Abstract
Age-dependent neurodegenerative diseases, including Parkinson’s disease, are characterized by a group of pathomorphological signs, such as neuronal death in certain brain structures, gliosis, and iron accumulation. However, literature data confirm that these signs can also be observed in normal (physiological) aging. The aim of the study is to evaluate qualitative and quantitative morphochemical changes in neurons and neuroglia in the human striatum in physiological aging, as well as changes in the localization of iron(II) compounds. It is found that neuronal cell bodies become significantly smaller in size at older ages, as compared with mature years, although the density of their distribution does not differ; the distribution density of total neuroglia and astrocytes, however, becomes higher at older ages. In addition, accumulations of iron(II) compounds are diagnosed along the vascular walls and inside the cytoplasm of neurons and glial cells. The indicators of physiological aging obtained in this research may serve as a basis for comparative studies of neurodegenerative processes in normal and pathological aging and for the pathomorphological diagnosis of age-associated neurodegenerative diseases, including Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Aging is associated with significant disorders in the structure and functions of different brain regions and is one of the main risk factors for developing neurodegenerative diseases, such as Parkinson’s (PD) and Alzheimer’s diseases. Normal (physiological) aging and PD have some similar functional and neuroanatomical changes [9]. Development of bradykinesia, shuffling gait, involuntary motor activity, and disorders in the coordination of movements in both PD and normal aging are thought to be associated with disorders of dopaminergic regulation in the nigrostriatal system [3, 10]. Pathomorphological changes, which are common during physiological (normal) aging and the aging associated with various neurodegenerative diseases, such as PD, also include the neurodegeneration of the nigrostriatal system, the pathological protein aggregation, and the accumulation of iron and lipofuscin [14, 19, 24]. Changes in the amount and morphology of glial elements play a significant, although not completely clear, role in the pathogenesis of both PD and normal aging [17]. For example, astrocytes not only fulfill the trophic function and are one of the elements of the blood-brain barrier, but also actively participate in the formation and support of synapse functions [8]. According to different data, they may either contribute to [12] the damage of neurons or protect [6, 16] them during a neurodegenerative process. Considerable attention has been given in the literature over the last years to the study of accumulation of iron compounds in basal nuclei during neurodegenerative processes. Iron ions participate in many reactions, and, therefore, disorders in their metabolism lead to oxidative stress, inflammation, and, as a result, neurodegeneration [13, 24]. According to magnetic resonance imaging (MRI) data, the iron content in the striatum increases in both PD [13, 24] and normal aging [11, 22], however, its localization in the human striatum tissue has not been described so far at the cell level. Despite the abundance of studies dedicated to pathomorphological changes in the substantia nigra and the striatum of PD patients and in Parkinsonism models, it is still unclear whether these changes are actually signs of pathological aging. Hence, it is especially important to study the processes taking place in the brain during normal aging.
The aim of the study was to evaluate qualitatively and quantitatively the morphochemical changes in neurons and neuroglia, as well as to determine the localization of iron (II) compounds in the human striatum in physiological aging.
MATERIALS AND METHODS
We studied the post mortem brain of 34- to 57‑year-old people (three men and two women) and older persons (two men and three women) who died at ages of 76 to 89 from diseases not associated with neurological and mental disorders. Autopsy material was taken 5–11 h after death and fixed with 4% formalin on a phosphate buffer (рН 7.2–7.4).
The autopsic brain specimens of 0.5 cm thickness containing the caudate nucleus and putamen were cut into frontal sections of 40 μm thickness, using a Series 1000 microtome (USA). One part of the sections were placed on slides and stained with either cresyl violet by the Nissl method or by Kluwer–Barrera Luxol fast blue. Another part of the sections was placed in a phosphate buffer (pH 7.2–7.4) and used for performing histochemical, immunohistochemical, and immunofluorescent reactions. Iron(II) compounds were detected on floating sections by the Perls method; cell nuclei were counterstained with cresyl violet or carmine. Astrocytes were localized immunohistochemically using mouse monoclonal antibodies against glial fibrillary acidic protein (GFAP, Sigma 1 : 200). 3,3-Diaminobenzidine with cobalt chloride was used as a chromogen. At least three sections were taken from each case for morphometry, and ten randomly selected fields of view were studied for each section, using a Leica DMLB light microscope with a digital photocamera and the Leica Qin software to analyze images. A NikonEclipse Ni–U luminescent microscope with a Nikon DS-Qi digital camera was used to visualize the immunofluorescent reaction. Neurons, as well as total neuroglia (total number of astrocytes and oligodendrocytes), were counted and the area of neuronal bodies was measured on the Nissl stained sections in the microscope field of view at 40× magnification. The glia-to-neuron ratio was defined as the ratio between the total number of gliocytes and the number of neurons. The density of the GFAP-positive astroglia and the areas of astrocyte bodies were measured at 25× and 40× magnifications, respectively, on the GFAP-stained sections. The significance of differences between the compared parameters was determined by the nonparametric Mann–Whitney test.
