Abstract
The properties of a sandy Ferric Podzol over fluvioglacial deposits and the root mass of its 0–20 cm layer with Pinus sylvestris L. fine roots (≤3 mm in diameter) have been studied in a mid-taiga cowberry-type pine stand. Keeping in mind the structure of the phytogenic field of a tree, the study is subdivided into trunk, crown, and gap zones. The root mass of the forest floor and the mineral horizons are found to change in different directions in the these zones. In the forest floor, the trunk zone is more saturated with roots than the gap zone, while the trend in the soil mineral layer was the reverse. The highest root biomass is associated with sites that are the richest in soil nutrients and organic matter (NPKC). The cellulolytic activity and ecophysiological parameters of the microbiota vary in the studied horizons. Cellulolytic activity in the forest floor is the highest near tree trunks, while the spatial variation of this parameter in the mineral body of the soil is less pronounced. A correlation is detected between the contribution of root respiration to total СО2 emissions from the soil surface and the biomass of fine roots (≤3 mm) in the 0–20 cm soil layer. The effect of the soil thermal regime on root mass in different zones of the phytogenic field is studied. Soil temperature variation due to the redistribution of solar energy by the canopy results in a higher root mass in areas receiving more heat. The zone with better heated soils is the canopy gaps. The effect of the temperature of the root mass of the soil is more explicit in the mineral layer. The results of this study can be used in environmental monitoring and in predictive assessments of the effects of climate change on boreal forest ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Thin tree roots supply plants with water and mineral nutrients, ensure the growth of underground biomass, participate in the synthesis of organic matter and the carbon cycle, and play a key role in regulating biochemical cycles (Sun, 2015). In boreal forests, all forest-forming species have ectomycorrhizae, which are characterized by the absence of root hairs and the isolation of the sucking ends of the roots from the soil by a mushroom cover (Shubin, 1973; Taylor et al., 2000). This means that most of the mineral nutrients entering the plant from the soil pass through the mushroom–root interface (Ostonen et al., 2011). The study of the ecology of thin roots is important for understanding the processes occurring in ecosystems in response to climate change (Yuan and Chen, 2010). Currently, the roots, especially thin ones, are actively studied in many aspects. (Vanninen and Mäkelä, 1999; Helmisaari et al., 2007; Karpechko, 2009; Lehtonen et al., 2016; Usoltsev, 2018; etc.): the relationship between the biomass of roots and various indicators characterizing the aboveground part of the stand is revealed and the impact of environmental factors on the growth and functionality of root systems is assessed. However, due to the significant complexity and high laboriousness of root measurements, knowledge of their ecology is still very limited in comparison with the aboveground parts of trees (Yuan and Chen, 2010; Usoltsev, 2018).
The root mass of a stand is a dynamic indicator that depends on environmental conditions. Among the most important environmental factors affecting the underground biomass, one can distinguish both inherent in the stand itself (age, species composition, and other characteristics) and external (radiation balance, average annual temperature, precipitation, soil conditions, etc.) (Eissenstat and Yanai, 1997; Yuan and Chen, 2010).
Many researchers have shown the influence of elements of mineral nutrition and soil acidity on the mass of fine roots (Hokkanen et al., 1995; Helmisaari et al., 2007; Yuan and Chen, 2010). More fertile soils contain less root mass than less fertile ones do (Vanninen and Mäkelä, 1999; Helmisaari et al., 2007; Helmisaari et al., 2009). With an increase in soil pH, the mass of roots increases (Leuschner et al., 2003; Helmisaari et al., 2009; Lawrence et al., 2012). A decrease in root mass in acidic soils is associated with a decrease in microbiological activity, while soils with a higher pH can stimulate the growth of thin roots (Haynes, 1982; Francis, 1986). Elements of mineral nutrition have a significant effect on the growth of plant root systems. The elements necessary for plants can be in the soil in a form that is difficult for them to access. Mycotrophy enhances the ability of plants to extract nutrients from mineral and organic compounds. At the same time, the symbiont fungus satisfies its needs for carbohydrates and biologically active substances at the expense of the plant (Shubin, 1973). It was found that, with an increase in the content of total nitrogen and phosphorus in the soil, the content of roots decreases (Yuan and Chen, 2010). It should be noted that the above studies are devoted to the effect of soil properties on the mass of roots at the level of individual phytocenoses, which are compared with each other; at the same time, it was established that, for example, the acid-base properties of soils can change even at a distance of 2 m within one forest community (Hokkanen et al., 1995). This indicates the need to study the patterns of variation in the mass of roots “within” the phytocenosis.
