Abstract—It has long been recognized that parabiosis and paranecrosis are two close cytological theories that have demonstrated the intermediate state of the cell between life and death from various scientific positions. However, they have not previously been shown by anyone at the same time on the same object. This became the goal of our electron microscopic work. Active and non-excitable membranes of nerve and glial cells under pessimal inhibition were studied. The main sign of paranecrosis was considered to be denaturation and aggregation of membrane protein, manifested itself in a decrease in its degree of dispersion and dehydration. Parabiosis was caused by the pessimal frequency of electroactivation of the sympathetic ganglion of white rats. As a result, the axolemma turned into a thick membrane, reinforced with a fringe and the appearance of desmosomes. Protein adherence appeared on the inner side of the neurolemma in the form of pyramids, which, by retracting, distorted the membrane. Pyramid-like loose aggregates of intermembrane protein were formed in its bends on the outer sides of the glial and axolemm membranes, which, merging, turned into a kind of hourglass and septa. The septa were localized in the intercellular slits of axons and glia and often crossed both membranes. In chemical synapses, the shell of dendrites turned out to be denser than that of presynaptic axons. The process of protein aggregation and retraction locally narrows the intercellular axo-axonal and axo-glial cleft. Gap and tight junctions (GJ and TJ) are formed. In this way, for the first time we obtained the experimental formation of intercellular membrane contacts that correspond to all morphological features. All reactive changes that occur de novo are considered as one reversible process of denaturation and aggregation of the mass of intrinsic and near-membrane proteins developed under the influence of frequency electrical stimulation. The pulse of the drug is restored within minutes. It is assumed that the revealed changes, paranecrosis, are a morphological manifestation of parabiosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The membrane is a thin lipid bilayer (70 nm) with membrane-embedded and peripheral proteins, which form a single inseparable tangle of protein molecules. Active neuronal and glial non-excitable membranes are interconnected. They are divided only for scientific and educational purposes. The membranes are very dynamic. Membrane proteins can move between lipids, rotate and diffuse between layers. The binding role of lipids in the membrane is played by the interaction of hydrophobic fatty acids. Most membrane water is associated with proteins. Half the weight of membranes is protein. The study of morphological constructions and the role of denaturation and aggregation of perimembrane proteins during electrical stimulation to pessimal inhibition is the aim of this study. We aim to reveal structural changes in the nervous system (paranecrosis) in parabiosis.
It is known that electrical activity enhances the regeneration of damaged brain nuclei (Nekhendzy et al., 2006; Jiang et al., 2012). Subthreshold stimulation of fiber networks from the premotor areas is used to study cortico-subcortical connections (Herbet et al., 2013; Schucht et al., 2013). Various modifications of electrical stimulation are widely used in the treatment of epilepsy (Xu et al., 2016; Mardani et al., 2018) and recovery of the spinal cord after injuries (Rath et al., 2018; Calvert et al., 2019; Gerasimenko et al., 2019). Unfortunately, we were unable to find any data on the morphology of the nervous system during normal or pessimal electroactivation. Most studies of the effect of electrical stimulation on the nervous system consist of the study of chemical synapses, the size of buds and soma, the spread of synaptic vesicles, and the theory of synaptic circulation of mediator vesicles.
In the body almost all tissue cells are in contact with each other. Adjacent membranes are necessarily interconnected chemically and physically. Consequently, multiple detailed descriptions of single cell membranes are largely incomplete or even defective (Finkelstein and Ptitsyn, 2012). There is a clear need to study adjacent neuron-glial membrane interactions in various living conditions. The aim of the study was to find out whether the nervous and glial systems have any morphological equivalents during electrical stimulation and how the reactions of active axon membranes and non-excitable glial membranes to the impact of frequency impulses differ. Data on the protein environment and reactions of adjacent neuronal glial membranes are insufficient. There are no complete data on intercellular structural relationships between neurocytes and gliocytes. Although structures such as additional loose pyramidal protrusions, bridges, septa, and fringes can affect neuron excitability (Ogawa and Rasband, 2008).
