Abstract
The staged crystallization of aluminophosphate AlPO4-11 from a commercially available aluminum source based on boehmite is studied for the first time by means of X-ray diffraction, 27Al and 31P magic-angle spinning nuclear magnetic resonance, low-temperature nitrogen adsorption–desorption, and scanning election microscopy. It is shown that the synthesis of AlPO4-11 proceeds via the formation of an intermediate phase based on crystalline aluminophosphate with a layered structure. It is found that AlPO4-11 with a high degree of crystallinity and phase purity forms in 6–24 h at 200°C. Lengthening the time of crystallization at 200°C to more than 48 h results in the transformation of AlPO4-11 into nonporous cristobalite. The results can be used to develop means for directional control of the phase purity and degree of crystallinity of commercially important silicoaluminophosphate molecular sieves SAPO-11 with desired properties. These can be used as a base for the synthesis of promising domestic catalysts for the commercial hydroisomerization of higher n-paraffins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Zeolites and zeolite-like materials are commonly used as heterogeneous catalysts, adsorbents, and ion-exchange materials, due to the presence of acid sites, a developed microporous structure, and a molecular sieve effect in them [1–6].
Due to the presence of a one-dimensional channel system of pores and medium-strength acid sites in aluminophosphate (AlPO4-11) and silicoaluminophosphate molecular sieves (SAPO-11), these materials are of considerable interest as promising catalyst systems for the hydroisomerization of higher n-paraffins. However, issues over the crystallization mechanism of these materials remain poorly studied.
In 1982, Wilson et al. [8, 9] reported the synthesis of a new class of zeolite-like materials based on aluminophosphates AlPO4-n (n indicates the type of structure). The AlPO4-n structure is composed of strictly alternating AlO4 and PO4 tetrahedra linked through their oxygen atoms. Aluminophosphates with one-dimensional, two-dimensional, and three-dimensional channel structures have been synthesized: AlPO4-11, AlPO4-40, and AlPO4-18, respectively [10]. The sizes of the entrance windows in aluminophosphates can vary in the range of 3–12 Å. These materials are of considerable interest as matrices for the development of single-site catalysts for liquid-phase oxidation, since they are capable of isomorphously incorporating variable-valence metal atoms into their crystal lattices [11–16]. At the same time, introducing atoms of such elements as Si or Mg into the aluminophosphate lattice produces acid sites that are weaker than those in aluminosilicates [17–20].
Among the wide variety of aluminophosphates, of particular interest is aluminophosphate AlPO4-11 with a one-dimensional system of 10-membered elliptic channels 0.40 × 0.65 nm in size [10]; it has been used as a base for synthesizing silicoaluminophosphate SAPO-11 and developing promising catalyst systems for the hydroisomerization of higher n-paraffins [21], the methylation of aromatic hydrocarbons [22], the isomerization of butylene to isobutylene [23], and the isomerization of cyclohexanone oxime to caprolactam [24].
Researchers have given much attention to studying the crystallization mechanism of AlPO4-11; an understanding of this mechanism is required for developing ways of controlling the phase purity and degrees of crystallinity and dispersion of the synthesized aluminophosphates, and thus silicoaluminophosphate molecular sieves with desired properties. Using ex situ X-ray diffraction (XRD) and 27Al and 31P magic-angle spinning nuclear magnetic resonance (MAS NMR), the authors of [25, 26] studied the crystallization of AlPO4-11 from aluminophosphate gels of different concentrations, prepared using pseudoboehmite (AlO((OH)) as an aluminum source. It was shown that in the dilute gels, AlPO4-5 forms at the initial stage of crystallization; afterward, it transforms into AlPO4-11. The intermediate formation of AlPO4-5 is not observed in concentrated gels.
The crystallization of AlPO4-11 from aluminophosphate gels prepared using phosphoric acid and Al(OH)3 was studied by the authors of [27] by means of two-dimensional 27Al and 31P MAS NMR and Fourier transform Raman spectroscopy. It was found that at room temperature, amorphous aluminophosphate can form even at the initial stage of gel preparation. The formation of an intermediate semicrystalline phase was also observed, but the structure of this phase was not determined.