Trees, as an edifier of forest ecosystems, affect various components of the biogeocenosis, including determining the properties of the soil (Gersper and Holowaychuk, 1970; Boettcher and Kalisz, 1990; Liski, 1995). Crowns affect the distribution of sediments and mineral nutrients dissolved in them over the area, which affects the edaphic properties of soils (Karpachevsky et al., 1998; Archegova and Kuznetsova, 2011, Karpechko et al., 2015; Karpechko et al., 2018). Plant litter affects the acidity of soils and the thickness of the humus layer (Hokkanen et al., 1995; Lawrence et al., 2012). Local changes in soil properties associated with the influence of trees can be reflected in the distribution of roots in the soil layer.
The soil creates conditions for the development of plant root systems; in turn, the roots affect the properties of the soil. Moreover, the influence of living and dead roots is manifested in different ways. Living roots act on the soil, supporting the rhizosphere biota through exfoliating cells and exogenous excretions, as well as absorbing mineral nutrients and water from it (Fisher and Gosz, 1986; Vancura, 1988; Whipps, 1990). Dead roots are a substrate for saprotrophic fungi and bacteria (Ehrenfeld et al., 1997).
Previously, the change in root saturation within phytocenosis was studied using the example of an oxalis spruce forest (Sennov et al., 1994). It is found that the root saturation of the upper horizons is associated with the saturation of the soil with bases. There are data on the spatial variability of the carbon content in the forest litter and in the upper mineral soil layer (Podvezhennaya and Ryzhova, 2010). It was found that the vegetation of the lower layers affects the variability of the thickness and reserves of forest litter, as well as the carbon content only in the upper 5-cm soil layer. Changes in the structure and properties of forest litter, as an important component of biogeocenosis, were studied in the trunk–crown–window system (Lukina and Orlova, 2008; Semenyuk et al., 2020). The authors established the trends of spatial changes in the basic physical and chemical properties of soils in different zones of the phytogenic field of old-growth forests.
Soil microbiota provides plants with vitamins, organic acids (Kaviya et al., 2019), and the work of enzyme systems (Yang et al., 2008) and participates in the decomposition of organic matter and the synthesis of humus (Fedotov and Lysak, 2014). The operation of the microbiological complex of cellulolytics depends on the type of soil, the thickness of the soil profile (Purtova et al., 2020), and the root exudates of plants (Kurachev and Baturina, 2005).
Insufficient knowledge of the spatial heterogeneity of the root saturation of the soils of the stand and its dependence on edaphic conditions determine the relevance of our work. We tested the following hypotheses: the mass of thin roots differs in different zones of the tree’s phytogenic field and there is a connection between the distribution of fine roots within the phytocenosis in the zones of the phytogenic field of a tree with factors of soil fertility (content of mineral nutrients and temperature). The following main tasks were set: for different zones of the phytogenic field of a tree, to establish the change in the temperature of the upper soil horizons; to assess the content of the main elements of mineral nutrition of plants; to reveal the features of the microbiological activity of soils; and to determine root saturation.
The resulting data can be used for monitoring the natural environment and as diagnostic indicators of the quality of soils in undisturbed forest ecosystems in Eastern Fennoscandia.
MATERIALS AND METHODS
The research was carried out in the middle taiga subzone of the Republic of Karelia on the territory of the Kivach nature reserve, which was founded in 1931. The climate of the study area is continental with features of the sea (Nauchno-prikladnoy spravochnik…, 1988). According to long-term observations, the average annual air temperature is +2.0 to +2.5°С, the average temperature in January is ̶̶ 9–10°С, and in it is July +16 to +17°С. According to long-term studies, the amount of precipitation is 550–750 mm per year (Romanov, 1961).