RESULTS AND DISCUSSION
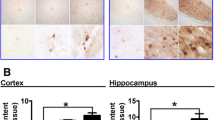
Neurons having the body area smaller than 200 μm2 were predominant in the striatum of the investigated age groups (95%), and their sizes were smaller at older ages, compared with mature ages: 129 ± 0.9 and 140.5 ± 1 μm2, respectively (Fig. 1а). No differences were detected in the density of neurons in the striatum of the examined age groups (Fig. 1b), but the density of neuroglia was almost one third higher at older ages than at mature ages (Fig. 1c), which was reflected in the ratio between neuroglia and neurons. The glia-to-neuron ratio at mature and older ages were, respectively, 2.2 and 2.8 (Fig. 1d). GFAP-positive astrocytes were predominantly localized near bundles of fibers, in the internal capsule area, and around large blood vessels. Fibrous astrocytes were identified inside bundles of fibers, and protoplasmic ones in the gray matter of the striatum. The staining intensity of the fibrous astrocytes, especially for older ages, was higher than that of the protoplasmic ones. The density of protoplasmic astrocytes was significantly higher on the portions of the sections not containing bundles of nerve fibers in the older age group compared to mature age (Fig. 2а). The arrangement of astrocytes preserved the domain nature, and their processes almost did not overlap (Fig. 2b). Three morphotypes of astrocytes were identified: with a small soma and numerous long fine processes (Fig. 2b, the long arrow), another large soma and short thick processes (Fig. 2b, the triangle arrow), and the transitional type (Fig. 2b, the short arrow). All types of astrocytes were identified in both older and mature age groups, but activated astrocytes with hypertrophic somata were more frequently related to older age (Fig. 2c). Lipofuscin aggregates were more frequently observed in older than in mature age groups in the cytoplasm of neurons (Fig. 1h) and some cells of astroglia (Fig. 2e). The content of iron(II) compounds (Figs. 3a and b) detected along the walls of vessels (Fig. 3b) and sometimes in the cytoplasm of neurons (Fig. 3c) and glial elements (Figs. 3d, 3e, 3f, and 3g) was higher at older than mature age (Figs. 3а, 3b).
Morphometric changes in the human striatum with aging. The size of neuron body (а); neuronal density (b); the density of total glia (c); glia-to-neuron ratio (d) in the old-age group (dark bar), compared to the mature one (white bar); neurons and glia in a mature person (e) and an old-aged person (f), the arrow indicates clusterization of oligodendrocytes along the blood vessel; a cluster of glial cells around the dead neuron (g) and lipofuscin in the cytoplasm of neurons (h); Nissl staining; *р < 0.05, Mann–Whitney test.
GFAP-positive astrocytes in the striatum in old age. (а) The cell density and (c) the size of the body of GFAP-positive astrocytes in the striatum in the mature group (white bar) and in the older age group (dark bar), *р < 0.05, Mann–Whitney test; (b) the distribution of GFAP-positive protoplasmic astrocytes in the striatal tissue: long arrows—astrocytes of the first (resting) morphotype with poorly stained somata and long thin processes; the arrowheads—astrocytes of the second (activated) morphotype with intensively stained large body and short thick processes; the short arrow—astrocyte of a transitional morphotype, immunohistochemical staining for GFAP; (d) GFAP-positive astrocyte of the resting morphotype (green), not containing lipofuscin in the cytoplasm (the arrowheads), lipofuscin autofluorescence in the neuron bodies (thick arrows); (e) GFAP-positive astrocyte of the activated morphotype with the hypertrophic soma and the aggregation of lipofuscin granules in the cytoplasm (the thin arrow), lipofuscin autofluorescence in the neuron bodies (thick arrows); immunofluorescence.