The crystallization of AlPO4-11 from dry gels, also prepared using phosphoric acid and Al(OH)3, was studied via ex situ XRD and 27Al and 31P MAS NMR in [28]. It was shown that a semicrystalline phase containing fragments of 10-membered rings forms at the initial stages of crystallization. The cited authors assumed that the local structure of this phase was fairly close to that of AlPO4-11, and the former was rapidly transformed into the latter. However, the structure of the intermediate phases was not fully determined either.
Despite the considerable number of works on the synthesis of AlPO4-11 [25–36], the mechanism of this aluminophosphate’s crystallization thus remains poorly understood. The aim of this work was to study the key stages of the formation of AlPO4-11 via crystallization from an aluminophosphate gel prepared using an industrial feedstock (boehmite) as an aluminum source.
EXPERIMENTAL
Aluminophosphate Synthesis Procedure
All aluminophosphate samples were prepared via hydrothermal synthesis from a reaction gel of the folldue composition: 1.0Al2O3 · 1.0P2O5 · 1.0 di-n-propylamine (DPA) · 50H2O. The sources of phosphorus and aluminum were phosphoric acid (85% H3PO4, Reakhim, Russia) and boehmite (81% Al2O3, OOO Ishimbay Specialized Chemical Plant of Catalysts, Russia), respectively; DPA (99%, Acros Organics) was used as a template. The aluminophosphate gel was prepared as follows: 50.0 g of phosphoric acid was diluted with 178.0 g of distilled water; 27.5 g of boehmite was added to the resulting solution; the mixture was vigorously stirred for 1 h. Next, 22 g of DPA was added to the resulting gel, and the mixture was held in a thermostat at 90°C for 24 h. It was shown in [37] that the original gel must be held at 90°C to ensure the subsequent selective crystallization of AlPO4-11. The prepared gel is designated AlPO4(90°C). The crystallization of aluminophosphates was conducted at 200°C in a stainless steel autoclave (SEN, V = 1000 mL) with a special fluoroplastic coating and a sampling system. The products of crystallization were sampled after 1, 3, 6, 12, 24, 48, and 72 h. The aluminophosphate samples synthesized from the AlPO4(90°C) gel were designated as AlPO4-11-n, where n is the period of crystallization.
Investigation Procedure
X-ray diffraction analysis of the samples was performed on a Rigaku Ultima IV diffractometer using monochromatized CuKα radiation. Scanning was done in a 2θ angular range of 3°–80° in increments of 0.5 deg/min; acquisition time was 2 s per point. The XRD patterns were processed using the Rigaku PDXL software and the PDF2 database.
The coordination environment of the aluminum and phosphorus atoms was determined from 27Al and 31P MAS NMR spectra. The spectra were recorded on a Bruker Avance-400 NMR spectrometer under conditions of a single-pulse experiment, while spinning the sample at the magic angle (≈7 KHz) in zirconia rotors (Ø4 mm). The Larmor frequency was 104 (27Al) and 162 MHz (31P); the π/2 pulse length was 2 (31P) and 2.5 μs (27Al).
Micropore volume was determined from the heptane vapors in a desiccator, as described in [38]. Specific surface area and total pore volume were measured via low-temperature nitrogen adsorption–desorption on an ASAP 2020 surface area and porosimetry system. Calculations of the specific surface area using the Brunauer–Emmett–Teller (BET) approach [39] were made at a relative partial pressure of Р/Р0 = 0.2.
The morphology of the aluminophosphates was studied via scanning electron microscopy (SEM) on a JEOL JSM-6490LV electron microscope. Images were recorded in the secondary electron imaging mode at an accelerating voltage of 20 kV and a working distance of 10 mm. Before recording, the samples were placed on the surface of an aluminum table with a diameter of 25 mm and fixed there using a conductive adhesive tape.
