Abstract
The present review is devoted to the sorption properties of magnetite towards hexavalent chromium ions and the possibility of its use as a sorbent for removing these toxic ions from contaminated aqueous solutions. The behavior of magnetite nanoparticles in aqueous solutions has been also investigated. It has been shown that two processes occur simultaneously during the sorption of chromium(VI) ions by magnetite: ordinary adsorption and chemisorption (redox reaction between hexavalent chromium ions and magnetite). The latter is accompanied by the oxidation of iron(II) in magnetite to iron(III) and the reduction of chromium(VI) ions to chromium(III) with the formation of maghemite and a number of other compounds, including mixed chromium(III) compounds with iron, in the surface layer of magnetite (or onto its surface). It has been demonstrated that the kinetics of the redox process between chromium(VI) and magnetite is described by the first-order reaction equation with respect to the concentration of chromium(VI) ions in a solution. As a result of chemisorption, unlike the case of traditional sorbents, chromium(VI) is irreversibly bound by magnetite, which eliminates the reentry of its ions into the environment. Here, the sorption capacity of magnetite irreversibly decreases in the course of its saturation with chromium. In addition, the sorption capacity of magnetite towards chromium(VI) ions also decreases along with increasing pH of the purified solution, and at pH > 11 it becomes almost zero.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Purification of natural and waste water from heavy metal ions represents an urgent ecological task due to their high toxicity [1, 2]. Hexavalent chromium ions are among the most dangerous environmental pollutants [3–5], which arrive into the environment mainly with the wastewaters of industrial enterprises. Hexavalent chromium is used in the production of stainless steels, textile paints, and preservatives for wood. Chromium salts are widely used in electrotyping for chromium plating, as well as in the leather and a number of other industries. Here, hexavalent chromium compounds easily penetrate into the cells of living organisms. For human beings, they constitute one of the causes of lung and stomach cancer. They can destroy DNA, cause dermatitis and, kidney diseases, damage the liver and nerve tissue, and serve as a cause of many other diseases [1–5]. For this reason, in the European Union, the RoHS directive introduced a restriction on the use of hexavalent chromium compounds in industry, and in Russia the maximum permissible concentration of chromium(VI) ions in wastewater discharged into the environment was limited to 0.02 mg/L.
Currently, there are a number of methods for removing chromium(VI) ions and other heavy metals from contaminated aqueous solutions [6–10]. However, many of them involve using various chemical reagents. This results in a change in the salt content and ionic composition of the treated water, which makes it difficult to subsequently use it in public utilities and industry. Therefore, the search for new methods of purifying natural and waste water from chromium(VI) ions and various other pollutants continues.
One of the most promising ways to remove chromium(VI) from solutions is the sorption method. Its main advantage consists in the fact that it introduced virtually no new foreign ions and substances into a solution to be purified [10–12]. This allows using this method for the regeneration of a number of technological solutions (for example, in electrotyping), as well as to use it for purifying polluted natural waters for their subsequent use in public utilities.
The sorption capacity of adsorbents is largely determined by their specific surface area, which increases along with the decrease of the size of sorbent particles. However, the processes of separating a sorbent from a solution by traditional methods of settling and filtering are getting consequently more complicated. Therefore, it is important to fabricate sorbents that (1) have good sorption properties and (2) are easily separated from a solution. This task can be solved by using a powder of small ferromagnetic particles as a sorbent, which can be separated from a solution under effect of a gradient magnetic field after the sorption of pollutants. Magnetite (Fe3O4) is one example of such a ferromagnetic sorbent.
One should mention that, over recent years, there have appeared a large number of published works devoted to the study of the properties of magnetite and other iron oxides as sorbents for the treatment of wastewater and natural water from various pollutants [13–20]. However, in the present work, the main attention has been paid to magnetite and the possibility of its use as a sorbent for the removal of hexavalent chromium from polluted natural and waste water. The prospect of using magnetite as a sorbent is also determined by the fact that it is an inexpensive material that can be produced, in particular, from spent etching solution, which is a wastewater of a number of metallurgy and hardware plants [21]. It should be also noted that, at present, a fairly large amount of experimental data have been accumulated on the sorption of hexavalent chromium ions from aqueous solutions by magnetite and the behavior of magnetite particles in aqueous solutions, and the present work was an attempt to generalize them. Special attention was paid to the analysis of the mechanism and kinetics of chromium(VI) sorption by magnetite.
STATE OF CHROMIUM(VI) IONS IN AQUEOUS SOLUTIONS
Hexavalent chromium(VI) exists in a solution mainly as ions of chromic acid H2CrO4. Moreover, the forms of the state of the ions change depending on the pH of a solution. The equilibria between different forms of chromium(VI) ions are described by the following equations [8, 11, 12, 22–24]:
At pH < 1, the solution is dominated by molecules of chromic acid H2CrO4; in the pH range from 1 to 6.5, by \({\text{HCrO}}_{4}^{ - }\) ions; and at pH > 6.5, by \({\text{CrO}}_{4}^{{2 - }}\) ions. With an increase in the concentration of chromium(VI) above 1 g/L, \({\text{CrO}}_{4}^{{2 - }}\) dimers dominate in the solution:
However, for such high concentrations of chromium(VI) ions in a solution, it seemed economically unfeasible to use the sorption method to remove them due to the increasing cost of purification.
SOME PHYSICAL AND CHEMICAL PROPERTIES OF MAGNETITE. BEHAVIOR OF ITS NANOPARTICLES IN AQUEOUS SOLUTIONS
Magnetite (Fe3O4) comprises a double iron oxide FeO·Fe2O3. Its main distinguishing feature from other iron oxides is that iron atoms exist in it simultaneously in two degrees of oxidation: +2 and +3. This feature provides this compound with unique properties, high magnetization in particular. In addition, magnetite exhibits good chemical and thermal resistance. The transformation of magnetite into other iron oxides (maghemite (γ-Fe2O3) and hematite (α-Fe2O3)) initiates only when it is heated up to a temperature above 200°C:
Magnetite is stable in an alkaline environment and poorly soluble in acids [25]. This allows us to consider it as a promising sorbent suitable for use in solutions of different salt composition, acidity, and alkalinity.
Magnetite can be synthesized by mixing solutions of divalent and trivalent iron salts with the following alkalinization of the solution [13–15, 20, 23]:
This method is the most common and simple. There are other methods of producing magnetite [21, 26–29]. For example, magnetite can be synthesized by precipitation of divalent iron with alkali, followed by heating and aeration of the resulting suspension [21, 29, 30]. The total equation of the set of chemical reactions occurring in this case is as follows:
This method makes it possible to obtain magnetite from waste etching solutions of metallurgical and hardware plants [21], which reduces the production cost of this sorbent. Here, nanoscaled particles are formed as in most other methods of magnetite synthesis [31, 32].
The average particle size in the resulting magnetite powders can be determined microscopically or calculated based on their specific surface area (assuming a spherical shape of particles) determined by the BET method, as well as on the coherent scattering region by the X-ray phase analysis. At this, all the obtained values were in good agreement with each other. This indicated the low porosity of the synthesized magnetite particles and the absence of large porous agglomerates among them [31, 32].
When a magnetite nanopowder is placed in an aqueous solution, its particles form rather large aggregates and acquire an electric charge, the magnitude and sign of which depend on the pH and salt composition of a solution [14, 28, 32–37]. Here, according to [32], nanomagnetite powder with a particle size of about 26 nm in an aqueous solution of sodium sulfate forms stable aggregates with an average size of about 23 µm (Fig. 1a). When exposed to ultrasound, these aggregates are partially destroyed and their average size drops to 2.68 µm, whereas the specific surface area calculated according to the sedimentation analysis method increases by almost an order of magnitude (Fig. 1b). However, there was no complete dispersion of aggregates to the initial state [32].
Effect of ultrasound on the granulometric composition of aggregates of magnetite nanoparticles in Na2SO4 solution (400 mg/L) [32]: (a) granulometric composition of aggregates before ultrasound treatment of the suspension; (b) the same after ultrasound treatment. Q is the mass fraction of aggregates of diameter (size) d; dav is the average diameter of aggregates, and Ssp is their specific surface area.
Measurement of the ζ potential showed that the point of zero charge of magnetite particles, depending on the composition of a solution, its temperature, and the age of magnetite, lies in the range of pH 2–8 (most often at pH 6–7) [25, 28, 33–37]. Here, the authors of [28] determined the ζ potential and the size distribution function of magnetite nanoparticles with an average size of 84 nm dispersed by ultrasound in distilled water at different pH values of the solution. The resulting dependences are shown in Fig. 2 and 3.
Dependence of the ζ potential (ZP) of magnetite particles in distilled water on its pH [28].
Dependence of the size distribution function (V) of aggregates of magnetite nanoparticles in distilled water on its pH [28].
As can be seen from Fig. 2, in neutral (at pH ≈ 7) and alkaline (pH > 7–8) media, magnetite particles have a negative charge, which is typical for metal oxides [38]. In an acidic solution (at pH < 7), the charge of magnetite particles becomes positive [28]. In this case, the observed change in the charge of magnetite particles (with a change in the pH of a solution) has some effect on the shape of a function of their size distribution shown in Fig. 3. It can be seen that it has two maxima: one at 1 µm and another at 5 µm [28]. Changing the pH of the solution changes the magnitude of these peaks. However, their position almost does not change. All this indicates that, in a magnetite suspension, the forces of mutual magnetic attraction between particles prevail over the forces of electrostatic repulsion.
Due to the formation of rather large aggregates of magnetite nanoparticles, their sedimentation in an aqueous solution proceeds rather quickly. Here, the pH value of a solution also somewhat influences it [28]. As can be seen from Fig. 4, the light transmission of the magnetite nanopowder suspension in distilled water starts to increase just a few seconds after the stirring of the solution is stopped, which indicates the beginning of the precipitation of the magnetite powder. When the pH of the solution decreases, the light transmission growth curves shift to the left and upward, which indicates an increase in the sedimentation rate of aggregates of magnetite nanoparticles (Fig. 4).
Changes in the light transmission of distilled water during sedimentation of the nanomagnetite powder in it, depending on the pH of the solution [28].
The observed effect is not entirely clear, since the formation of the largest aggregates from magnetite nanoparticles should be expected at pH of about 6.5, which corresponds to the zero charge point of the magnetite surface (see Fig. 2). At this pH value, there are no electrostatic repulsive forces between the magnetite particles, only the forces of mutual magnetic attraction act. For this reason, the largest possible aggregates should be formed. Therefore, the sedimentation rate of the magnetite powder should be the highest. However, in practice, a different effect is observed: with an increase in pH, the sedimentation rate somewhat slows down, while it accelerates in an acidic solution (Fig. 4). This is all presumably related to the fact that the zero charge point of this magnetite sample is shifted to the acidic region (pH < 6).