MATERIALS AND METHODS
The object of the study were the truncus sympathicus of 10 white Wistar rats. The experiments were carried out in accordance with the requirements of the Council of the European Community (86/609/EEC) 1986 and the recommendations of the ethical committee of the Pavlov Institute of Physiology. Russian Academy of Sciences (protocol No. 02/02 dated February 2, 2022). After dissection of the truncus sympathicus, a single lumbar ganglion was exposed. Its interganglionic branches were placed in fixed glass pipettes filled with Ringer’s solution. Excitation was carried out with an ESU-1 electrical stimulator (USSR). The current was drawn from the connectors using isolated non-polarizable silver chloride electrodes and an amplifier. The response was recorded on an oscilloscope. The truncus sympathicus preparation was stimulated to achieve the pessimum frequency. The preparation was stimulated at a frequency of 150 pulses/s (1–2 V, 0.2 ms). After the experiment, the impulse appeared at the ganglion at 9–12 min. After cessation of stimulation, action potentials (AP) recovered within minutes. After the electrophysiological experiment, the lumbar ganglion was fixed for 1 h in a 2.5% solution of glutaraldehyde (Acros Organics, USA) and 4% paraformaldehyde, prepared in 0.1 M cacodylate buffer pH 7.4 (Sigma, Israel) at 4°C. Further fixation was carried out in a 1% solution of chilled osmium tetroxide (Sigma-Aldrich, Germany). After dehydration in ethanol solutions, the material was poured into a mixture of araldites (Fluka, Switzerland). Ultrathin sections were prepared on a Leica Microsystems ultratome (Austria). Viewing and photography were carried out using an FEI Tecnai G2 Spirit BioTWIN electron microscope (Netherlands) at a voltage of 80 kV. It is well known only bilayer phospholipid membranes and denatured proteins that form internal and near-membrane structures can usually be impregnated and contrast well in electron microscopy of the nervous system (Gayer, 1974; Mironov et al., 1994). This allows, even in the absence of histochemistry, the interpretation of certain morphological changes such as protein denaturation and aggregation during electrical stimulation.
Histological methods of fixation are designed to study the normal structure of organs, tissues and cells. When structures change, histological methods should register the effect according to the final result. Histological fixation is not intended to study the processes and dynamics of structures. During denaturation and aggregation, the known natural properties of proteins are taken into account, such as retraction of aggregates and an increase in optical density (visibility of the protein) during its accumulation and fixation. A morphological study of a known physiological process suggests a hypothetical connection between the variants of structures as their dynamics.
In the Materials and Methods section, it is necessary to make a reservation that all the clear and solid structures presented in the article, fixed in the preparation, are semi-liquid, gel-like formations in dynamics. Peri-membrane accumulations of proteins, which form new additional morphological structures in experiments, will henceforth be referred to as “fringes” by the authors. Using the term “parabiosis,” N.E. Vvedensky had in mind significant intravital changes in the neuron (para—Greek.—“near”). In English-language journals, the authors unanimously use the term “parabiosis” as an experimental surgical connection of the vascular systems of two animals (Conese et al., 2017).