This work was carried out in a lingonberry pine forest; the tree layer is represented exclusively by Pinus sylvestris L., 190–210 years in age. The phytocenosis is characterized by the even distribution of trees over the area. The study area is located at an altitude of about 60 m above sea level on the flat surface of the terrace of the fluvioglacial system; it is composed of coarse and medium-grained sands with inclusions of gravel and pebbles (Demidov et al., 2006). The soil of the study area is a sandy illuvial–ferruginous podzol formed on fluvioglacial sandy deposits (Shishov et al., 2004). The morphological structure of the soil profile is as follows: O (forest litter)–E (podzolic horizon)–BF (illuvial horizon)–BC1–BC2–C. Under the moderately decomposed litter O, podzolic horizon E is formed with a thickness of up to 5 cm: grayish white, in the upper part the horizon is lighter gray, in some loci it is saturated with humus. The illuvial–ferruginous horizon BF (up to 7 cm in thickness) has a gray-brown color, the color becomes more intense in some places. In the middle part of the soil profile (up to 42 cm), there is a gradual decrease in ocher color and a transition to the parent sandy rock. The content of the sandy fraction in the soil profile is 70–90%; the amount of silt varies from 0.5 to 4.1%. The high content of coarse–sandy granulometric elements prevents the formation of the structure of soils and determines their low water-holding capacity. Soils are strongly acidic (Table 1). The horizons of forest litter and podzolic are the most acidic (pHKCl 3.2–3.5). In the illuvial horizon, pHKCl increases to 4.3–4.4. The hydrolytic acidity of soils (Нg) is high, especially in the forest litter (up to 100 mequiv/100 g of soil). Нg decreases with depth and the degree of soil saturation with bases (V) increases. In general, the V values of the studied soils are low, from 4% of soil in the forest litter to 10% in the illuvial horizon (Table 1).
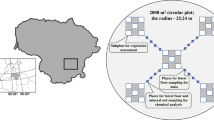
The spatial variation of indicators of soil properties and saturation with its roots within the phytocenosis was studied relative to the position to the tree. To determine the zones for sampling soil samples, we used the concept of a horizontal radial-belt structure of the phytogenic field of a tree (Uranov, 1965), which consists of concentric zones (Chernyaeva and Viktorov, 2016). Three areas were identified: trunk—the zone of the near-trunk rise, crown—the projection of the crown onto the earth’s surface, and window—the intercrown space (the area free from the projections of the crowns).
In each zone of the phytogenic field of the tree, soil samples were taken for chemical analysis separately from each genetic horizon (O, E, BH) at a depth of 25 cm in 12 replicates. The thickness of the horizons was as follows: forest litter 3–6 cm, eluvial horizon 2–6 cm, and illuvial horizon up to 16 cm. The determination of the content of C, N, P, and K in the soil was carried out according to the traditional methods in soil science: the content of organic carbon by the Tyurin method, total nitrogen by the Kjeldahl method, and the determination of mobile phosphorus and potassium compounds in the soil by the Kirsanov method (Vorobyeva, 1998). The stock of organic carbon and NPK for the 0–25 cm soil layer was calculated based on the data on the content of organic carbon, total nitrogen, mobile forms of phosphorus and potassium, and the bulk density of soils.
The temperature of the three upper soil horizons at depths of 2, 5, and 10 cm was measured with a Hanna Instruments Checktemp-1 thermometer from May to October (2–3 times a month).
To determine the mass of roots, soil monoliths 10 × 10 × 20 cm in size were taken. The limitation of the depth of the sample to 20 cm is explained by the concentration of the greatest mass of roots (according to various estimates, up to 90%) in this soil layer (Bobkova, 1987; Vanninen and Mäkelä, 1999; Yuan and Chen, 2010; etc.). The total number of samples taken was 63 pcs. (21 pcs. in each zone of the phytogenic field). Thin roots were removed separately from the litter and the underlying mineral soil layer by hand, without washing the entire volume of soil. If necessary, the roots were soaked and washed of soil. The thinnest roots were removed with tweezers. In this work, the fractions of roots ≤3 and ≤1 mm and in diameter, which included mycorrhizal ends, were isolated. The roots were dried to an absolutely dry state at a temperature of 105°C and weighed using an OHAUS PX224 analytical balance.