Localizations of iron(II) compounds in the human striatum at mature (а) and older ages (b to g). (а) Rare individual iron aggregates at a mature age; (b) aggregates of iron(II) compounds along a large blood vessel at an older age; (c) iron(II) in the cytoplasm of a neuron together with lipofuscin; (d) in microglia cells; (e) in an activated astrocyte; (f, g) in oligodendrocytes; staining by the Perls method with counterstaining by the Nissl method (c, d, and e) or carmine red (а, b, and f, and g).
Morphometric study has shown a significant decrease in the mean size of neuronal bodies at older, compared with mature, age, which confirms the cell atrophy and which characterizes not only basal nuclei with aging, but also the subcortical brain structures [1, 23]. However, the neuronal density in the human striatum did not differ between mature and older ages. Analogous results have also been obtained in animals [4]. Whereas some authors reported a decrease in the neuronal density in the human striatum, other researchers, on the contrary, noted that this indicator increased with aging, which was probably associated with a reduction in the total size of the striatum with aging [18]. The data on neurodegeneration in the striatum in Parkinsonism are also controversial [5].
The conducted study has shown gliosis in the striatum of old-aged people, which was manifested in the increased density of both the total glia and GFAP-positive astrocytes. Astrogliosis, in turn, was manifested in an increase in the density of GFAP-positive astrocytes, a higher intensity of staining observed in their bodies and processes, and changes in the morphology of astrocytes. The greater amount of GFAP-positive astrocytes more associated with older than mature age, may be due to an increase in the expression of this protein in cells, making them visible on immunohistochemically stained preparations, rather than an increase in the number of the cells themselves [20]. We identified three cell morphotypes among the GFAP- astrocytes. The first type of identified cells corresponds to resting astroglia described in the literature, whereas the second type corresponds to activated astroglia [15]. Activation of astroglia is seen in both normal aging [8, 15] and a variety of neurodegenerative diseases, including PD [7, 16]. A number of studies have shown that reactive astrocytes can perform the inflammatory, as well as anti-inflammatory, functions [21]. The absence of reduction in the neuronal density and the higher levels of GFAP expression shown by this study correspond to the suggestion that astrogliosis may be a factor protecting neurons from death during the development of a neurodegenerative process [6]. Our data correspond to the contemporary ideas that a reduction in the volume of brain structures is rather associated with involutive changes in the neuropil and the shrinkage of neuron cell bodies than with neuronal death [2, 23].
Lipofuscin has been found in the older age group not only in neurons, where it occupies a significant portion of the cytoplasm, but also in astroglial cells. Overloading with lipofuscin indicates disturbances in the processes of lipid degradation [19].
CONCLUSIONS
We have histochemically shown the accumulation of iron in the striatal tissue at older ages, which corresponds to the MRI data [11, 22]. It has been shown for the first time at the cell level that iron-containing inclusions are mainly localized along the walls of vessels, as well as inside the cytoplasm of neurons and glial cells. The iron accumulation in the striatal tissue contributes both to normal aging [11, 22] and to neurodegenerative diseases, including Parkinson’s disease [13, 24].
Thus, the obtained data on morphochemical changes in the striatum in normal aging provide a basis for the study of neurodegenerative processes and the confirmation of diagnosis of neurodegenerative diseases associated with aging, including Parkinson’s disease.
REFERENCES
Agapov, P.A., Bogolepova, I.N., and Malofeeva, L.I., Change of the size of neurons and density of neurons and glia of the 7 area of the women’s brain crease in the aging processes, Mezhdunar. Zh. Prikl. Fundam. Issled., 2017, no. 5-2, pp. 274–280.
Pal’tsyn, A.A. and Komissarova, S.V., Age-related changes of the brain, Patol. Fiziol. Eksp. Ter., 2015, vol. 59, no. 4, pp. 108–116.
Błaszczyk, J.W., Nigrostriatal interaction in the aging brain: new therapeutic target for Parkinson’s disease, Acta Neurobiol. Exp., 2017, vol. 77, no. 1, pp. 106–112.
Brasnjevic, I., Hof, P.R., Steinbusch, H.W.M., and Schmitz, C., Accumulation of nuclear DNA damage or neuron loss: Molecular basis for a new approach to understanding selective neuronal vulnerability in neurodegenerative diseases, DNA Repair, 2008, vol. 7, no. 7, pp. 1087–1097.
Bugiani, O., Perdelli, F., Salvarani, S., et al., Loss of striatal neurons in Parkinson’s disease: a cytometric study, Eur. Neurol., 1980, vol. 19, no. 5, pp. 339–344.