RESULTS
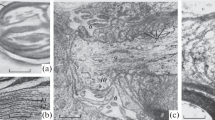
In response to electrical stimulation, both the active and non-excitable membranes of the ganglion truncus simpathicus change, but not simultaneously. After pessimal frequency stimulation axons were wider (with thick membranes) than gliocyte membranes (Fig. 1). The general expansion of the axolemma concerns large areas, but this is not a total thickening. It is noteworthy that this observation corresponds to the well-known statement about the accumulation of sodium ions on the inside of the axolemma during activation. The thickness of the axolemma increases due to the membranous sections of the axoplasm (fringe). Such a membrane is also a characteristic feature of the first section of the axon of the neuron—the spike generator. It can be concluded that these morphological and physiological phenomena are possibly interrelated. This appearance of thick membranes in normal conditions corresponds to those axolemmas that often deal with electrical AP. Sometimes this layer of fringe forms structures like small protein pyramids apex inward towards the axoplasm and resembles the thick axoplasm of Ranvier’s nodes, the one-sided desmosome, the chemical synapse specialization membrane, and the desmosome of the borders of cardiac myocytes, i.e., the membranes that often deal with electrical impulses. Apparently, following the thickening of axon membranes, a local, more intense protein membrane, the desmosome, appears in our experiment (Fig. 2). Such structures on a thick membrane can be unilateral and bilateral. At the same time, the intermembrane gap may have transverse loose accumulations of protein (Fig. 2a). It is important to note that in our experiment we are talking about the same morphological process during drug activation. During the frequency pessimum, denaturation of membrane proteins develops, which is consistent with an increase in adhesion and the appearance of axonal membrane protein aggregates.
Comparison of active and non-excitable rat sympathetic trunk ganglion membranes after activation. (а) Аctive and non-excitable membranes are normal; (b) thick active membrane; (c) fringe expands the axolemma; 1—thick axolemma; 2—thin gliolemma; 3—connections of the inner sheets of gliolemmas; arrows, cone-shaped and other fringe deposits under the axon membrane; A—axon; G—glia, here and in Figs. 2–5, 7. Electron microscopy. SW. 26 000.
Aggregation of moving protein masses is always by retraction. Therefore, when a single desmosome is sharply expressed on a thick axolemma, it begins to bend the membrane, forming an invagination similar to endocytosis (Fig. 3a). This often occurs in the presence of an adhesive bond between the axolemma and gliolemma or in the absence of the latter. It differs from endocytosis only by the attachment of a compacted fringe to the axolemma not from the outside, but from the inside of the membrane. But gradually, protein aggregates appear in the form of triangular shadows and inside the intercellular glio-axonal spaces (Fig. 3a, 2) outside the gliolemma. They appear to begin to grow from the glial membrane. Moreover, the thickness of the gliolemma also increases locally. This is combined with the manifestation in the intercellular gap of aggregates full of cross bridges (Fig. 4), bridges, and septate junctions of denatured protein. Obviously, glia are also involved in the process of axoplasm lability. Different forms of these bridges are visible, from barely noticeable shadow structures of the gliolemma and intercellular cone-shaped aggregates on opposite sides of the membranes (hourglass) to connections and the formation of clear rectangular septa (Fig. 4d). Next to the groups of septa, there are multiple, invaginations of the axolemma, that are similar to endocytosis, which indicate the continuation of the same process of axolemma change, despite the many manifestations of protein aggregation.
Endocytosis with glia inside and without it. (a, d) Cone-shaped fringe with endocytosis; (b, c) curvature of the axolemma in the region of the unilateral desmosome; 1—cone-shaped protein aggregates on the inner side of the axolemma; 2—developing cone-shaped aggregation inside and outside the intercellular glio-axonal gap; 3—thick membrane 4—pyramidal cone-shaped curvature of the axoplasm; arrows, cone-shaped fringe on the cytoplasmic side of the axolemma. Electron microscopy. SW: (а) 35 000; (b, c)100 000.
Neuroendocytosis and membrane septa during frequency electroactivation. (a) Desmosome and neuroendocytosis appear simultaneously on the same axolemma; (b) intermembrane septa during electrical stimulation; (c) cone-shaped aggregates of invaginations of the axolemma inside the cell body; (d) bridges at different stages of formation from “cones” to “hourglasses” and rectangular intermembrane bridges; 1—cone-shaped intracytoplasmic protrusions of the fringe; 2—shadow of a forming septum; 3—neuroendocytosis. Electron microscopy. SW: (а) 18 000; (b) 35 000; (с) 28 000; (d) 40 000.