RESULTS AND DISCUSSION
Dependence of the Phase Composition of the Products of Crystallization on the Period of Synthesis
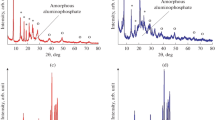
Figure 1 shows XRD patterns of the original gel and the products of crystallization formed at varying times of synthesis. Different phases were observed in the original AlPO4(90°C) gel sample: DPA phosphate (30 vol %), undissolved boehmite (10 vol %), amorphous aluminophosphate (60 vol %), and variscite (10 vol %). It is evident that the subsequent crystallization of the gel for 1 h at 200°C (sample AlPO4-11-1) results in the formation of an intermediate AlPO4 crystalline aluminophosphate phase (PDF 00-201-0795). According to XRD, this material has a rhombic syngony with an average coherent scattering region (CSR) of ≈10 nm. After 3 h of crystallization (sample AlPO4-11-3), the main phase was AlPO4-11, and a small amount of unreacted boehmite was observed. It should be noted that 24 h of crystallization led to complete dissolution of the unreacted boehmite and the formation of AlPO4-11 with a degree of crystallinity of ≈100% (sample AlPO4-11-24). Lengthening the period of crystallization past 24 h (sample AlPO4-11-48) produced a new phase of crystalline aluminophosphate with a cristobalite structure. After 72 h (sample AlPO4-11-72), complete recrystallization of the AlPO4-11 into cristobalite was observed.
More detailed information on the chemical nature of aluminophosphates formed during the initial period of crystallization could be obtained via 27Al and 31P MAS NMR spectroscopy. Figure 2 shows 27Al and 31P MAS NMR spectra for the original gel and the products obtained at different periods of synthesis.
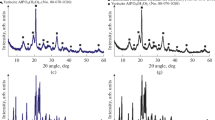
It is evident that the 27Al MAS NMR spectra of the original gel (Fig. 2a) exhibit characteristic signals at 48, 12, and −9 ppm. The authors of [33–36] attributed the signal at 48 ppm to tetrahedrally coordinated aluminum atoms that were part of the alumophosphate sol particles. The signal at 12 ppm is characteristic of octahedrally coordinated aluminum atoms contained in undissolved boehmite. The signal at −9 ppm corresponds to octahedrally coordinated aluminum atoms that were part of nonporous aluminophosphates. Crystallizing the AlPO4(90°C) gel for 1 h considerably weakened the signal at 12 ppm and amplified the signal at −9 ppm. The results are attributed to the interaction between previously unreacted boehmite and phosphoric acid, and the formation of an intermediate crystalline aluminophosphate. This assumption is in good agreement with the XRD data (see Fig. 1). Three hours of crystallization substantially reduced the fraction of the signal at −9 ppm and produces a new signal at 42 ppm, which is characteristic of tetrahedral aluminum in the crystal lattice of AlPO4-11. Twenty-four hours of crystallization resulted in complete disappearance of the signal at −9 ppm and amplification of the signal at 42 ppm.
According to 31P MAS NMR (Fig. 2b), the spectra of the original gels exhibit signals at 38, 27, 5, −8, and −15 ppm. The authors of [40, 41] attributed the signal at 5 ppm to phosphates of nitrogen-containing organic compounds. This signal is apparently associated with DPA phosphate, the presence of which was confirmed by XRD data for the original gel (Fig. 1, sample AlPO4-11-1). The signals at −8 and −15 ppm were attributed to tetrahedrally coordinated phosphorus atoms in aluminophosphates with different degrees of replacing –OH groups with aluminum atoms [33–36]. The signals at 27 and 38 ppm were attributed to tetrahedrally coordinated P atoms in the crystalline structure of the zeolite.
The spectral data show that the original AlPO4(90°C) gel exhibited signals at 5 and 8 ppm and an intense signal at 15 ppm. These signals suggest that most of the P atoms were present in the form of aluminophosphates with different fractions of P–O–Al bonds. This is in good agreement with the XRD and 27Al MAS NMR data. After 1 h of crystallization, the spectrum exhibited the main signal at −15 ppm. This indicates the formation of an intermediate crystalline aluminophosphate phase (Fig. 1, sample AlPO4-11-1). After 3 h of crystallization, an intense signal at 27 ppm, associated with the presence of tetrahedrally coordinated P atoms in the lattice of AlPO4-11, appeared in the spectrum. Lengthening the period of crystallization to 24 h produced a single signal at 27 ppm (spectrum not shown), testifying to the high degree of crystallinity of the synthesized material.