Potentiometric titration of a magnetite suspension with a caustic soda solution indicates its ability to adsorb hydroxyl ions on its surface (Fig. 5) [31].
The effect of magnetite on the change in the pH of a sodium sulfate solution (400 mg/L) when introducing NaOH solution to it [31]. VNaOH is the volume of 0.1 N NaOH solution added.
Figure 5 shows that the suspension of magnetite, unlike a pure solution of sodium sulfate, behaves as a buffer solution, preventing an increase in the pH of the solution when adding alkali to it, which indicates the adsorption of ОН– ions by magnetite. Hydrogen ions are adsorbed by the magnetite surface, apparently in smaller amounts. This is evidenced by the data in Fig. 2. From the dependence presented there, one can see that, along with a decrease in the pH of the solution, the ζ potential of magnetite particles becomes positive, increases, and gradually approaches the plateau at a value of about +20 mV, which indicates a saturation of the magnetite surface with H+ ions. With an increase in the pH of the solution, a different picture is observed: the charge of magnetite particles becomes negative and continues to increase in absolute value, which indicates the ability of the magnetite surface to further absorb OH– ions. This agrees with the potentiometric titration curve of the magnetite suspension with alkali in Fig. 5. It seems clear that the plateau was not yet reached. The dependences of changes in the ζ potential of magnetite particles on the pH of the solution, similarly to in Fig. 2, were also observed in [24, 33]. There, too, at a pH of about 4, the ζ potential curves approached the plateau at a value of about +20 mV, and when the pH increased above 6.5, the charge of magnetite particles became negative and continued to decrease. A curve similar to one in Fig. 2 for the change in the ζ potential of magnetite particles from the pH of the solution was obtained in [34]. However, there, in contrast to [28, 33], at pH 8.5–9.5, it was possible to reach a plateau for the ζ potential at about –140 mV. With an increase in the temperature of the solution, the ζ potential of magnetite particles changed: at pH < 6, it increased to +50–+90 mV, and, in the alkaline region, it shifted to about –100 mV [34].
SORPTION OF CHROMIUM(VI) IONS BY MAGNETITE
After adding the magnetite powder to an aqueous solution containing hexavalent chromium ions, the concentration of the latter in the solution decreases. Therefore, the observed phenomenon can only be explained by the process of sorption of chromium(VI) ions on the surface of magnetite particles. Below, we consider the influence of various factors on this process, as well as possible mechanisms of its course. Note that sorption can be reversible and irreversible in nature. In the first case, it is determined by simple physical (or ion-exchange) adsorption, in which a dynamic equilibrium is established in the solution between a sorbent and a sorbate, and the sorbent can always be regenerated to its original state. In the second case, sorption is caused by chemisorption, i.e., an irreversible chemical reaction between a sorbate and a sorbent, leading to the formation of a new chemical compound involving a sorbate and a sorbent. Here, to regenerate the sorbent, it is necessary to remove (chemically or mechanically) its surface layer with this newly formed chemical compound.
The Influence of Various Factors on the Sorption of Chromium(VI) Ions by Magnetite
Sorption of hexavalent chromium ions by magnetite powders synthesized by various methods has been studied in a number of works [15, 16, 19, 20, 24, 25, 28, 31, 32, 36, 37, 39–42]. This list is incomplete; here, we have mentioned works in which, in our opinion, the most significant results were obtained on this topic.
The sorption value is strongly influenced by the acidity of a solution. Thus, according to [36], the efficiency of the chromium(VI) sorption by magnetite decreases along with an increase in the pH of a solution. Hexavalent chromium ions are best sorbed by magnetite at low pH values. However, at pH < 3, the dependence reaches a plateau. When the pH of the solution is increased up to a value above 9, the efficiency of chromium(VI) sorption by magnetite decreases linearly down to very low values [36].
The authors of [39] also found a linear decrease in the efficiency of sorption of chromium(VI) ions by magnetite with an increase in pH from 2 to 11. The dependence of the sorption efficiency of hexavalent chromium by magnetite, similarly to the data from [36], was obtained in [40]. The entering on the plateau there also occurs at pH < 3, but, at higher pH, a decrease in the efficiency of sorption of ions of chromium(VI) by magnetite proceeds not linearly, but in accordance with a dependence similar to a hyperbole. A similar dependence of the decrease in the efficiency of chromium(VI) sorption by magnetite with an increase in the pH of a solution was also established in [41], where the sorption value dropped to almost zero at pH > 11. It is interesting to note that the sorption of hexavalent chromium ions by maghemite shows the same almost linear decrease in the sorption efficiency with an increase in the pH of the solution and with a plateau at pH < 3 [18].
The authors of [32] investigated the effect of the structure and particle size of magnetite synthesized by different methods on the sorption of chromium(VI) ions by it. The physical and chemical characteristics of the synthesized magnetite powders are shown in Table 1.
The sorption of chromium(VI) was studied by periodically stirring a solution with magnetite for 7 days [32]. The results are shown in Fig. 6. For an objective comparison of the sorption capacity of the synthesized magnetite powders towards hexavalent chromium ions (taking into account their different specific surfaces), the experimental data obtained were presented in the following coordinates: residual concentration of chromium(VI) ions in the solution–total surface of the magnetite powder added to the solution [32].
Changes in the concentration of chromium(VI) ions in the solution after the sorption by magnetite at a temperature of 25 ± 2°C depending on the total surface area of the magnetite powders added to the solution. The initial concentration of chromium(VI) ions in the solution is 50 mg/L, S is the total surface area of magnetite particles placed in the model solution, and C is the concentration of hexavalent chromium ions in the solution [32].
Figure 6 shows that all experimental points for different magnetite powders can be approximated by two straight lines. The lower line contains the experimental data for magnetite nanopowders that were synthesized by the gas-phase synthesis (samples 1, 2, and 4) and by the chemical precipitation from an aqueous solution (sample 3), while the upper line contains points for magnetite nanopowders synthesized by laser sputtering (samples 5 and 6) [32]. It can be seen that the latter powders have a lower sorption capacity. However, for all synthesized magnetite powders, regardless of the method of their preparation, there was no influence of the structure and particle size on their sorption efficiency observed. The reason for the lower sorption efficiency of samples 5 and 6 was the deviation of their composition from stoichiometry. The corresponding measurements and calculations showed that sample 5 agreed with the composition of Fe2.8O4, while sample 6 agreed with that of Fe2.82O4 [32]. Apparently, even such a small deviation from stoichiometry has a significant effect on the sorption capacity of magnetite towards hexavalent chromium ions. However, when the solution temperature increases, the differences in the sorption efficiency of magnetite powders practically disappear and all the experimental points are approximated by a single straight line (Fig. 7) [32]. At this, there is a significant decrease in the residual concentration of chromium(VI) ions in the solution (compare Figs. 6 and 7), which indicates an increase in the sorption efficiency with growing temperature.
The residual concentration of chromium(VI) ions in the solution after sorption with magnetite at a temperature of 60–80°С depending on the total surface area of the magnetite powders added to the solution. The holding time for the solutions with magnetite is 24 h [32].
The effect of temperature on the efficiency of chromium(VI) sorption by magnetite is more clearly shown in Fig. 8.
Effect of temperature on the residual content of chromium(VI) ions in the solution during their sorption by magnetite depending on the weight (m) of the magnetite powder placed in the solution. The holding time for the solutions with magnetite is 24 h [32].
One can see that, with an increase in the temperature of the solution, the residual concentration of chromium(VI) ions in it consistently decreases, approaching zero at 60°C, which indicates an increase in the sorption efficiency. A similar result was obtained in [37, 41].
As was shown above, magnetite nanoparticles form micron-sized aggregates in an aqueous solution. In this case, overlapping of their surfaces is possible, as well as, accordingly, the reduction of the effective surface available for sorption. However, experimental examination of this hypothesis showed that this does not happen [32]. Figure 9 shows data on the sorption of hexavalent chromium by the magnetite suspension before and after its ultrasound treatment. It can be seen that the effect of ultrasound on the suspension, as a result of which the calculated specific surface of the formed aggregates of magnetite nanoparticles increased by almost an order of magnitude (see Fig. 1), did not lead to a subsequent decrease in the residual concentration of chromium(VI) ions in the solution. This indicates that the ultrasound did not change the actual surface of the magnetite particles available for sorption. This is also indirectly evidenced by the data in Fig. 6, where there is a good correlation between the residual concentration of chromium(VI) in the solution and the total surface area of magnetite powders introduced into the solution, regardless of their particle size. Therefore, despite the aggregation of magnetite nanoparticles in the solution, their entire surface is available for sorption.
Effect of ultrasound on the sorption of chromium(VI) ions by magnetite at a temperature of 25 ± 2°C [32].
The decrease in the efficiency of removal of hexavalent chromium ions by magnetite with increasing pH of the solution is a result of, according to most researchers, the electrostatic interaction between the positively charged surface of the magnetite particles (at pH < 6–7) and negatively charged ions of chromium(VI), which in a wide range of pH exist mostly in the form of ions \({\text{HCrO}}_{4}^{-}\) and \({\text{CrO}}_{4}^{{2-}}.\) At high pH values of the solution, the charge of the magnetite becomes negative and, accordingly, absorption of chromium(VI) by magnetite decreases sharply (similarly charged particles are known to repulse). However, in our opinion, this explanation is not entirely correct, since the change in the charge of the magnetite surface is supposed to affect only the kinetics of the absorption of chromium(VI) ions by magnetite, and not its sorption capacity towards them. Therefore, the only reasonable explanation for the observed phenomenon, in our opinion, is a decrease in the surface of magnetite available for sorption of chromium(VI) ions due to partial adsorption of hydroxyl ions on it. We have already noted above that the sorption capacity of magnetite for OH– ions is apparently quite substantial. Therefore, at high pH values of the solution, due to their adsorption, they can block part of the magnetite surface for chromium(VI) ions.
Improving the efficiency of sorption of chromium(VI) by magnetite with increasing temperature of the solution indirectly indicates that there is a significant contribution of chemisorption to the overall sorption process, since, during a ordinary adsorption, the opposite effect is observed—a decrease in sorption with growing temperature.