Some septa have a certain order (Fig. 5). Protein osmiophilic aggregates are clearly combined with enlightenments, with contrasting white spaces devoid of protein. Perhaps this is a rupture of a long dense protein impregnated intermembrane strand into single fragments with retraction. But only some septa are built in this way, and most of them have irregularities in the length of the gaps between the septa and different OsO4 impregnation densities (Fig. 5) this distinguishes them from natural, “normal,” permanent septa described by other authors. Our experiment suggests that the septa, which are constantly found in invertebrates and in vertebrates in other parts of the brain, in the nodes of Ranvier, may also be temporary structures, not permanent, as many authors suggest. By form, it appears to be possible to reveal the dynamics of their construction. It is important to note that protein aggregations are able not only to connect the glial and axonal membranes of nerve fibers, they are able to penetrate through both adjacent membranes, neuronal and glial(Fig. 5c). That is, glia is also involved in this process of frequency pessimum formation.
Intercellular septa after pessimal stimulation according to Vvedensky. (a) Membrane septa of different lengths and gliolemma of equal thickness with axolemma; (b) septa of various shapes; (c) septa crossing the neuronal and glial membranes; 1—glio-axonal superlong septum; arrows are triangular aggregates. Electron microscopy. SW: (а) 40 000; (b) 24 000; (с) 30 000.
In a single process of protein denaturation and aggregation, structural changes in the membranes of chemical synapses should also be noted. They are also intensively connected to glia by the same bridges during frequent electrical stimulation. Pyramid-like axonal accumulations of protein are especially noticeable in presynaptic specializations (Fig. 6), although before stimulation, fringes prevailed in the dendrite specialization in control. Postsynaptic dendrites have a large membrane thickness, as do axons. The synaptic specialization of the dendrite is very dense. The intercellular gap is narrowed due to the accumulation of many protein bridges. The septa here are very thin, but there are many of them.
In addition to local septa, preparations also show intercellular clefts occupied by osmiophilic black (Fig. 7) or simply elongated large intercellular spaces. In some of these structures, both adjacent membranes are narrowed and have the same thickness. They are no different from SC or PC: 7 clear lines (Fig. 7d) or 5 if the fringe masks the membranes (Fig. 7c), or 3 lines if both membranes are occupied by osmium black, as well as aqueous intermembrane expansions (Fig. 7a). During aggregation and denaturation, proteins are known to release a significant amount of free water. Dehydration is an indispensable condition for paranecrosis. The expansion of the gap after the formation of the GJ is a sign of released water of the protein on the sides of the contact.
Narrowed intercellular gaps filled with aggregated proteins (gap and tight junctions) and extensions on the sides of the contacts. (a) Gap junction and water expansion of the intercellular gap on the sides of the gap junction; (b) axo-glial gap contact; (c) axo-axonal tight contact; (d) axo-axon gap contact; 1—triangular intercellular protein aggregates; 2—water expansion of the intermembrane gap; arrows—slot-like and tight contacts; A—axon; G—glia. Electron microscopy. Magnification: (а, d) 38 000; (b, с) 24 000.
Axonal-glial contacts can be occupied by a continuous impregnating protein mass and some convergence of neighboring constricted membranes upon activation. It should be noted that in Fig. 7, intercellular axo-glial GJs or PCs were found by us during pessimal stimulation of autonomic ganglia for the first time. In our experiments, they occupy small areas of membranes, but much larger than septa. The intercellular gaps narrow, forming GJ and PC. This is the first experimental production of gap axo-glial intermembrane junctions while maintaining glia, that is, between the active and non-excitable membranes. If narrowing does not occur, such intercellular gaps filled with osmium black are usually called continuous junctions by morphologists. Apparently, they represent stages in the formation of gap junctions.
DISCUSSION
Describing histological fixation as excessive paranecrotic irritation of proteins, leading to death, D.N. Nasonov and L.Ya. Alexandrov (1940) assumed that these two states could not be separated during fixation, and that it was realistic to obtain and fix only paranecrotic changes. It is possible that this was one of the reasons for the lack of morphological studies of parabiosis. However, it turned out that a number of fixatives, due to their physical abilities, such as OsO4, glutaraldehyde and some other substances are able to create a “thin sediment” and rapid hardening of the drug, preventing the redistribution of proteins, ahead of “colloidal changes in the protoplasm” (Gayer, 1974; Mironov et al., 1994). Thus, electron microscopy gained access to paranecrosis and parabiosis, although this, of course, does not exclude the appearance of small artifacts in preparations during fixation and beyond the resolution of ultramicroscopes.