Dependence of the Pore Structure of Crystallization Products on the Period of Synthesis
Table 1 shows characteristics of the pore structure of the aluminophosphate samples prepared at varying periods of crystallization. It is evident the intermediate crystalline aluminophosphate that formed after 1 h (sample AlPO4-11-1) was a porous material with a total pore volume of VƩ = 0.45 cm3/g and a BET specific surface area of 80 m2/g. Figure 3 shows the adsorption–desorption isotherm and the pore size distribution for this sample. It is evident that this material was a meso/macroporous system with a broad pore size distribution in the range of 2–300 nm. A reduction in the total pore volume (to 0.15 cm3/g) and in the specific surface area (to 132 m2/g) was observed after 3 h of crystallization (sample AlPO4-11-3). Lengthening the period of crystallization to 24 h (sample AlPO4-11-24) produces AlPO4-11 with a high degree of crystallinity, a pore volume of \({{V}_{{{{{\text{C}}}_{7}}{{{\text{H}}}_{{16}}}}}}\) = 0.14 cm3/g with respect to heptane, a total pore volume of VƩ = 0.16 cm3/g, and a specific surface area of SBET ≈ 110 m2/g. These results are in good agreement with the published data for aluminophosphate AlPO4-11 [25–32]. Lengthening the period of crystallization past 48 h (sample AlPO4-11-48) further reduces the total pore volume to VƩ = 0.09 cm3/g and the specific surface area to SBET ≈ 60 m2/g.
The above data suggest that the intermediate crystalline aluminophosphate (AlPO4-11-1 sample) formed in the initial period of crystallization is characterized by a developed meso/macroporous structure. The subsequent transformation of this aluminophosphate into AlPO4-11 lowers the specific surface area and the volume of micro- and mesopores. The recrystallization of AlPO4-11 into cristobalite further reduces in the pore volume and the specific surface area.
Dependence of the Morphology of Crystallization Products on the Period of Synthesis
Figure 4 shows SEM images of aluminophosphate samples prepared at varying periods of crystallization. It is evident that the AlPO4-11-1 sample consists of thin layered crystals 10–20 nm thick, which is in good agreement with the CSR value of around 10 nm. According to SEM, the pore size of this aluminophosphate is 100–300 nm. It should be noted that in the 2θ range of 3°–7°, the XRD pattern of the sample (AlPO4-11-1) exhibits none of the signals characteristic of layered materials, due to its large interplanar distances. According to XRD and 27Al and 31P MAS NMR, this sample is a crystalline aluminophosphate. According to XRD and 27Al and 31P MAS NMR, after 3 h of crystallization (AlPO4-11-3 sample), the dominant phase is AlPO4-11 (90%) with the crystal morphology in the form of thin plates 1–2 μm in size. Upon reaching the maximum degree of crystallinity after 6 h (sample AlPO4-11-6), the crystal morphology remained unchanged in the form of thin rectangular plates.
As noted above, AlPO4-11 was transformed into cristobalite after 48 h of crystallization. The image of the AlPO4-11-48 sample shows large (30–40 μm) crystals in the form of truncated octahedra; in addition, a small number of the remaining parts of AlPO4-11 crystals is observed.
According to XRD, 72 h of crystallization resulted in the complete recrystallization of AlPO4-11 into cristobalite.
Crystallization of AlPO4-11 from an Aluminophosphate Gel Using Boehmite
The obtained results revealed the stages of formation of AlPO4-11 from a boehmite-based aluminophosphate gel (Fig. 5). At the first stage (stage I, 90°C, 24 h), DPA phosphate reacted with undissolved boehmite to form an amorphous aluminophosphate gel and variscite; this ensured the subsequent selective crystallization of AlPO4-11. At the second stage (stage II), the aluminophosphate gel was transformed into a crystalline aluminophosphate with a layered structure. The duration of this stage at 200°C was around 1 h; in the literature [1], it is commonly referred to as the induction period, the stage at which nuclei form. At the third stage (stage III), AlPO4-11 crystals grew intensely for 3–6 h. The layered aluminophosphate simultaneously transformed almost completely into AlPO4-11, while unreacted boehmite and the amorphous phase dissolved. The AlPO4-11 content was 90% after 6 h of crystallization. Upon lengthening the period of crystallization from 6 to 24 h (stage IV), the AlPO4-11 content was ≈100%; in the literature, this stage is referred to as aging of the system. Lengthening the period of synthesis further to 72 h (stage V) resulted in the recrystallization of AlPO4-11 into cristobalite (see Fig. 4).