Kinetics of Chromium(VI) Sorption by Magnetite
The data available in the published works on the kinetics of sorption of chromium(VI) ions by magnetite are quite contradictory. Thus, according to [36], the sorption of chromium(VI) ions with an initial concentration in a solution of 50–150 mg/L by a magnetite nanopowder with a particle size of about 10 nm at a pH close to neutral proceeds quite quickly. The main amount of chromium(VI) in the solution is sorbed by magnetite after about 5 min from the start of the process, and the remaining amount after 60 min [36]. The sorption efficiency decreases with an increase in the initial concentration of chromium(VI) in the solution, which is probably due to the exhaustion of the sorption capacity of the magnetite nanopowder placed in the solution. A similar dependence of the change in the sorption efficiency of chromium(VI) ions by magnetite on time was observed in [40], where the sorption was studied at pH 2.5 and the initial concentration of chromium(VI) in a solution of 50 mg/L.
A slightly different result was obtained in [37]. There, the sorption of hexavalent chromium ions by magnetite with an average particle size of about 100 nm was studied at pH ≈ 2. The dependences from Fig. 5 of this work recalculated in other coordinates are provided in Fig. 10, which shows that, in this case, as opposed to the results of [36], the sorption of chromium ions(VI) by magnetite proceeds very slow and, even 380 min after the process started, the experimental curves of decreasing concentrations of chromium ions(VI) in the solution do not reach the plateau, which supposed to indicate the completion of the sorption. As the solution temperature increased, the sorption efficiency increased [37]. This is indicated by a decrease in the residual concentration of chromium(VI) in the solution with an increase in the temperature of the process (Fig. 10).
Kinetics of the sorption of chromium(VI) ions by magnetite at different temperatures of the solution. The initial concentration of chromium(VI) ions in the solution was 20 mg/L, pH 2, and the concentration of magnetite in the solution was 2 g/L [37].
A similar result was obtained in [19]. There, the total duration of the chromium(VI) sorption process with a mixed sorbent consisting of maghemite and magnetite at pH 3 was 200 min. Here, the experimental curve of the sorption efficiency on the time of stirring the solution with the sorbent reached the plateau approximately in 100 and 150 min after the start of the process for the initial concentration of hexavalent chromium ions in the solution of 1 and 2 mg/L, respectively. The concentration of the sorbent in the solution was 0.4 g/L.
In [32], the sorption of hexavalent chromium ions from a model solution (400 mg/L Na2SO4 + K2CrO4) at room temperature, neutral pH, and periodic stirring of the solution was investigated for 80 days (Fig. 11).
Kinetics of the sorption of chromium(VI) ions by magnetite at a temperature of 25 ± 2°C [32].
One can see that, in the initial period of the sorption process, there was a sharp decrease in the concentration of chromium(VI) ions in the solution, and then its slow gradual decrease continued further [32]. The same significant decrease in the concentration of chromium(VI) in the initial period of the process, followed by its slow decrease with further holding of magnetite in a solution, was also stated by the authors of [31]. The same effect can be seen in Fig. 10 (see above) for the curves corresponding to temperatures of 40 and 50°C.
A noticeable decrease in the concentration of chromium(VI) ions in the solution during the initial period of their sorption at pH 4 by a sorbent consisting of a mixture of maghemite and magnetite was also observed in [41]. After that, the chromium(VI) content in the solution gradually decreased for about 150 min and, then, reached a constant value. The initial concentration of chromium(VI) ions in the solution was 1.5 mg/L, and the sorbent concentration was 0.4 g/L.
Thus, the process of chromium(VI) sorption by magnetite can be divided into two stages: fast and slow, the presence of which can be easily explained if the magnetite powders had a high porosity. In this case, the first, fast stage of the process would correspond to the adsorption of chromium(VI) ions on the outer surface of the magnetite particles, whereas the second, slow one would correspond to their diffusion transfer and subsequent adsorption on the inner surface of the pores. However, as was mentioned above, magnetite powders are low-porosity. Therefore, intrapore diffusion transfer is not the reason for the observed high duration of the sorption process during the second stage of the process.
The presence of two stages of the sorption of hexavalent chromium ions on magnetite can be explained by assuming that two processes occur simultaneously during the sorption of chromium(VI) by magnetite: (1) simple physical (or ion exchange) adsorption and (2) chemical reaction of chromium(VI) interaction with magnetite (chemisorption). Moreover, at the initial stage of sorption, the first process prevails. In addition, the kinetics of the process seems to be significantly influenced by the ratio between the initial concentration of chromium(VI) ions in the solution and the concentration of magnetite (more precisely, the total surface area of the magnetite powder) in it. So, if the initial concentration of chromium(VI) in a solution is small, and the concentration of magnetite is high, then it seems obvious that, due to the course of adsorption, a rapid decrease in the residual concentration of chromium(VI) ions in the solution will be observed, followed by reaching a plateau. If there is a reverse ratio between the concentrations of chromium(VI) and magnetite in a solution, then, after a rapid initial decrease in the concentration of chromium(VI) ions in the solution due to adsorption, a period of its slow decrease due to chemisorption will follow (this type of dependence is shown in Fig. 11). Naturally, an increase in temperature will lead to an increase in the sorption efficiency due to the acceleration of chemisorption (Figs. 8 and 10).
In general, the adsorption kinetics can be described by equations of the first (pseudofirst) or second (pseudosecond) order. These equations were also used to describe the kinetics of chromium(VI) sorption by magnetite. Thus, the authors of [40] described the kinetics of the sorption of chromium(VI) ions by magnetite at pH 2.5 with high accuracy by the pseudo-second-order equation:
where τ is time, s; k2 is the rate constant, g/(mg s); qτ is the sorption value at a moment of time τ, mg/g or mg/m2; and q is the sorption equilibrium value, mg/g or mg/m2.
Here, rate constant k2 equals 0.6 g/(mg min) = 0.01 g/(mg s). The authors of [40] concluded that the sorption of chromium(VI) on the magnetite sample studied by them had a chemisorption character. However, this contradicts their other result, according to which the sorption of hexavalent chromium ions in their experiments is described by the Langmuir isotherm, which implies the existence of a dynamic equilibrium between the sorbent and the sorbate, which is impossible with chemisorption.
The authors of [41] studied the kinetics of the sorption of chromium(VI) ions, as mentioned above, on a mixture of magnetite and maghemite. The study was conducted at three different temperatures and pH 4. The decrease in the concentration of chromium(VI) in the solution from time was described by a first-order equation, the integral form of which is as follows [41]:
where k1 is the rate constant, s–1.
The value of k1 for temperatures of 10, 22 and 55°C had values of 0.014 min–1 (or 2.3 × 10–4 s–1), 0.02 min–1 (or 3.3 × 10–4 s–1) and 0.03 min–1 (or 5 × 10–4 s–1), respectively, which indicated a slight increase in the rate of the sorption process with increasing solution temperature [41].
Isotherms of Chromium(VI) Adsorption by Magnetite
One of the first known works on the sorption of chromium(VI) with magnetite was carried out in 1994 [39]. The study was conducted at pH 9.8, and the duration of the sorption experiments was 30 min. The experimental data obtained were well described by the Langmuir isotherm for monomolecular adsorption (correlation coefficient of 0.98):
where qm is the capacity of the adsorption monolayer, mg/g or mg/m2, and KL is the adsorption equilibrium constant, L/mg.
The calculation based on the experimental data presented in Fig. 1 of [39] provides the following values of the parameters of this equation: qm = 10.5 mg/g and KL = 0.017 L/mg (Fig. 12).
The authors of [36] described the sorption of chromium(VI) by magnetite by the empirical Freundlich equation:
where KF and n are constants (n > 1).
With an increase in the temperature of the solution from 10 to 40°A, there was a small increase in constant KF observed in this equation from the value of 10.38 to 12.20. Constant n, conversely, decreased from 3.23 to 3.12. The correlation coefficient varied from 0.986 to 0.994 [36]. However, the calculation based on the data in Fig. 2 of this work shows that the dependence presented there can also be well described by the Langmuir equation with a correlation coefficient of 0.981 and the following parameters: qm = 18.94 mg/g = 0.096 mg/m2 (the specific surface area of magnetite was 198 m2/g) and KL = 0.017 L/mg (Fig. 13).
A similar result was obtained in [19]. There, the sorption of hexavalent chromium ions at pH 4.5 was also described by the Freundlich isotherm with constants KF = 6 and n = 1.71. The duration of the sorption process was 24 h. It was not a pure magnetite used as a sorbent, but its mixture with maghemite (30.8% maghemite and 69.2% magnetite). Recalculation of the experimental data [19] presented in Fig. 7a of this work shows that they are also well described by the Langmuir equation (correlation coefficient of 0.99) with the following parameters: qm = 7.2 mg/g = 0.15 mg/m2 (specific surface area of the sorbent is 49 m2/g) and KL = 2.96 L/mg (Fig. 14).
The sorption of chromium(VI) by the synthesized magnetite powder with a specific surface area of 86.6 m2/g at room temperature (25 ± 2°C) and an initial pH value of 2.5 was studied in [40]. The isotherm of the sorption of chromium(VI) ions by magnetite in this work is well described by the Langmuir equation (correlation coefficient of 0.999) with the following parameters: qm = 21.7 mg/g = 0.251 mg/m2 and KL = 0.262 L/mg. The duration of the sorption process was 120 min.
In [41], as in [19], the sorption of chromium(VI) was studied on a mixture of iron oxides: 70% of maghemite + 30% magnetite. The specific surface area of such a mixed sorbent was equal to 49 m2/g. Here, the sorption isotherm was well described by the Langmuir equation with the following parameters: qm = 6.9 mg/g = 0.141 mg/m2 and KL = 3.1 L/mg (the following data were provided: pH 4 and temperature of 22°C) [41]. The duration of the sorption process was 24 h. As the solution temperature increased, a small increase in qm and KL values was observed.
To sum up, the data in the published works indicate that the sorption of chromium(VI) ions by magnetite with a sorption process duration of no more than 24 h is well described by the Langmuir equation. The values of the parameters of this equation obtained in the above-mentioned works (or calculated from the experimental results of these works) are shown in Table 2.
We did not construct a similar table for the parameters of the empirical Freundlich equation found in [19, 36], since it was shown above that the experimental data of these works were also satisfactorily described by the Langmuir equation, which is well theoretically justified and the parameters of which have a clear physical meaning. Let us note that the situation in which the sorption isotherm is described with approximately equal accuracy by the Freundlich and Langmuir equations is typical for many sorbents.