It is known that during frequency electrical stimulation according to Vvedensky and the onset of pessimal frequency, the sympathetic ganglion of the rat undergoes a wave of all-round depolarization. The excitable membrane plays the role of an AP carrier, while the gliolemma is, as it were, inactive, but our experiments also show its participation in the frequency pessimum. First of all, attention is drawn to the denaturation and aggregation of mobile proteins. The circummembrane proteins of the axolemma gradually form its thickness and pyramid-like aggregates on the cytoplasmic side of the neurite membrane, on the same inner side of the membrane where Na+ ions accumulate with frequency activation. In this way additional structures appear. Protein aggregates in the axon, as well as in glia, end with connections of membrane adhesions in the form of rectangular septa that pierce both membranes. The appearance of new structures is, perhaps, the first case when, morphologically, it is possible to obtain not a change in the experiment, but the de novo construction of protein formations.
The value of the fundamental research carried out by N.E. Vvedensky undoubtedly plays an important role in modern neurophysiology. Even in the first studies of Professor M.A. Masson (Masson, 1837) in the auto-experiments of pain sensations during frequency stimulation, a surprising phenomenon was discovered—the cessation of pain at high frequencies of activation. This phenomenon is currently widely used in human and animal medicine (Fisher et al., 2015; Ling et al., 2019; Janjua et al., 2021). His research didn’t stop there. With continuous activation, more precisely after it, N.E. Vvedensky discovered indistinct and incomprehensible noises in the stethoscope (Vvedensky, 1952). He understood that this was a nervous impulse, but to explain it with a developing pessimal frequency in the 19th century was impossible. Nowadays, with the help of oscilloscopes, it is possible to show this unique, mysterious impulse after blocking nerve conduction. The appearance of new electrical synapses that we discovered during electrical stimulation (Fig. 7) is a possible explanation for this phenomenon. Deeper study of the frequency pessimum may yet yield additional results.
The morphological data obtained also have general biological significance. This concerns the unexpected appearance in the nervous system during electrical stimulation of tertiary and quaternary structures of proteins (septum, GJ, etc.). Septa are organelles long described in cytology. Now they are recognized as independent and permanent cellular structures, the origin and nature of which are unknown (Rice et al., 2021). They are described in invertebrates and vertebrates between epithelial cells, in the nervous system, and also in humans in pathology. Septa are even considered by many as an exclusive feature of some invertebrate species. They are described in daphnia, cockroaches, crayfish, beetles, termites, caterpillars, and worms, mainly between the epitheliocytes and the cuticle of the animal. They are also described in the epithelium of organs, for example, salivary glands. We analyzed 52 studies, and all of them consider these structures to be permanent formations of a normal cell, but our experience indicates their temporary, possibly alterated, formation.
These septa have one thing in common. They necessarily occur together with other cellular organelles, such as desmosomes, gap, tight and continuous junctions, as if they arose for one reason, at the same time, that is, just like in our case, after electrical stimulation. The only thing that we have not seen in the articles of other authors is structures similar to endocytosis, which are found in large numbers during pessimal electrical stimulation. Recently, a large number of articles have appeared on the function of the paranodal septa intercept of Ranvier (Vallat et al., 2017; Manso et al., 2019; Faivre-Sarrailh, 2020). Unfortunately, all the ideas presented in the articles about the function of these septa are expressed in the form of unfounded assumptions. Based on in vivo studies of intercepts, we believe that the variability of intercept septa are also an indicator of their temporary appearance associated with the experimental conditions (Sotnikov and Revenko, 2022).