CONCLUSIONS
The kinetics of the crystallization (200°C) of an aluminophosphate gel prepared from phosphoric acid and boehmite into commercially important AlPO4-11 with a high degree of crystallinity and phase purity was studied via XRD, 27Al and 31P MAS NMR, low-temperature nitrogen adsorption–desorption, and SEM.
It was found that the formation of AlPO4-11 proceeds through the stages of
• holding the gel at 90°C (24 h) to ensure the subsequent selective crystallization of AlPO4-11;
• an induction period around 1 h long to ensure the formation of an intermediate phase based on crystalline aluminophosphate with a layered structure;
• intense growth of AlPO4-11 crystals for 3–6 h; and
• recrystallization of AlPO4-11 into cristobalite.
It was found that AlPO4-11 with a high degree of crystallinity and phase purity forms in 6–24 h at 200°C. Upon lengthening the period of crystallization at 200°C to more than 48 h, we observed the transformation of AlPO4-11 into nonporous cristobalite.
The results will be used to develop means for the directional control of the phase purity and degree of crystallinity of commercially important silicoaluminophosphate molecular sieves with desired properties.
REFERENCES
Cejka, J., Corma, A., and Zones, S., Zeolites and Catalysis: Synthesis, Reactions and Applications, Weinheim: Wiley-VCH, 2010.
Degnan Jr, T. F., Stud. Surf. Sci. Catal., 2007, vol. 170, pp. 54–65.
Moliner, M., Martínez, C., and Corma, A., Angew. Chem., Int. Ed., 2015, vol. 54, no. 12, pp. 3560–3579.
Chal, R., Gérardin, C., Bulut, M., and van Donk, S., ChemCatChem, 2011, vol. 3, no. 1, pp. 67–81.
Zeolite Characterization and Catalysis: A Tutorial, Chester, A.W. and Derouane, E.G., Eds., New York: Springer 2009.
Wright, P.A., Microporous Framework Solids, Cambridge: Royal Society of Chemistry, 2008.
Xu, R., Pang, W., Yu, J., Huo, Q., and Chen, J., Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure, Singapore: Wiley, 2007.
Wilson, S.T., Lok, B.M., Messina, C.A., Cannan, T.R., and Flanigen, E.M., J. Am. Chem. Soc., 1982, vol. 104, no. 4, pp. 1146–1147.
US Patent 4310440, 1982.
Baerlocher, C., McCusker, L.B., and Olson, D.H., Atlas of Zeolite Framework Types, New York: Elsevier, 2007.
Thomas, J.M., Raja, R., Sankar, G., and Bell, R.G., Nature, 1999, vol. 398, no. 6724, pp. 227–230.
Inui, T., Matsuda, H., Okaniwa, H., and Miyamoto, A., Appl. Catal., 1990, vol. 58, no. 1, pp. 155–163.
Thomas, J.M., Xu, Y., Catlow, C.R.A., and Couves, J.W., Chem. Mater., 1991, vol. 3, no. 4, pp. 667–672.
Liu, Y., Liu, C., Liu, C., Tian, Z., and Lin, L., Energy Fuels, 2004, vol. 18, no. 5, pp. 1266–1271.
Rastelli Jr, H., Lok, B.M., Duisman, J.A., Earls, D.E., and Mullhaupt, J.T., Can. J. Chem. Eng., 1982, vol. 60, no. 1, pp. 44–49.
Thomas, J.M., Raja, R., Sankar, G., and Bell, R.G., Acc. Chem. Res., 2001, vol. 34, no. 3, pp. 191–200.
Pastore, H.O., Coluccia, S., and Marchese, L., Annu. Rev. Mater. Res., 2005, vol. 35, pp. 351–395.
Liu, Y., Yan, A.Z., and Xu, Q.H., Appl. Catal., 1990, vol. 67, pp. 169–177.
Borade, R.B. and Clearfield, A., J. Mol. Catal., 1994, vol. 88, no. 2, pp. 249–266.
Tao, S., Li, X., Gong, H., Jiang, Q., Yu, W., Ma., H., Xu, R., Tian, Z., Microporous Mesoporous Mater., 2018, vol. 262, pp. 182–190.
Walendziewski, J. and Pniak, B., Appl. Catal., A, 2003, vol. 250, no. 1, pp. 39–47.
Zhu, Z., Chen, Q., Xie, Z., and Yang, W., Microporous Mesoporous Mater., 2006, vol. 88, nos. 1–3, pp. 16–21.