It can be seen from Table 2 that, in the mg/g dimension, the values of the capacity of the adsorption monolayer (qm) of magnetite powders differ significantly from each other. However, in the mg/m2 dimension, these differences are smoothed out and there is a relationship between the capacity of the adsorption monolayer of magnetite powders towards hexavalent chromium ions and the pH of the solution. So, at pH 2.5, the capacity of the adsorption monolayer of magnetite powders is 0.251 mg/m2; at pН 4–4.5, it is already 0.141–0.15 mg/m2; and, at pH > 7, it decreases to 0.096 mg/m2. This correlation is in good agreement with the above mentioned published data on a decrease in the efficiency of sorption of chromium(VI) ions by magnetite with an increase in the pH of the solution. This relationship is shown graphically in Fig. 15. However, there are no grounds to consider the same well-defined correlation for the adsorption equilibrium constant, since the value of KL for pH 2.5, found in [40], does not agree with the data obtained by other researchers (see Table 2).
Dependence of the adsorption capacity of magnetite on the pH of the solution (according to Table 2).
Let us also note the similarity of the found values of the capacity of the adsorption monolayer of mixed sorbents (magnetite + maghemite) with the corresponding values for pure magnetite powders. Hence, it can be concluded that the adsorption capacity of maghemite with respect to hexavalent chromium ions is close to the corresponding capacity of magnetite.
Factors Indicating the Chemisorption Character of Chromium(VI) Ion Sorption by Magnetite
The chemisorption nature of the sorption of chromium(VI) by magnetite is stated in almost all the above-mentioned works on this process. At the same time, a contradiction is observed: sorption is most often described by the Langmuir isotherm, which corresponds to the course of simple physical (or ion-exchange) adsorption and assumes the presence of a dynamic equilibrium between an adsorbed substance and a sorbent, which is not possible with chemisorption, as already noted above.
Thus, in [39], the isotherm of sorption of chromium(VI) ions by magnetite was described by the Langmuir equation, but the authors noted the presence of chemisorption, since repeated washing of magnetite after the sorption with water leads only to partial desorption of chromium(VI) ions from it.
A similar contradiction was found in [42], where a complex composite sorbent was used for the sorption of chromium(VI) containing magnetite, hematite (α‑Fe2O3), as well as specially treated eucalyptus sawdust. The sorption of chromium(VI) on this mixed sorbent was also best described by the Langmuir isotherm. Here, the capacity of the sorbent increased with an increasing temperature of the solution, which indirectly indicated the contribution of chemisorption to the overall sorption process (since, in the case of simple physical adsorption, the opposite effect would be observed—reduction of the capacity of the sorbent with increasing temperature). The X-ray photoelectron spectroscopy (XPS) of the sorbent after the sorption showed that chromium in it was partially in the trivalent state; i.e., chromium(VI) was reduced during the sorption process. Chromium(III) and iron(III) ions predominated on the surface of magnetite contained in the sorbent [42]. All this, in fact, also constitutes evidence of the chemisorption of chromium(VI) ions on the magnetite surface. The authors suggested that this is a reaction of oxidation of iron(II) to iron(III) and reduction of chromium(VI) to chromium(III) [42]. In this case, according to the authors, the formation of a mixed compound FexCr(1 – x)(OH)3 on the magnetite surface is possible, since the ionic radii of iron(III) (0.067 nm) and chromium(III) (0.065 nm) are close to each other. Therefore, in the magnetite lattice, it is possible that Fe(III) ions are replaced with Cr(III) [42]. Chemisorption is also evidenced by the absence of chromium(III) ions in the solution after the sorption process is completed.
The X-ray photoelectron spectroscopy of the magnetite surface after the sorption of chromium(VI) performed in [40] also showed peaks typical for Cr(III). In addition, a small change in the positions of Fe2p iron peaks was found, which, according to the authors of [40], could indicate the substitution of iron(III) ions for chromium(III) ions in the magnetite lattice due to the proximity of their ionic radii. Cyclic experiments were also carried out on the regeneration of magnetite with an alkali solution, followed by repeated sorption of chromium(VI) ions on it. These experiments showed that the sorption capacity of magnetite decreased from cycle to cycle, which indicated the irreversible nature of chromium(VI) sorption on magnetite. The authors also note that, as opposed to magnetite, the sorption of chromium(VI) on maghemite is reversible [40].
The authors of [32] also noted the irreversible nature of chromium(VI) sorption on magnetite.
A thorough analysis of a mixed sorbent (magnetite + maghemite) after the sorption of chromium(VI) on it was carried out in [41] using X-ray diffraction, X-ray photoelectron (XPE), and Raman spectroscopy. This study showed that, after the sorption, the iron(II) content in magnetite decreased from 8.2 to 3.6%, and the amount of maghemite in the sorbent increased from 70 to 89%. Here, peaks corresponding to chromium(III) were detected in the X-ray photoelectron spectra. The Raman spectroscopy data showed that a mixed compound of chromium and iron was formed in the sorbent [41]. All this also indicates that there is a redox reaction between iron(II) in magnetite and chromium(VI) ions during their sorption on the magnetite surface.
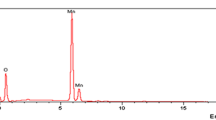
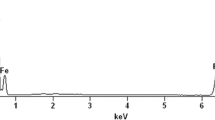
The appearance of chromium(III) in the magnetite sediment after the sorption of hexavalent chromium ions on it was also detected in [43]. The magnetite powders were prepared by three methods: gas-phase, laser sputtering, and chemical precipitation from an aqueous solution. Their physical and chemical properties were provided above in Table 1. These magnetite powders were thoroughly analyzed before and after sorption of chromium(VI) ions on them using the X‑ray photoelectron spectroscopy [43]. For example, Fig. 16 shows the data on the chemical composition of the surface of magnetite powder particles after the chromium(VI) sorption [43]. It can be seen that chromium appeared in the composition of all three magnetite powders after the sorption process was completed. The relative concentration of absorbed chromium calculated relative to the iron content in the surface layers of magnetite is 1–2%.
Content of iron, chromium, and oxygen on the surface of magnetite particles synthesized by three methods: (a) gas-phase, (b) laser sputtering, and (c) chemical precipitation from the aqueous solution after the sorption of chromium(VI) ions on them [43].
Figure 17 shows the typical for all the samples Cr2p chromium spectrum in the composition of magnetite after the sorption of chromium(VI) ions on it, when photoemission is excited by both MgKα and AlKα radiation. It can be seen that, in both cases, the spectrum contains the band Cr2p3/2 corresponding to the binding energy of 576.6 eV. The position and shape of the Cr2p spectrum clearly indicate that chromium on the surface of the magnetite particles has an oxidation state of +3. In addition, after the sorption of chromium(VI) ions, a band from Fe(OH)2 was present in the O1s spectra of all three samples, while the Fe2p spectra were similar in shape to those in Fe2O3 and Fe(OH)3 [43].
X-ray photoelectron spectra of Cr2p chromium of magnetite powder after the sorption of chromium(VI) ions on it under excitation of photoemission by (1) MgKα and (2) AlKα radiation [43].
The study of the magnetite powders composition after the sorption of chromium(VI) by electrochemical method performed in [44] also corroborated the presence of chromium in it in the trivalent state.
A sharp increase in the efficiency of the chromium(VI) sorption by magnetite with an increase in the temperature of the solution, found in a number of studies [31, 32, 37, 41] (see also Figs. 8, 10), also evidences the presence of chemisorption during the absorption of chromium(VI) ions by magnetite. This is also indicated by the prolonged duration of the sorption process, which, at room temperature, can last for dozens of hours and days (Fig. 11). At the same time, according to [31], the pH of the solution changes (Fig. 18).
Changes in the pH of the solution depending on the total surface area of the magnetite powder placed in the solution with chromium(VI) ions and the duration of the sorption process (temperature 25 ± 2°C) [31].
As can be seen from Fig. 18, during prolonged holding of the magnetite powder in the solution, the pH of the latter, regardless of the concentration of magnetite in it, initially shifted to the alkaline region (the initial solution had a pH of ≈ 7.5). However, then, after 7–38 days of exposure, further changes in the alkalinity of the solution proceeded unevenly. Thus, at S ≈ 20–40 m2, the pH of the solution still increased up to pH ≈ 9.2–9.4, and at S > 40 m2, on the contrary, it decreased down to pH ≈ 8–9. 2. After 134 days of exposure, a further decrease in the pH of the solution was observed. After 302 days, at S ≈ 20 m2, the solution had a pH of 8.2; at S > 20 m2, рН ≈ 7.3 [31]. The observed phenomenon was presumably determined not only by the process of chemisorption of chromium(VI) by magnetite, but also by the interaction of the latter with water, and this requires further study.
With prolonged holding of magnetite in the solution with chromium(VI) ions, the sorption process can no longer be described by the Langmuir isotherm [31]. This is evidenced by the dependences shown in Fig. 19.
It can be seen that, for the duration of the sorption process of chromium(VI) ions by magnetite of 240 min and 28 h, the experimental points are well approximated by straight lines with different angles of inclination to the abscissa axis, indicating that simple adsorption described by the Langmuir isotherm occurs and prevails during this time period [31]. Here, the parameters of the isotherm change with time, which indicates a nonequilibrium state of the system. With a longer sorption process (7 days or more), the dependences become already curved (Fig. 19), which can be interpreted as evidence of the predominance of chemisorption during this stage of the process (curves for the sorption duration exceeding 7 days are not shown in Fig. 19 so as not to overload it) [31]. These dependences confirm the above assumption that two processes occur simultaneously during the sorption of chromium(VI) by magnetite: a simple physical (or ion-exchange) adsorption and a chemical reaction of interaction of chromium(VI) ions with magnetite (chemisorption), which leads to the formation of a mixed chromium-iron compound. Here, during the initial stage of the sorption, the first process prevails; the second one then predominates. In the kinetic curves, the adsorption process corresponds, apparently, to a short initial stage of a sharp decrease in the concentration of chromium(VI) ions in the solution, while the chemisorption corresponds to its subsequent slow decrease (Fig. 11).
To sum up, the course of chemisorption during the sorption of chromium(VI) ions by magnetite is indicated by
– the irreversible nature of the sorption, which leads to a decrease in the capacity of magnetite towards chromium(VI) ions up to zero with repeated use of the sorbent, as well as the impossibility of its complete regeneration with alkali;
– prolonged duration of the sorption process, accompanied by a change in the pH of the solution;
– the impossibility of describing the sorption by the Langmuir isotherm when the process lasts for a long period of time;
– a sharp increase in efficiency of the sorption of chromium(VI) ions by magnetite with a growth in the temperature of the solution, which is not possible in the case of simple physical or ion-exchange adsorption;
– a change in the oxidation states: of chromium from +6 to +3 and of iron ions from +2 to +3 in magnetite, which indicates the redox reaction between chromium(VI) ions in a solution and iron(II) ions in magnetite; and
– the appearance of chromium(III) in magnetite, which indicates the formation of a mixed compound of chromium(III) with iron and the possible partial replacement of iron(III) in magnetite with chromium(III) due to the proximity of their ionic radii.