Permeability of phospholipid artificial membranes generally appears only when peptides and proteins are introduced into the membrane from an external solution (Khodorov, 1975). It has long been known that proteins destabilize membranes by forming small and large pores. The appearance of newly formed gap junctions in our preparations allows us to discuss the pore formation of proteins during electrical stimulation in a new way. Pore-forming proteins come from many unrelated families. They refer to ion pores and ion channels. It is known that they tend to change the size of perforations from 0.7 to 40 nm (Omersa et al., 2019). It has recently been shown that such proteins are able to pass through bacteria (Cosentino and García-Sáez, 2018) and intracellular parasites (Marchioretto et al., 2013; Guerra and Carruthers, 2017). It is possible that a change in protein conformation, as we have seen during electrical stimulation, also occurs with other membrane proteins. The variable diameter of the pores (channels) is achieved either by incorporating a different number of protein subunits or by stretching the area of the liquid membrane along the perimeter of the channels. That is, the pores may be not only ionic, but also larger (Laube et al., 1996). During aggregation during electrical stimulation of fibers and bodies of neurons, a new contact of mobile proteins is possible, which means that an increase in membrane permeability is also possible. Molecular modeling suggests that pore amyloid subunits can assemble into 24-dimensional pore-forming structures (Kagan, 2012). Potassium channels with two pores are widely distributed among neurons and glia (Luo et al., 2021).
It is known that during the fusion of intramembrane connexin proteins (numerous different Cx), GJ channels are formed. We hypothesize that during the aggregation of denatured proteins during electrical stimulation, the processes of combining proteins, which are always enriched in water caves and channels with high electrical conductivity of membranes, are also possible. I wonder if the septa disappear after the restoration of the activity of the drug? This remains to be seen in the future. Neuronal septa, as it turned out, also appear when ganglia are treated with 0.4% pronase (Sotnikov, 2019). This means that their appearance confirms the nonspecific properties of the protein. Characterizing parabiosis and paranecrosis, D.N. Nasonov, V.Ya. Alexandrov and N.E. Vvedensky also emphasized the nonspecific nature of paranecrosis and parabiosis of these phenomena. The protein structures noted in the article and the mixing of water during parabiosis, can apparently be considered a manifestation of paranecrosis.
CONCLUSIONS
These results confirm that the electrophysiological process, frequency pessimum is accompanied by dehydration, denaturation of the near-membrane protein, and also has an abundant morphological equivalent: dense membranes, desmosomes, endocytosis, near-membrane protein aggregates, septa and gap junctions.
REFERENCES
Calvert, J.S., Grahn, P.J., Strommen, J.A., et al., Electrophysiological guidance of epidural electrode array implantation over the human lumbosacral spinal cord to enable motor function after chronic paralysis, J. Neurotrauma, 2019, vol. 36, no. 9, pp. 1451–1460. https://doi.org/10.1089/neu.2018.5921
Conese, M., Carbone, A., Beccia, E., and Angiolillo, A., The fountain of youth: A tale of parabiosis, stem cells, and rejuvenation, Open Med. (Warsaw), 2017, vol. 12, pp. 376–383. https://doi.org/10.1515/med-2017-0053
Cosentino, K. and García-Sáez, A.J., Mim through mom: The awakening of Bax and Bak pores, EMBO J., 2018, vol. 37, no. 17, p. e100340. https://doi.org/10.15252/embj.2018100340
Faivre-Sarrailh, C., Molecular organization and function of vertebrate septate-like junctions, Biochim. Biophys. Acta, Biomembr., 2020, vol. 1862, no. 5, p. 183211. https://doi.org/10.1016/j.bbamem.2020.183211
Finkel’shtein, A.V. and Ptitsyn, O.B., Fizika belka (Protein Physics), Moscow: KDU, 2012.
Fisher, K.M., Jillani, N.E., Oluoch, G.O., and Baker, S.N., Blocking central pathways in the primate motor system using high-frequency sinusoidal current, J. Neurophysiol., 2015, vol. 113, no. 5, pp. 1670–1680. https://doi.org/10.1152/jn.00347.2014
Geyer, G., Ultrahistochemie, Stuttgart: Gustav Fischer, 1972.