Yang, S.-M., Lin, J.-Y., Guo, D.-H., and Liaw, S.-G., Appl. Catal., A, 1999, vol. 181, no. 1, pp. 113–122.
Singh, P.S., Bandyopadhyay, R., Hegde, S.G., and Rao, B.S., Appl. Catal., A, 1996, vol. 136, no. 2, pp. 249–263.
Cheng, T., Xu, J., Li, X., Li, Y., Zhang, B., Yan, W., Yu, J., Sun, H., Deng, F., and Xu, R., Microporous Mesoporous Mater., 2012, vol. 152, pp. 190–207.
Zhang, B., Xu, J., Fan, F., Guo, Q., Tong, X., Yan, W., Yu, J., Deng, F., Li, C., and Xu, R., Microporous Mesoporous Mater., 2012, vol. 147, no. 1, pp. 212–221.
Huang, Y., Richer, R., and Kirby, C.W., J. Phys. Chem. B, 2003, vol. 107, no. 6, pp. 1326–1337.
Chen, B. and Huang, Y., J. Phys. Chem. C, 2007, vol. 111, no. 42, pp. 15236–15243.
Balakrishnan, I. and Prasad, S., Appl. Catal., 1990, vol. 62, no. 1, pp. L7–L11.
Qisheng, H. and Xu, R., J. Chem. Soc., Chem. Commun., 1990, no. 10, pp. 783–784.
Ojo, A.F. and McCusker, L.B., Zeolites, 1991, vol. 71, no. 5, pp. 460–465.
Zhao, X., Gao, X., Zhang, X., and Hao, Z., Microporous Mesoporous Mater., 2017, vol. 242, pp. 160–165.
Zhao, Z., Zhang, W., Xu, R., Han, X., Tiana, Z., and Bao, X., Dalton Trans., 2012, vol. 41, no. 3, pp. 990–994.
Tapp, N.J., Milestone, N.B., and Bibby, D.M., Zeolites, 1988, vol. 8, no. 3, pp. 183–188.
Zhu, G., Qiu, S., Gao, F., Wu, G., Wang, R., Li, B., Fang, Q., Li, Y., Gao, B., Xu, X., and Terasaki, O., Microporous Mesoporous Mater., 2001, vol. 50, nos. 2–3, pp. 129–135.
Bandyopadhyay, M., Bandyopadhyay, R., Kubota, Y., and Sugi, Y., Chem. Lett., 2000, vol. 29, no. 9, pp. 1024–1025.
Agliullin, M.R., Khairullina, Z.R., Faizullin, A.V., Petrov, A.I., Badretdinova, A.A., and Kutepov, B.I., Abstract of Papers, Materialy 8 Vserossiiskoi tseolitnoi konferentsii (Proc. Eighth All-Russian Zeolite Conference), 2018, pp. 64–65.
Kel’tsev, N.V., Osnovy adsorbtsionnoi tekhniki (Principles of Adsorption Technique), Moscow: Khimiya, 1984.
Gregg, S.J., Sing, K.S.W., and Salzberg, H.W., J. Electrochem. Soc., 1967, vol. 114, no. 11, p. 279.
Tong, X., Xu, J., Li, X., Li, Y., Yan, W., Yu, J., Deng, F., Sun, H., and Xu, X., Microporous Mesoporous Mater., 2013, vol. 176, pp. 112–122.
Tong, X., Xu, J., Xin, L., Huang, P., Lu, H., Wang, C., Yan, W., Yu, J., Deng, F., Sun, H., and Xu, R., Microporous Mesoporous Mater., 2012, vo. 164, pp. 56–66.
ACKNOWLEDGMENTS
The synthesized materials were analyzed using equipment at the Agidel Shared Resource Center of the Institute of Petrochemistry and Catalysis of the Russian Academy of Sciences.
Funding
This work was supported by the Russian Science Foundation, project no. 18-73-00007.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Agliullin, M.R., Khairullina, Z.R., Faizullin, A.V. et al. Key Stages in the Formation of AlPO4-11 via the Crystallization of a Boehmite-Based Aluminophosphate Gel. Catal. Ind. 11, 87–94 (2019). https://doi.org/10.1134/S2070050419020028
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070050419020028