Let us add that, apparently, the different contribution of the adsorption and chemisorption to the overall process of chromium(VI) sorption by magnetite explains the available spread of data on the adsorption capacity of magnetite towards chromium(VI) (see Table 2) and discrepancies in the equations describing the kinetics of the sorption process.
Mechanism of Chemisorption of Chromium(VI) by Magnetite
Thus, the published data indicate that the sorption of hexavalent chromium ions by magnetite leads to a redox reaction, in which Fe(II) in magnetite is oxidized to Fe(III), and chromium(VI) on the surface of magnetite is reduced to chromium(III). Here, a new mixed compound of chromium(III) with iron(III) or, possibly, with iron(II) is formed in the thin surface layer of magnetite (or on its surface). For example, the authors of [42] hypothesized that this compound has the general formula FexCr(1 – x)(OH)3; however, this was not confirmed experimentally.
According to another study, iron chromite FeCr2O4 is formed in the surface layer of magnetite after its interaction with chromium(VI) ions at pH 2.5 [36]. Its formation was confirmed by the X-ray phase analysis. An equation for the reaction of hexavalent chromium ions with magnetite was proposed in [36]:
However, in our opinion, this equation does not comply with the resulting compound FeCr2O4. Therefore, it should have a different form:
In the molecular form, reaction (6) can only be written if we assume that, besides FeCr2O4, it produces iron(III)oxide, for example, maghemite, and hydroxide Fe(OH)3. This results in two equations with different ratios of reaction products and reacting substances:
In the case of these reactions, the interaction of chromium(VI) with magnetite should be followed by an increase in the pH of the solution, which is consistent with the experimental data of [31]. Bands corresponding to Fe2O3 and Fe(OH)3 (or ones very close to them) were found in the X-ray spectra in [43]. However, the formation of FeCr2O4 in the surface layer of magnetite was no longer confirmed by the X-ray phase analysis in any of the published works known to us.
Along with the above-mentioned works on the sorption of chromium(VI) by magnetite, where the chemical interaction between hexavalent chromium ions and iron(II) in magnetite was detected, there are published works in which sorption was virtually not taken into account and magnetite was considered as a reducing compound for chromium(VI) [45–49]. Here, in [45], the following equation for the redox reaction between chromium(VI) ions and magnetite was proposed:
This reaction can be rewritten in the molecular form:
The reduction of chromium(VI) by magnetite according to Eq. (10) should be accompanied by the appearance of maghemite (γ-Fe2O3) in the sediment and an increase in the pH of the solution. The latter, as was mentioned above, is consistent with the experimental data of [31]. The formation of maghemite in the sorbent was established by the authors of [41].
The thickness of the surface layer of magnetite reacted with chromium(VI) ions at pH 7 according to [45] is about 2.4 nm. Thereafter, the reaction of interaction of chromium(VI) with magnetite does not develop because of the passivating action of these new compounds formed on the surface of magnetite. As a result, calculations [45] showed that the maximum sorption capacity of magnetite for chromium(VI) ions did not exceed 1.1 mg/m2. By comparing this value with the data in Table 2, one can see that it is about an order higher than the values presented there for qm.
The authors of [46], as a result of the analysis of the X-ray spectra of magnetite after interaction with a solution of chromium(VI), concluded that either chromium(III) hydroxide or mixed iron(III) hydroxide (solid solution) with chromium(III) FexCr(1 – x)(OH)3 can be formed on the surface of magnetite (or in its surface layer).
According to the experimental data provided in [47], the thickness of the surface layer of magnetite that reacts with hexavalent chromium ions at pH 6 increases with increasing concentration of the latter in the solution and increasing time of interaction with magnetite. According to [47], the maximum thickness of the surface layer of magnetite reacting with chromium(VI) ions is approximately 1.5 nm. The X-ray absorption fine structure spectroscopy (XAFS) showed that chromium and iron are present on the magnetite surface in the oxidation states of +3. A large number of hydroxyl groups were also found there [47]. Based on this, the authors suggested that chromium forms CrOOH or (Cr,Fe)OOH oxyhydroxide on the magnetite surface [47]. Meanwhile, Fig. 1 of this work clearly shows the proximity of the X-ray absorption spectra of iron chromite FeCr2O4 and magnetite after the latter is soaked in Na2CrO4 solution. However, the authors of [47] did not discuss this fact in detail.
The interaction of magnetite with hexavalent chromium ions at pH 1, 7, and 13 was studied in [48]. Here, the formation of maghemite and goethite (α‑FeOOH) was detected. With an increase in the pH of the solution, the proportion of goethite in the sediment increased, while that of maghemite, on the contrary, decreased. The entire set of the processes occurring, according to the authors, can be described by the following chemical reactions [48]:
It can be seen that, here, just like in [47], the formation of mixed oxyhydroxide CrxFe(1 – x)OOH was assumed. Here, the reduction of chromium(VI) by magnetite, according to these reactions, occurs due to the partial dissolution of magnetite with the formation of maghemite in the solid phase. Let us note that reaction (11) can explain the decrease in the pH of the solution observed in [31] with prolonged holding of magnetite in it (see Fig. 18).
According to very detailed studies [49], two layers are formed on the surface of magnetite when it interacts with hexavalent chromium ions in the solution at pH 6. The upper layer in contact with the solution is chromium oxyhydroxide CrOOH or, possibly, hydroxide Cr(OH)3. The thickness of this layer is estimated to be about 1.5 nm [49]. Below, there is a layer of iron(III) oxide, and then there is unreacted magnetite. The authors of [49] were not able to estimate the thickness of the iron(III) oxide layer.
Therefore, the published data on the mechanism of interaction of magnetite with hexavalent chromium ions are quite contradictory. For convenience of their further analysis, they are summarized in Table 3.
From the data given in Table 3, it can be seen that the initial pH value of the solution practically does not affect the composition of the products of the redox reaction between magnetite and chromium(VI) ions. Most researchers agree (and this was proven in a number of works reviewed above) that one of these products is maghemite [40, 41, 45, 48, 49]. The authors of [48] discovered the formation of goethite in addition. Opinions differ regarding the composition of chromium-containing reaction products. These can be chromium oxyhydroxide CrOOH or hydroxide Cr(OH)3 [42, 45, 47, 49], or a mixed hydroxide (solid solution) of the composition FexCr(1 – x)(OH)3 [42, 46], or oxyhydroxide CrxFe(1 – x)OOH [47, 48]. All this is stated in the published works in the form of suggestions, since these compounds are very difficult to distinguish experimentally due to the proximity of their spectral, energy, and other characteristics. In most of the studies considered, in fact, only the appearance of chromium(III) on the surface of magnetite and the predominance of iron(III) ions there was found, which indicated the presence of a redox reaction between chromium(VI) ions in a solution and magnetite [42, 45, 46–49].
In [36], as was already mentioned, the X-ray diffraction analysis demonstrated the formation of iron chromite FeCr2O4. However, maghemite was not detected, which, according to reactions (7) and (8), was also supposed to be formed in the system.
The mechanism proposed in [48] for the formation of goethite and oxyhydroxide CrxFe(1 – x)OOH by reactions (11)–(13), in our opinion, is improbable, since the solubility of magnetite in a neutral and alkaline medium is very small. Therefore, the process of reduction of chromium(VI) by magnetite over the course of a combination of these reactions is supposed to be extremely slow, which does not correspond to the actual observed rate.
When comparing the experimental conditions for the reaction between chromium(VI) and magnetite in the studies reviewed above, one can see that they differ significantly (see Table 3). In particular, there are differences both in the initial pH value of the solution and in the ratio between the initial concentration of chromium(VI) ions and the content of magnetite in the C0/Csol solution, on which, in our opinion, the composition of the reaction products depends. Here, at low initial pH values of the solution and at a small value of the C0/Csol ratio, a rapid increase in the pH of the solution should be expected already during the first minutes of the process due to both the redox reaction between chromium(VI) and magnetite and the partial dissolution of the latter in an acidic medium. Therefore, the following interaction of chromium(VI) ions with magnetite in these experiments should probably occur already at a higher pH of the solution, close to neutral. In other words, at low concentrations of chromium(VI) in a solution and, on the contrary, a high concentration of magnetite in it, despite the low initial value of pH of a solution during the redox reactions between the ions of chromium(VI) and magnetite, as well as partial dissolution of the latter in acidic medium, will shift pH to a neutral value, with the magnitude of this shift determining the composition of the resulting reaction products.
It can be seen from Table 3 that most of the experiments on the reduction of chromium(VI) by magnetite were carried out precisely at a low C0/Csol ratio not exceeding 8. Therefore, after a short initial period, the main interaction of chromium(VI) with magnetite in the experiments of these works occurred, apparently, at higher and proximate pH values of the solution. This, in our opinion, explains the practical lack of influence of the initial pH value of a solution on the composition of the reaction products. Only in [36] were experiments carried out at a rather high C0/Csol ratio of 10–30 and with an increased initial concentration of chromium(VI) ions in a solution (50–150 mg/L). As a result, the increase in the pH of the solution during the reaction of chromium(VI) interaction with magnetite and its partial dissolution in the solution was apparently insignificant, and the reaction proceeded in an acidic medium. This presumably led to the formation of iron chromite FeCr2O4 in the solid phase. If this is the case, then it becomes clear why this compound was not detected in the other works discussed above.
Let us also add that, when hexavalent chromium in a solution reduced with iron(II) ions at pH > 3.9, a mixed X-ray amorphous hydroxide of the composition Fe0.75Cr0.25(OH)3 was also formed [50]. When chromium(VI) is reduced by metallic iron at pH > 4, the authors of [51] demonstrated the formation of compounds the composition of which can be described by the formula Cr0.67Fe0.33(OH)3 or Cr0.67Fe0.33OOH. However, in some cases, at an initial pH value of <2, the formation of chromite FeCr2O4 was also observed [52]. All the above, in our opinion, indirectly confirms the above hypotheses regarding the effect of the pH of a solution and the C0/Csol ratio on the composition of the products of the chromium(VI) reduction reaction with magnetite.