Gerasimenko, Y., Preston, C., Zhong, H., et al., Rostral lumbar segments are the key controllers of hindlimb locomotor rhythmicity in the adult spinal rat, J. Neurophysiol., 2019, vol. 122, no. 2, pp. 585–600. https://doi.org/10.1152/jn.00810.2018
Guerra, A.J. and Carruthers, V.B., Structural features of apicomplexan pore-forming proteins and their roles in parasite cell traversal and egress, Toxins (Basel), 2017, vol. 9, no. 9, p. 265. https://doi.org/toxins9090265
Herbet, G., Lafargue, G., Bonnetblanc, F., et al., Is the right frontal cortex really crucial in the mentalizing network? A longitudinal study in patients with a slow-growing lesion, Cortex, 2013, vol. 49, no. 10, pp. 2711–2727. https://doi.org/10.1016/j.cortex.2013.08.003
Janjua, T.A.M., Nielsen, T.G.N.D.S., Andreis, F.R., et al., The effect of peripheral high-frequency electrical stimulation on the primary somatosensory cortex in pigs, IBRO Neurosci. Rep., 2021, vol. 11, pp. 112–118. https://doi.org/10.1016/j.ibneur.2021.08.004
Jiang, F., Yin, H., and Qin, X., Fastigial nucleus electrostimulation reduces the expression of repulsive guidance molecule, improves axonal growth following focal cerebral ischemia, Neurochem. Res., 2012, vol. 7, no. 9, pp. 1906–1914. https://doi.org/10.1007/s11064-012-0809-y
Kagan, B.L., Membrane pores in the pathogenesis of neurodegenerative disease, Prog. Mol. Biol. Transl. Sci., 2012, vol. 107, pp. 295–325. https://doi.org/10.1016/B978-0-12-385883-2.00001-1
Khodorov, B.I., Obshchaya fiziologiya vozbudimykh membran. Rukovodstvo po fiziologii (General Physiology of Excitable Membranes. Physiology Guide), Moscow: Nauka, 1975.
Laube, G., Röper, J., Pitt, J.C., et al., Ultrastructural localization of Shaker-related potassium channel subunits and synapse-associated protein 90 to septate-like junctions in rat cerebellar Pinceaux, Brain Res. Mol. Brain Res., 1996, vol. 42, no. 1, pp. 51–61. https://doi.org/10.1016/s0169-328x(96)00120-9
Ling, D., Luo, J., Wang, M., et al., Kilohertz high-frequency alternating current blocks nerve conduction without causing nerve damage in rats, Ann. Transl. Med., 2019, vol. 7, no. 22, p. 661. https://doi.org/10.21037/atm.2019.10.36
Luo, Y., Huang, L., Liao, P., and Jiang, R., Contribution of neuronal and glial two-pore-domain potassium channels in health and neurological disorders, Neural Plast., 2021, vol. 2021, p. 8643129. https://doi.org/10.1155/2021/8643129
Manso, C., Querol, L., Lleixa, C., et al., Anti-neurofascin-155 IgG4 antibodies prevent paranodal complex formation in vivo, J. Clin. Invest., 2019, vol. 129, no. 6, pp. 2222–2236. https://doi.org/10.1172/JCI124694
Marchioretto, M., Podobnik, M., Dalla Serra, M., and Anderluh, G., What planar lipid membranes tell us about the pore-forming activity of cholesterol-dependent cytolysins, Biophys. Chem., 2013, vol. 182, pp. 64–70. https://doi.org/10.1016/j.bpc.2013.06.015
Mardani, P., Oryan, S., Sarihi, A., et al., ERK activation is required for the antiepileptogenic effect of low frequency electrical stimulation in kindled rats, Brain Res. Bull., 2018, vol. 14, pp. 132–139. https://doi.org/10.1016/j.brainresbull.2018.04.013
Masson, M.A., De l’induction d’un courant sur lui-même, Ann. Chim. Phys., 1837, vol. 66, pp. 5–36.