Bylicky, M.A., Mueller, G.P., and Day, R.M., Mechanisms of endogenous neuroprotective effects of astrocytes in brain injury, Oxid. Med. Cell. Longevity, 2018, vol. 2018, p. 16.
Charron, G., Doudnikoff, E., Canron, M.-H., et al., Astrocytosis in Parkinsonism: considering tripartite striatal synapses in physiopathology? Front. Aging Neurosci., 2014, vol. 6, pp. 258.
Clarke, L.E., Liddelow, S.A., Chakraborty, C., et al., Normal aging induces A1-like astrocyte reactivity, Proc. Natl. Acad. Sci. U.S.A., 2018, vol. 115, no. 8, pp. E1896–E1905.
Collier, T.J., Kanaan, N.M., and Kordower, J.H., Aging and Parkinson’s disease: Different sides of the same coin? Mov. Disord., 2017, vol. 32, no. 7, pp. 983–990.
Costa, K.M., The effects of aging on substantia nigra dopamine neurons, J. Neurosci., 2014, vol. 34, no. 46, pp. 15 133–15 134.
Daugherty, A.M. and Raz, N. Accumulation of iron in the putamen predicts its shrinkage in healthy older adults: a multi-occasion longitudinal study, NeuroImage, 2016, vol. 128, pp. 11–20.
Halliday, G.M. and Stevens, C.H., Glia: initiators and progressors of pathology in Parkinson’s disease, Mov. Disord., 2011, vol. 26, no. 1, pp. 6–17.
Hare, D., Ayton, S., Bush, A., and Lei, P., A delicate balance: iron metabolism and diseases of the brain, Front. Aging Neurosci., 2013, vol. 5, pp. 34.
Hirsch, E.C., Vyas, S., and Hunot, S., Neuroinflammation in Parkinson’s disease, Parkinsonism Relat. Disord., 2012, vol. 18, suppl. 1, pp. 210–212.
Jyothi, H.J., Vidyadhara, D.J., Mahadevan, A., et al., Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta, Neurobiol. Aging, 2015, vol. 36, no. 12, pp. 3321–3333.
Liu, B., Teschemacher, A.G., and Kasparov, S., Neuroprotective potential of astroglia, J. Neurosci. Res., 2017, vol. 95, no. 11, pp. 2126–2139.
Lynch, A.M., Murphy, K.J., Deighan, B.F., et al., The impact of glial activation in the aging brain, Aging Dis., 2010, vol. 1, no. 3, pp. 262–278.
Mann, D.M.A., Sense and Senility: The Neuropathology of the Aged Human Brain, Ch. 2 Pathological Changes in the Elderly Human Brain, Neuroscience Intelligence Unit Series, Boston: Springer-Verlag, 1997.
Moreno-García, A., Kun, A., Calero, O., et al., An overview of the role of lipofuscin in age-related neurodegeneration, Front. Neurosci., 2018, vol. 12, p. 464.
Sofroniew, M.V., Molecular dissection of reactive astrogliosis and glial scar formation, Trends Neurosci., 2009, vol. 32, pp. 638–647.
Sofroniew, M.V. and Vinters, H.V., Astrocytes: biology and pathology, Acta Neuropathol., 2010, vol. 119, pp. 7–35.
Steiger, T.K., Weiskopf, N., and Bunzeck, N., Iron level and myelin content in the ventral striatum predict memory performance in the aging brain, J. Neurosci., 2016, vol. 36, no. 12, pp. 3552–3558.
Thulborn, K., Lui, E., Guntin, J., et al., Quantitative sodium MR Imaging of the human brain at 9.4 tesla provides assessment of tissue sodium concentration and cell volume fraction during normal ageing, NMR Biomed., 2016, vol. 29, no. 2, pp. 137–143.
Zecca, L., Youdim, M.B., Riederer, P., et al., Iron, brain ageing and neurodegenerative disorders, Nat. Rev. Neurosci., 2004, vol. 5, pp. 863–873.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by N. Tarasyuk
Rights and permissions
About this article
Cite this article
Ivanov, M.V., Kutukova, K.A. & Khudoerkov, R.M. Morphochemical Changes in the Human Striatum in Aging. Adv Gerontol 9, 303–307 (2019). https://doi.org/10.1134/S207905701903007X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207905701903007X