Kinetics of Chemisorption of Chromium(VI) by Magnetite
As was mentioned above, the published data on the kinetics of adsorption of hexavalent chromium ions on magnetite were quite contradictory. The kinetic equations found there are, in our opinion, empirical, since they suggest that the sorption of chromium(VI) ions by magnetite has a purely adsorptive nature [40, 41]. They do not take into account the fact that, in this case, two processes occur simultaneously during the sorption: adsorption and chemisorption. The ratio of the rates of these processes can be different and is largely determined by the conditions of a sorption experiment (temperature, pH, initial concentration of hexavalent chromium ions in a solution, the concentration of magnetite in it, etc.). Therefore, depending on the sorption conditions, its kinetics can be described by different adsorption kinetic equations.
Meanwhile, there are published works [25, 31, 37, 45, 53] in which the kinetics of chromium(VI) chemisorption on magnetite or, rather, the kinetics of the redox process between hexavalent chromium ions and magnetite were studied. However, the possible adsorption of chromium(VI) ions on the magnetite surface during the initial period of the process was not considered.
It can be seen from the above data on the mechanism of chemisorption that, apparently, this process is a whole set of simultaneously or sequentially occurring chemical reactions. Therefore, the study of its kinetics could allow us to establish the chemical reaction that is the basis of it and determines its rate. These results are also of great practical importance for the industrial application of magnetite as a sorbent for the removal of toxic chromium(VI) ions from contaminated solutions. However, it is necessary to observe and fulfill a number of conditions so that the data obtained are reliable and can be unambiguously interpreted. First of all, it is necessary to consider or minimize the contribution of adsorption during the interaction of chromium(VI) with magnetite, due to which a significant decrease in the concentration of chromium(VI) in the solution can occur during the initial stage of the process. This can be done, for example, by conducting a sorption process at an elevated temperature. Further, the initial concentration of chromium in the solution should be such that its subsequent decrease due to the chemisorption can be recorded with sufficient accuracy. In addition, it should be taken into account that, in this case, a heterogeneous reaction takes place, the speed of which is determined not only by the concentration of chromium(VI) ions in a solution, but also by the value of the total surface of magnetite powder placed in a solution. Its active surface that is available for interaction with hexavalent chromium ions, as the redox reaction between chromium(VI) and magnetite proceeds, decreases due to the passivating effect of new chemical compounds formed in the surface layer of magnetite (or on its surface), reducing, as a result, the overall rate of the process. Therefore, to avoid this effect, in the kinetic experiments, such an amount of magnetite powder should be introduced into the solution with chromium(VI) that the reduction of its active surface during chemisorption can be disregarded.
The above conditions were most fully met in [31], in which an excessive amount of magnetite was introduced into a model sodium sulfate solution (400 mg/L) containing hexavalent chromium ions (K2CrO4), so that the reduction of its surface available for chemisorption during the sorption process could be disregarded. To reduce the impact of adsorption, the experiments were carried out at an elevated temperature of a solution. To describe the kinetics of the interaction of chromium(VI) ions with magnetite, the following equation was proposed [31]:
where v is the solution volume, m3; k is the rate constant of the chemisorption reaction; E is the activation energy of the chemisorption reaction, J/mol; R is the universal gas constant; T is the absolute temperature, K; z is the order of the equation; and K is the integral rate constant of the chemisorption process rate.
Integration of Eq. (14) at z = 1 yields the following formula:
where, as above, C0 is the initial concentration of hexavalent chromium ions in the solution.
The results of the experiments performed in the coordinates of Eq. (15) are shown in Fig. 20 and 21 [31].
The linear shape of the dependences shown in Figs. 20 and 21 indicates that Eq. (14) adequately describes the experimental data at z = 1. Here, at temperatures of 60 and 80°C, the approximating lines do not pass through the beginning of the ordinate axis and the first experimental points deviate from the theoretically expected linear dependence (Fig. 20). This indicates a significant contribution of adsorption to the initial moment of the process in these experiments. However, after the temperature increases up to 98°C, such a deviation is no longer observed (Fig. 21). It seems obvious that, in the latter case, an increase in the process temperature up to 98°C led to the chemisorption rate exceeding the adsorption rate. As a result, the deviation of the approximating lines from the origin of the ordinate axis observed at temperatures of 60 and 80° C disappeared [31].
To generalize the experimental data on chemisorption at different temperatures, formula (15) can be converted into the following shape [31]:
The generalized experimental results for several temperatures at pH 10.5 in the coordinates of Eq. (16) are shown in Fig. 22. It can be seen that all the experimental points are well approximated by a straight line, which again confirms the validity of Eq. (15) [31].
The conducted experiments demonstrated that the parameters of the chemisorption process depended on the initial pH value of the solution in which the sorption proceeds (Table 4) [31].
The data presented in Table 4 show that the chemical reaction of interaction of chromium(VI) with magnetite has sufficiently high activation energy. Therefore, at a nanometer size of magnetite particles, the diffusion transfer of hexavalent chromium ions in a solution to the surface of magnetite particles does not become a stage that determines the overall rate of the process and, even in a solution that is not stirred by a mixer, chemisorption proceeds in the kinetic mode.
The high values of correlation coefficients and Fisher’s criterion confirm that formula (14) reliably describes the obtained experimental data at z = 1 [31].
An increase in the activation energy of the chemisorption process and preexponential factor k in Eq. (14) was observed with an increase in the initial pH value of the solution (see Table 4). This is presumably determined by the displacement of the surface charge of magnetite particles to the negative region and the appearance of an additional electrostatic barrier during their interaction with magnetite.
Let us examine other similar works. Thus, the authors of [37] investigated the absorption of chromium(VI) by magnetite at pH 2 and at three different temperatures: 30, 40, and 50°C. The obtained dependences are shown in Fig. 5 in [37]. Their processing was performed according to several kinetic equations. Here, the highest correlation coefficient corresponded to the second-order equation with respect to the concentration of chromium(VI) ions in the solution. It was slightly lower for the first-order equation [37]. However, the impact of adsorption, which can distort the appearance of kinetic dependences at the beginning of the process, was not taken into account. For this reason, we recalculated these experimental data. The results of recalculation in the coordinates of Eq. (15) are shown in Fig. 23.
One can see that the dependences in Fig. 23 are similar to those obtained by the authors of [31] (see Figs. 20, 21). The first experimental points at the solution temperatures of 40 and 50°C, just like as in Fig. 20, deviate from a linear dependence, which can be explained, as above, by the influence of adsorption. However, at a temperature of 30°C, the linear dependence occurs from the very beginning of the process, which is not entirely clear, since here the influence of adsorption and, accordingly, the deviation from the linearity were believed to be the most pronounced.
In Fig. 24, the same experimental data are presented in the coordinates of Eq. (14) after its integration at z = 2. In this case, this equation takes the following form:
It can be seen that here, too, the first experimental points for temperatures of 40 and 50°C deviate from the expected linear dependence. This is also presumably determined by the influence of adsorption. And, just like in Fig. 23, there is a linear dependence for the experimental data at a temperature of 30°C. Thus, the results of [37] do not enable one to determine unambiguously which equation of the first or second order with respect to the concentration of chromium(VI) ions in the solution, describes the kinetics of absorption of hexavalent chromium by magnetite in the experiments of this work. In fact, this is not surprising, since it can be shown mathematically that, for a small range of changes in the С/С0 ratio, the equations (15) and (17) describe the experimental data with approximately the same accuracy. And, in order to get reliable differences between them, it is necessary to increase the range of changes in the С/С0 ratio. To illustrate what has been said in Fig. 25 in the coordinates of Eq. (17) is shown the experimental results obtained in [31] presented above in Fig. 21.
It can be seen from Fig. 25 that, in this case, the range of changes in the С/С0 ratio was quite wide, and in the coordinates of Eq. (17), there is a pronounced curvilinear dependence instead of linear, which allows us to unambiguously conclude that the second-order equation is not suitable for describing the experimental data obtained in [31] on the kinetics of interaction of chromium(VI) ions with magnetite.
Let us consider the experimental data on the kinetics of sorption of chromium(VI) ions by a magnetite nanopowder presented in Fig. 8 of [32]. They were shown above in Fig. 11. The results of their processing according to Eqs. (15) and (17) are shown in Fig. 26.
One can see that, due to the small range of changes in the С/С0 ratio, the results obtained with approximately the same accuracy can be described by Eq. (14) at z = 1 and z = 2.
The authors of [25] investigated the reduction of chromium(VI) ions in aqueous solutions with natural and synthetic magnetite at pH 4, 6, and 8. Some of the experimental data obtained are presented in Fig. 1 of this work. The results of their processing according to Eqs. (15) and (17) are shown in Fig. 27.
As follows from Fig. 27, chemisorption of chromium(VI) on natural magnetite can be also described with approximately the same accuracy by Eqs. (15) and (17). None of them can be definitively preferred. The reason for this is the same—an insufficiently wide range of changes in the С/С0 ratio in experiments with natural magnetite [25].
The results of processing of the experimental data on the interaction of hexavalent chromium ions with synthetic nanomagnetite presented in Fig. 1c from the same work are shown in Fig. 28.
As can be seen from Fig. 28a, the kinetics of reduction of chromium(VI) ions by synthetic magnetite at pH 6 and 8, same as in [32, 37], as well as when using natural magnetite (Fig. 27), can also be described with equal accuracy by Eqs. (15) and (17). However, at pH 4, where the range of changes in the С/С0 ratio was quite wide, the picture changes and it is possible to correctly describe the results obtained only by formula (15). While in the coordinates of Eq. (17), a curvilinear dependence is observed (see Fig. 28b).
The reduction of hexavalent chromium ions by magnetite at pH 7 was investigated in [45]. The results of processing the experimental data obtained there according to Eqs. (15) and (17) are shown in Fig. 29.
One can see that the results of this work clearly indicate that the kinetics of chemisorption of hexavalent chromium ions on magnetite is best described by Eq. (15).
Another work by the same authors shows that magnetite reduces chromium(VI) ions to chromium(III) on its surface, and Fig. 1 of this paper presents the data on the kinetics of absorption and reduction of chromium(VI) by magnetite [53]. The results of processing these data in the coordinates of Eqs. (15) and (17) are shown in Fig. 30. It can be seen that the same picture is observed here as above: in the coordinates of formula (15), there is a good linear dependence, which confirms the applicability of this equation to describe the redox reaction between hexavalent chromium ions and magnetite, and, in the coordinates of formula (17), there is a clearly defined curve, indicating that it is impossible to describe the process by this equation.