Mironov, A.A., Komissarchik, Ya.Yu., and Mironov, V.A., Metody elektronnoi mikroskopii v biologii i meditsine (Methods of Electron Microscopy in Biology and Medicine), St. Petersburg: Nauka, 1994.
Nasonov, D.N. and Aleksandrov, V.Ya., Reaktsiya zhivogo veshchestva na vneshnie vozdeistviya (The Reaction of Living Matter to External Influences), Moscow: Akad. Nauk SSSR, 1940.
Nekhendzy, V., Davies, M.F., Lemmens, H.J., and Maze, M., The role of the craniospinal nerves in mediating the antinociceptive effect of transcranial electrostimulation in the rat, Anesth. Analg., 2006, vol. 102, no. 6, pp. 1775–1780. https://doi.org/10.1213/01.ANE.0000219588.25375.36
Ogawa, Y. and Rasband, M.N., The functional organization and assembly of the axon initial segment, Curr. Opin. Neurobiol., 2008, vol. 18, no. 3, pp. 307–313. https://doi.org/10.1016/j.conb.2008.08.008
Omersa, N., Podobnik, M., and Anderluh, G., Inhibition of pore-forming proteins, Toxins (Basel), 2019, vol. 11, no. 9, p. 545. https://doi.org/10.3390/toxins11090545
Rath, M., Vette, A.H., Ramasubramaniam, S., et al., Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury, J. Neurotrauma, 2018, vol. 35, no. 21, pp. 2540–2553. https://doi.org/10.1089/neu.2017.5584
Rice, C., De, O., Alhadyian, H., et al., Expanding the junction: New insights into non-occluding roles for septate junction proteins during development, J. Dev. Biol., 2021, vol. 9, no. 1, p. 11. https://doi.org/10.3390/jdb9010011
Schucht, P., Moritz-Gasser, S., Herbet, G., et al., Subcortical electrostimulation to identify network subserving motor control, Hum. Brain Mapp., 2013, vol. 34, no. 11, pp. 3023–3030. https://doi.org/10.1002/hbm.22122
Sotnikov, O.S., Ob”edinennaya neironno-retikulyarnaya teoriya (Unified Neuronal-Reticular Theory), St. Petersburg: Nauka, 2019.
Sotnikov, O.S. and Revenko, S.V., Physiology of Ranvier nodes in living myelinated nerve fibers, Biochem. (Moscow), Suppl. Ser., 2022, vol. 16, no. 3, pp. 224–235.
Vallat, J.M., Yuki, N., Sekiguchi, K., et al., Paranodal lesions in chronic inflammatory demyelinating polyneuropathy associated with anti-Neurofascin 155 antibodies, Neuromuscular Disord., 2017, vol. 27, no. 3, pp. 290–293. https://doi.org/10.1016/j.nmd.2016.10.008
Vvedenskii, N.E., Polnoe sobranie sochinenii (Full Composition of Writings), Lenigrad: Izd. Leningr. Gos. Univ. im. A.A. Zhdanova, 1952, vol. 3, p. 84.
Xu, Z., Wang, Y., Chen, B., et al., Entorhinal principal neurons mediate brain-stimulation treatments for epilepsy, EBioMedicine, 2016, vol. 14, pp. 148–160. https://doi.org/10.1016/j.ebiom.2016.11.027
ACKNOWLEDGMENTS
I express my deep gratitude to Dmitry Anatolyevich Mekhilyainen for his help in preparing the article.
Funding
The work was supported by the State Program 47 SE “Scientific technological development of the Russian Federation” (2019–2030), topic no. 0134-2019-0001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The author declares that he has no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Sotnikov, O.S. Changes in Active and Non-Excitable Adjacent Nerve Membranes after Electroactivation. Biol Bull Rev 13, 104–111 (2023). https://doi.org/10.1134/S2079086423020081
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086423020081