absorbance allied with C=O and C–O regions. The synthesized graphene oxide exhibits prominent peaks between 3000–3700 cm–1 due to hydroxyl, carboxyl, and water environment [38–44]. The sharp peaks at 3667 and 1491 cm–1 are assigned to O–H stretching vibration. The peak present at 1723 cm–1 is because of C=O stretching of carboxyl groups. The peaks observed at 1337 and 918 cm–1 are due to the C–O–H and C–O stretching vibrations. The sharp peak noticed at 1491 cm–1 also arises due to the asymmetric C=C stretching of sp2 hybridized carbon. Its intensity relies on the environment and is attributed to the skeletal vibrations from graphitic domains.
CONCLUSIONS
Few-layer graphene oxide with wrinkled morphology is synthesized from the botanical precursor Cinnamomum camphora. The synthesized graphene oxide sample is analyzed by AFM, SEM, XRD, Raman spectroscopy, XPS, and FTIR characterization techniques. AFM results confirm the formation of few-layer graphene oxide. The formation of about 9–10 stacking layers of graphene oxide is confirmed by X-ray spectral analysis. Raman spectral analysis confirms the formation of crystalline and graphitized graphene oxide sheets with few structural disorders. The FT-IR spectrum discloses the presence of carbonyl, carboxyl, and hydroxyl functional groups. The presence of these functional groups is further verified using the XPS analysis data. In conclusion, graphene oxide prepared from abundant, low-cost, and readily available Cinnamomum camphora exhibits high quality with carbon-rich few-layered structure and good crystallinity with low defects.
REFERENCES
X. Shi, C. Zhang, Q. Liu, et al., BMC Genomics. 17, 1 (2016).
Y. Zhou and W. Yan, Genet. Resour. Crop Evol. 63, 1049 (2016).
M. S. Aref and S. S. Salem, Biocatal. Agric. Biotechnol. 27, 101689 (2020).
G. Oza et al., Sci. Rep. 6, 1 (2016).
X. Yang, Q. Li, H. Wang, et al., J. Nanopart. Res. 12, 1589 (2010).
H. Andrianiaina, L. C. Razanamahry, and J. Sackey, et al., Mater Today–Proc. (2020, in press).
V. Chabot, D. Higgins, A. Yu, et al., Energy Environ Sci. 7, 564 (2014).
W. S. Hummers, Jr. and R. E. Offeman, J. Am. Chem. Soc. 80, 1339 (1958).
F. Y. Ban, S. R. Majid, N. M. Huang, and H. N. Lim, Int. J. Electrochem. Sci. 7, 4345 (2012).
S. Bykkam and K. Rao, Int. J. Adv. Biotechnol. 4, 142 (2013).
M. Agharkar, S. Kochrekar, S. Hidouri, and M. A. Azeez, Mater. Res. Bull. 59, 323 (2014).
H. Saleem, M. Haneef, and H. Y. Abbasi, Mater. Chem. Phys. 204, 1 (2018).
L. Stobinski et al., J. Electron Spectrosc. Relat. Phenom. 195, 145 (2014).
R. Singh and T. Jawaid, Pharmacogn. J. 4, 1 (2012).
I. Ali et al., Environ. Int. 127, 160 (2019).
S. Chen et al., Ecotoxicol. Environ. Safety 163, 594 (2018).
A. García-Mira Ferrari, C. W. Foster, and P. J. Kelly, et al., Biosensors 8, 1 (2018).
R. Inchulkar Shrikant, K. Yuvraj, S. Chauhan Nagendra, et al., Arch Pharm. Pract. 1, 81 (2019).
Y. Saito, P. Verma, K. Masui, et al., J. Raman Spectrosc. 40, 1434 (2009).
Y. Liu, Q. Ouyang, H. Li, et al., J. Agric. Food Chem. 66, 6188 (2018).
F. J. Akinseye, Int. J. Adv. Res. Biol. Sci. 4, 164 (2017).
S. F. Oliveira, G. Bisker, N. A. Bakh, et al., Carbon 95, 767 (2015).
B. Manoj, Res. J. Biotechnol. 8, 49 (2013).
T. Riya, U. Jyothi, N. V. Aparna, et al., Appl. Nanosci. 10, 4207 (2020).
B. Manoj, Asian J. Chem. 26, 4553 (2014).
M. Kumar and Y. Ando, Diamond Relat. Mater. 12, 1845–1850 (2003).
S. Claramunt, A. Varea, D. López-Díaz, et al., J. Phys. Chem. C 119, 10123 (2015).
S. Eigler, C. Dotzer, and A. Hirsch, Carbon 50, 3666 (2012).
S. Grimm, M. Schweiger, S. Eigler, and J. Zaumseil, J. Phys. Chem. C 120, 3036 (2016).
F. Paquin, J. Rivnay, A. Salleo, et al., J. Mater. Chem. C 3, 10715 (2015).
B. Manoj, J. Environ. Res. 6, 653 (2012).
A. N. Mohan and B. Manoj, Mater. Chem. Phys. 232, 137 (2019).
A. Sadezky, H. Muckenhuber, H. Grothe, et al., Carbon 43, 1731 (2005).
A. V. Ramya, Anu N. Mohan and B. Manoj, Asian J. Chem. 28, 1501 (2016).
F. Tuinstra and J. L. Koenig, J. Chem. Phys. 53, 1126 (1970).
A. M. Raj and M. Balachandran, Energ. Fuel 34, 13291 (2020).
R. A. Venkatesan and M. Balachandran, Environ. Sci. Pollut. Res. 27, 43845 (2020).
V. Mututu, A. K. Sunitha, R. Thomas, et al., Int. J. Electrochem. Sci. 14, 3752 (2019).
A. N. Mohan and B. Manoj, New J. Chem. 43, 13681 (2019).
A. N. Mohan and B. Manoj, Chem. Eur. J. 26, 8105 (2020).
B. Manoj and A. G. Kunjomana, IOP Conf. Ser.: Mater. Sci. Eng. 73, 012096 (2015).
S. Ijima, Nature (London, U.K.) 345, 56 (1991).
B. Manoj, A. M. Raj, and G. T. Chirayil, Sci. Rep. 7, 1 (2017).
K. Krishnamoorthy, M. Veerapandian, K. Yun, and S. Kim, Carbon 53, 38 (2012).
ACKNOWLEDGMENTS
The authors are thankful to the Centre for Research, CHRIST (deemed to be University), Bengaluru, India, for providing the necessary facilities to conduct this research. The authors also acknowledge CeNSE IISc, Bengaluru, IUCNN MG University, Kottayam and SAIF, CUSAT, Cochin for the help and facilities provided for the characterization of the samples.
Funding
This research did not receive any external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Ramya, A.V., Joseph, N. & Balachandran, M. Facile Synthesis of Few-Layer Graphene Oxide from Cinnamomum camphora . Nanotechnol Russia 16, 183–187 (2021). https://doi.org/10.1134/S2635167621020130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2635167621020130