To sum up, the analysis of the published experimental data indicates that the kinetics of reduction of chromium(VI) by magnetite (i.e., the process of chemisorption of hexavalent chromium ions on magnetite) is well described by the first-order reaction equation with respect to the concentration of chromium(VI) in solution. It seemed of interest to further compare the values of integral rate constant of the chemisorption process K in the works discussed above (where possible). The results of this comparison for a temperature of 25°C are graphically presented in Fig. 31.
As can be seen, there are significant differences between the values of K calculated from the experimental data in [25] and [31, 32]. Moreover, the values of the integral rate constant of the chemisorption process, which were calculated based on the results of [45, 53], were equal to 1.78 × 10–10 and 1.74 × 10–10 m/s, respectively, which are several orders of magnitude higher than the analogous values in [25, 31, 32]. Therefore, they are not shown in Fig. 31. Despite this, Fig. 31 still shows some patterns. Here, the calculated points for K in [31, 32] are in agreement with each other and fall on the same curve, although these works used magnetite samples synthesized by different methods. They had only one thing in common—in both cases, sorption was carried out in a solution of sodium sulfate with a concentration of the latter of 400 mg/L. A good correlation is also observed between the values of K calculated from the experimental data [25]. All of them, for both natural and synthesized magnetite, fall on one curve (the dotted line in Fig. 31). Only one point for natural magnetite at pH 8 deviates significantly from this dependence, which seems to be due to an experimental error. Figure 31 also shows that the dependences from works [31, 32] and [25] have the same shape and run almost parallel to each other, although they differ in values by several times. The observed differences in the value of K are probably explained by the influence of the chemical composition of the solution in which the sorption process took place. For example, chemisorption in [31, 32], as was mentioned above, was studied in a solution of sodium sulfate (400 mg/L); in [25], it was studied in a solution of 0.01 M NaCl. The authors of [45, 53] studied the interaction of chromium(VI) ions with magnetite in 0.1 M NaCl solution. According to [54], the composition and concentration of the background electrolyte have a significant effect on the sorption of chromium(VI) by magnetite.
The found first order (in regards to the concentration of hexavalent chromium ions in a solution) of the process of reduction of chromium(VI) by magnetite, at first glance, does not agree with the equations of this reaction discussed above. However, it is apparently explained by the fact that the reduction of chromium(VI) ions to chromium(III) by iron (II), which is part of magnetite, actually occurs as a result of not one, but three consecutive redox reactions [7]:
These reactions proceed on the magnetite surface. Here, a chromium(VI) ion after interacting with a Fe(II) ion on one site of the magnetite surface by reaction (18), in order to continue its further reduction to chromium(IV), must migrate to another site of its surface, where there is an unoxidized Fe(II) ion. After being reduced there by reaction (19) to Cr (IV), it must then again jump to a new site of the magnetite surface, on which there is also an Fe(II) ion, and so on. In those sites on the magnetite surface where iron(II) was reduced to iron(III), the structure of the solid phase obviously changes and maghemite is formed. Thus, it was shown in [55] that magnetite transforms into maghemite in an oxidizing medium. The appearance of maghemite in the composition of magnetite powder after the sorption of hexavalent chromium ions is confirmed, as mentioned above, in multiple works [40, 41, 45, 48, 49].
It can be easily seen that each of reactions (18)–(20) has the first order with respect to the concentration of chromium ions in the solution, with the slowest of them determining the overall rate of the process. According to some researchers, this is reaction (19), since it is associated with a change in the coordination number of ligands in chromium ions, with a transition from a tetrahedral configuration to an octahedral one [7, 56].
Ratio between the Adsorption and Total Sorption Capacity of Magnetite towards Chromium(VI) Ions
When using magnetite as a sorbent to remove toxic hexavalent chromium ions from contaminated solutions, it is crucial to know its adsorption and total sorption capacity towards them. The authors of [31] estimated this value for a sulfate solution (400 mg/L sodium sulfate). The resulting dependences are shown in Fig. 32.
Dependence of the adsorption (○) and total sorption ( ) capacity of magnetite towards chromium(VI) ions on the initial pH value of the solution to be purified [31].
) capacity of magnetite towards chromium(VI) ions on the initial pH value of the solution to be purified [31].
These dependences are described by the following equations [31]:
where qf is the total sorption capacity of magnetite, mg/m2.
Here, the adsorption capacity indicates the amount of chromium(VI) ions absorbed by the magnetite surface during the first minutes of the sorption process, when the contribution of chemisorption can be disregarded. The total sorption capacity implies the maximum amount of chromium(VI) ions that can be sorbed by magnetite during its very long exposure to a solution to be cleaned or at an elevated sorption temperature, when the chemisorption process prevails.
As can be seen from Fig. 32, with an increase in the initial pH value of the purified solution, the adsorption and total sorption capacities of magnetite towards chromium(VI) decrease and at pH 11 they are almost equal zero. At pH 3.3, the full sorption capacity of magnetite is almost an order of magnitude higher than its adsorption capacity.
If we compare Figs. 32 and 15, it is easy to see that the values of the sorption capacity of magnetite towards hexavalent chromium ions shown in Fig. 15 occupy an intermediate position between the dependences of the adsorption capacity and the total sorption capacity shown in Fig. 32. So, according to Fig. 15, the sorption capacity of magnetite towards chromium(VI) at pH 6 is approximately 0.1 mg/m2, while, according to Fig. 32, the adsorption capacity of magnetite at the same pH value of the solution is about 0.05 mg/m2 and the total sorption capacity is about 0.35 mg/m2. The observed ratio is rather consistent, since the values of the sorption capacity in Fig. 15 correspond to the duration of the sorption process of about 24 h. It seems clear that, with such a duration of the process, the contribution of chemisorption becomes noticeable, which leads to an increase in the found value of the sorption capacity. Therefore, it exceeds the same value of the adsorption capacity in Fig. 32 (0.05 mg/m2). However, since the chemisorption process was not yet completed, it is lower than the total sorption capacity of magnetite at a given pH of the solution (0.35 mg/m2).
According to [45], the total sorption capacity of magnetite at pH 7 towards chromium(VI) ions, as mentioned above, is about 1.1 mg/m2. This value is about three times higher than the estimation that follows from Fig. 32 and is probably determined, yet again, by the difference in the chemical composition of the solution, where the interaction of magnetite with chromium(VI) occurs. It was already mentioned above that the concentration and composition of the background electrolyte greatly affected the rate of the chemisorption process. In [45], the background electrolyte was a 0.1 M NaCl solution, in which the integral rate constant of the chemisorption process is about two orders of magnitude higher than the same value for a sodium sulfate solution with a concentration of 400 mg/L. As a result, in [45], the depth of the magnetite layer that entered into the redox reaction with hexavalent chromium ions was apparently greater than in [31], which led to a higher value of the total sorption capacity in this work.
Let us note another interesting fact. It follows from Fig. 32 that, with an increase in the pH of the solution, the total sorption capacity of magnetite towards chromium(VI) ions decreases. This phenomenon is not entirely clear, since, in this case, a heterogeneous reaction occurs and the value of the total surface area of magnetite particles does not change with a change in the pH of the solution. Therefore, the acidity of the solution is not supposed to affect the total sorption capacity of the magnetite.
This phenomenon can be explained if we assume that it is caused by two factors. On the one hand, this is a slowdown in the rate of redox reaction between chromium(VI) and magnetite with an increase in the pH of the solution (see Fig. 31), and, on the other hand, a possible decrease in the effective surface of magnetite due to the adsorption of hydroxyl ions on it. We already noted above that, with an increase in the pH of the solution, the adsorption of these ions on the magnetite surface increases (Figs. 2, 5).
CONCLUSIONS
To sum up, magnetite represents a promising sorbent for the removal of toxic hexavalent chromium ions from contaminated natural and waste waters.
It has been shown that two processes occur simultaneously during the sorption of chromium(VI) ions by magnetite: ordinary adsorption and chemisorption (redox reaction between hexavalent chromium ions and magnetite). The latter is accompanied by the oxidation of iron(II) in magnetite to iron(III) and the reduction of chromium(VI) ions to chromium(III) with the formation of maghemite and a number of other compounds, including mixed chromium(III) compounds with iron, in the surface layer of magnetite (or on its surface). The chemical composition of these compounds is determined by the initial acidity of the solution from which chromium(VI) ions are sorbed and the ratio between their concentration and the amount of magnetite powder introduced into the solution.
As a result of chemisorption, unlike the case of traditional sorbents, hexavalent chromium ions are irreversibly bound by magnetite, which prevents their reentry into the environment.
The kinetics of the redox process between chromium(VI) and magnetite is described by the first-order reaction equation with respect to the concentration of chromium(VI) ions in a solution. The parameters of this equation are determined by the acidity and composition of the chromium-containing solution. With an increase in pH, the rate of the interaction of chromium(VI) ions with magnetite decreases.
The sorption capacity of magnetite, as it is saturated with chromium, irreversibly decreases. To recover it, it is necessary to remove the surface layer of magnetite particles (for example, by partial dissolution in acid). The sorption capacity of magnetite towards chromium(VI) ions also decreases with increasing pH of the purified solution, and at pH > 11 it is almost zero.
REFERENCES
Salnikow, K. and Zhitkovich, A., Chem. Res. Toxicol., 2008, vol. 21, pp. 28–44. https://doi.org/10.1021/tx700198a
Rukovodstvo po professional’nym zabolevaniyam (Manual on Occupational Diseases), Izmerov, N.F., Ed., Moscow: Meditsina, 1978.
Costa, M., Crit. Rev. Toxicol., 1997, vol. 27, pp. 431–442. https://doi.org/10.3109/10408449709078442
Zhitkovich, A., Chem. Res. Toxicol., 2011, vol. 24, pp. 1617–1629. https://doi.org/10.1021/tx200251t
Spravochnik po professional’noi patologii (Handbook on Occupational Pathology), Gratsianskaya, L.N. and Kovshilo, V.E., Ed., Moscow: Meditsina, 1981.
Kurniawan, T.A., Chan, G.Y.S., Lo, W.-H., and Babel, S., Chem. Eng. J., 2006, vol. 118, pp. 83–98. https://doi.org/10.1016/j.cej.2006.01.015
Buerge, I.J. and Hug, S.H., Environ. Sci. Technol., 1997, vol. 31, pp. 1426–1432.
Jin, W., Du, H., Zheng, S., and Zhang, Y., Electrochim. Acta, 2016, vol. 191, pp. 1044–1055. https://doi.org/10.1016/j.electacta.2016.01.130
Mitrakas, M.G., Pantazatou, A.S., Tzimou-Tsitouridou, R., and Sikalidis, C.A., Desalin. Water Treat., 2011, vol. 33, nos. 1–3, pp. 77–85. https://doi.org/10.5004/dwt.2011.2620
Owlad, M., Aroua, M.K., Daud, W.A.W., and Baroutian, S., Water, Air, Soil Pollut., 2008, vol. 200, no. 1, pp. 59–77. https://doi.org/10.1007/s11270-008-9893-7
Mohan, D. and Pittman, C.U., Jr., J. Hazard. Mater., 2006, vol. 137, pp. 762–811. https://doi.org/10.1016/j.hazmat.2006.06.060
Venditti, F., Cuomo, F., Ceglie, A., Ambrosone, L., and Lopez, F., J. Hazard. Mater., 2010, vol. 173, pp. 552–557. https://doi.org/10.1016/j.jhazmat.2009.08.121
Kairbekov, Zh.K., Yemelyanova, V.S., Shakiyeva, T.V., Dossumova, B.T., Dzhatkambayeva, U.N., and Shakiyev, E.M., Adv. Mater. Res., 2015, vols. 1079–1080, pp. 88–94. https://doi.org/10.4028/www.scientific.net/AMR.1079-1080.88
Moghimi, A., Int. J. Bio-Inorg. Hybrid Nanomater., 2016, vol. 5, no. 3, pp. 203–212.
Horst, M.F., Alvarez, M., and Lassalle, V.L., Sep. Sci. Technol., 2016, vol. 51, no. 3, pp. 550–563. https://doi.org/10.1080/01496395.2015.1086801
Saharan, P., Chaudhary, G.R., Mehta, S.K., and Umar, A., J. Nanosci. Nanotechnol., 2014, vol. 14, pp. 627–643. https://doi.org/10.1166/jnn.2014.9053
Xu, P., Zeng, G.M., Huang, D.L., Feng, C.L., Hu, S., Zhao, M.H., Lai, C., Wei, Z., Huang, C., Xie, G.X., and Liu, Z.F., Sci. Total Environ., 2016, vol. 424, pp. 1–10. https://doi.org/10.1016/j.scitotenv.2012.02.023
Hu, J., Chen, G., and Lo, I.M.C., J. Environ. Eng., 2006, vol. 132, pp. 709–715. https://doi.org/10.1061/(ASCE)0733-9372(2006)132:7(709)
Chowdhury, S.R. and Yanful, E.K., J. Environ. Manage., 2010, vol. 91, pp. 2238–2247. https://doi.org/10.1016/j.jenvman.2010.06.003
Zhao, J., Guan, X., and Unuma, H., J. Ceram. Soc. Jpn., 2007, vol. 115, no. 8, pp. 475–478.
Vainshtein, I.A., Ochistka i ispol’zovanie stochnykh vod travil’nykh otdelenii (Purification and Utilization of Waste Water from Etching Departments), Moscow: Metallurgiya, 1986, p. 82.
Gheju, M., Water, Air, Soil Pollut., 2011, vol. 222, pp. 103–148. https://doi.org/10.1007/s11270-011-0812-y
Pang, Y., Zeng, G., Tang, L., Zhang, Y., Liu, Y., Lei, X., Li, Z., Zhang, J., Liu, Z., and Xiong, Y., Chem. Eng. J., 2011, vol. 175, pp. 222–227. https://doi.org/10.1016/j.cej.2011.09.098
Gallios, G.P. and Vaclavikova, M., Environ. Chem. Lett., 2008, vol. 6, pp. 235–240. https://doi.org/10.1007/s10311-007-0128-8
Villacís-García, M., Villalobos, M., and Gutiérrez-Ruiz, M., J. Hazard. Mater., 2015, vol. 281, pp. 77–86. https://doi.org/10.1016/j.jhazmat.2014.07.007
Hasany, S.F., Ahmed, I., Rajan, J., and Rehman, A., Nanosci. Nanotechnol., 2012, vol. 2, no. 6, pp. 148–158. https://doi.org/10.5923/j.nn.20120206.01
Kotov, Yu.A., Osipov, V.V., Ivanov, M.G., Samatov, O.M., Platonov, V.V. Azarkevich, E.I., Murzakaev, A.M., and Medvedev, A.I., Tech. Phys., 2002, vol. 47, no. 11, pp. 1420–1426.
Medvedeva, I., Uimin, M., Yermakov, A., Mysik, A., Byzov, I., Nabokova, T., Gaviko, V., Shchegoleva, N., Zhakov, S., Tsurin, V., Linnikov, O., Rodina, I., Platonov, V., and Osipov, V., J. Nanopart. Res., 2012, vol. 14, no. 3, pp. 740–750. https://doi.org/10.1007/s11051-012-0740-9
Bernal, J.D., Dasgypta, D.R., and Mackay, A.S., Clay Miner. Bull., 1959, vol. 4, no. 21, pp. 15–30.
Kijama, M., Clay Miner. Bull., 1974, vol. 47, no. 7, pp. 1646–1650. Kijama, M., Bull. Chem. Soc. Jpn., 1974, vol. 47, no. 7, pp. 1646–1650.
Linnikov, O.D. and Rodina, I.V., Sci. Rev. Chem. Commun., 2018, vol. 8, no. 1, pp. 1–17.
Linnikov, O., Rodina, I., Shevchenko, V., Medvedeva, I., Uimin, M., Schegoleva, N., Yermakov, A., Platonov, V., and Osipov, V., Desalin. Water Treat., 2014, vol. 52, nos. 1–3, pp. 324–330. https://doi.org/10.1080/19443994.2013.786654
Barale, M., Lefèvre, G., Carrette, F., Catalette, H., Fèdoroff, M., and Cote, G., J. Colloid Interface Sci., 2008, vol. 328, pp. 34–40. https://doi.org/10.1016/j.jcis.2008.09.007
Vidojkovic, S., Rodriguez-Santiago, V., Fedkin, M.V., Wesolowski, D.J., and Lvov, S.N., Chem. Eng. Sci., 2011, vol. 66, pp. 4029–4035. https://doi.org/10.1016/j.ces.2011.05.021
Tombácz, E., Illés, E., Majzik, A., Hajdú, A., Rideg, N., and Szekeres, M., Croat. Chem. Acta, 2007, vol. 80, nos. 3–4, pp. 503–515.
Hu, J., Lo, I.M.C., and Chen, G., Water Sci. Technol., 2004, vol. 50, no. 12, pp. 139–146. https://doi.org/10.2166/wst.2004.0706
Namdeo, M. and Bajpai, S.K., EJEAFChe, Electron. J. Environ., Agric. Food Chem., 2008, vol. 7, no. 7, pp. 3082–3094.
Frolov, Yu.G., Kurs kolloidnoi khimii. Poverkhnostnye yavleniya i dispersnye sistemy (Course of Colloid Chemistry. Surface Phenomena and Disperse Systems), Moscow: Khimiya, 1988.
Lesnikovich, A.I., Vorob’eva, S.A., and Karpenko, N.V., Zh. Prikl. Khim., 1994, vol. 67, pp. 500–502.
Yuan, P., Liu, D., Fan, M., Yang, D., Zhu, R., Ge, F., Zhu, J., and He, H., J. Hazard. Mater., 2010, vol. 173, pp. 614–621. https://doi.org/10.1016/j.jhazmat.2009.08.129
Chowdhury, S.R., Yanful, E.K., and Pratt, A.R., J. Hazard. Mater., 2012, vols. 235–236, pp. 246–256. https://doi.org/10.1016/j.jhazmat.2012.07.054
Zhu, Z., Zhu, Y., Yang, F., Zhang, X., Qin, H., Liang, Y., and Liu, J., Desalin. Water Treat., 2014, vol. 52, nos. 16–18, pp. 3133–3146. https://doi.org/10.1080/19443994.2013.798841
Kuznetsov, M.V., Linnikov, O.D., and Rodina, I.V., Inorg. Mater., 2012, vol. 48, no. 2, pp. 169–175. https://doi.org/10.1134/S0020168512020148
Buldakova, L.Yu., Linnikov, O.D., Rodina, I.V., and Yanchenko, M.Yu., Inorg. Mater., 2015, vol. 51, no. 2, pp. 138–141. https://doi.org/10.1134/S0020168515010033
Peterson, M., White, A.F., Brown, E., Jr., and Parks, G.A., Environ. Sci. Technol.,1997, vol. 31, no. 5, pp. 1573–1576.
Jung, Y., Choi, J., and Lee, W., Chemosphere, 2007, vol. 68, pp. 1968–1975. https://doi.org/10.1016/j.chemosphere.2007.02.028
Kendelewicz, T., Liu, P., Doyle, C.S., Brown, G.E., Nelson, E.J., and Chambers, S.A., Surf. Sci., 1999, vol. 424, pp. 219–231.
He Thomas, Y. and Traina, S.J., Environ. Sci. Technol., 2005, vol. 39, pp. 4499–4504.
Kendelewicz, T., Liu, P., Doyle, C.S., and Brown, G.E., Surf. Sci., 2000, vol. 469, pp. 144–163.
Eary, L.E. and Rai, D., Environ. Sci. Technol., 1988, vol. 22, pp. 972–977.
Li, X., Cao, J., and Zhang, W., Ind. Eng. Chem. Res., 2008, vol. 47, no. 7, pp. 2131–2139.
Chen, S.-S., Hsu, B.-C., and Hung, L.-W., J. Hazard. Mater., 2008, vol. 152, pp. 1092–1097. https://doi.org/10.1016/j.jhazmat.2007.07.086
Peterson, M., Brown, G.E., and Parks, G.A., Colloids Surf., A, 1996, vol. 107, pp. 77–88.
Meena, A.H. and Arai, Y., Geochem. Trans., 2016, vol. 17, pp. 1–13. https://doi.org/10.1186/s12932-016-0033-9
White, A.F., Peterson, M.L., and Hochella, M.F., Jr., Geochim. Cosmochim. Acta, 1994, vol. 58, no. 8, pp. 1859–1875.
Tong, J.Y. and King, E.L., J. Am. Chem. Soc., 1960, vol. 82, no. 15, pp. 3805–3809.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Marinin
Rights and permissions
About this article
Cite this article
Linnikov, O.D. Regularities of Chromium(VI) Ions Sorption by Magnetite (Review). Prot Met Phys Chem Surf 57, 235–259 (2021). https://doi.org/10.1134/S2070205121020064
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205121020064