Basal respiration (BR) was determined by the rate of CO2 release soil (without roots) over 24 h of incubation at 22°C and 60% of the total field moisture capacity. The microbial biomass carbon content (Cmic) was determined by the method of substrate-induced respiration (SIR) by the rate of the initial maximum respiration of microorganisms after soil enrichment with glucose (Anderson, Domsch, 1978). Microbial biomass carbon (Cmic) was calculated by the formula Cmic (μgC g–1 soil) = SID (μgC g–1 soil h–1) × 40.04 + 0.37.
The actual cellulolytic activity of soils was established by the application method in experiments in situ, the intensity of the process of destruction of cellulose was judged by the loss of the weight of the linen linen, and the period of exposure of the linen in the soil was 4 months (Metody…, 1991).
The intensity of respiration in the field was determined by the chamber method in seasonal dynamics (twice a month) from May to November. We used a series of measuring chambers, which are PVC pipes with a diameter of 10 cm cut into the soil to a depth of 25 cm (without removing the litter). A portable CO2 gas analyzer based on an NDIR sensor from SenseAir (Sweden) was used as the recording device. The contribution of root respiration to the total emission of carbon dioxide from the soil surface was assessed by the root removal method (Kuzyakov, Larionova, 2006). Measurements were carried out from May 2017 to November 2020.
Statistical processing of the data was carried out using Microsoft Office Excel 2007. The tables containing the values of the determined parameters show the mean values and their standard errors. To determine the statistical significance of the differences in the means, we used their pairwise comparisons by Student’s test and one-way analysis of variance (ANOVA). Hypotheses about normal distributions and equality of variances were preliminarily tested.
RESULTS
The mass of roots in different zones of the phytogenic field of the tree differed. In the forest litter, a higher content of roots of ≤1 mm and ≤3 mm in the trunk zone was revealed when compared to the window zone. For the mineral horizon of soils, the opposite tendency is characteristic: an increase in the mass of roots with a diameter of ≤3 mm is observed with distance from the trunk: in the trunk zone, the roots are 31% fewer than in the window zone (Table 2) (p < 0.05). For roots with a diameter of ≤1 mm in the mineral layer of the soil, this trend continues.
The highest activity of the microbial community of soils is confined to the crown zone, as is evidenced by the values of the carbon content of the microbial biomass (Cmic, Table 3) and the activity of the CO2 productive capacity of soils (R microbial, Table 4). However, the largest contribution of root respiration to the total CO2 emissions from the soil surface is typical for the window zone (R root, Table 4), where the mass of roots ≤3 mm in diameter in the 0–20 cm layer (in the forest litter and mineral layer) was maximum (Table 2).
CO production2 in soil is an important integral indicator of the biological activity of soils and includes heterotrophic or microbial, as well as root autotrophic, components. According to various researchers, root respiration accounts for 7–56% of the total CO2 emissions from the surface of the soil, depending on the type of soil and the plants growing on them (Larionova et al., 2003; Evdokimov et al., 2010). The variation in the amount of heterotrophic and autotrophic CO2 production is associated with the different sensitivity of the biotic soil component to temperature, humidity, and composition of organic matter (Hanson et al., 2000; Evdokimov et al., 2010).
This study made it possible to arrange the soils of different zones of the phytogenic field of the tree in a row in decreasing order of the contribution of root respiration to the total CO2 emissions from the soil surface as follows: window–crown–trunk (Table 4), while the absolute values of root respiration of the soils of the studied areas differed significantly (p < 0.05). The highest absolute values of CO2 emissions of both heterotrophic and autotrophic origin are confined to areas under the crown.
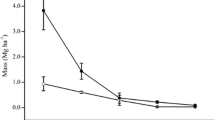
A relationship has been established between the value of the contribution of root respiration to the total CO2 emissions from the surface of soils and a mass of roots with a diameter of ≤3 mm in the 0–20 cm soil layer (Fig. 1). The highest values of the share of autotrophic soil respiration (45 ± 5%) are confined to the window zone; the stocks of thin roots in the window zone were also assessed as the highest.
The hydrothermal regime of soils is the main indicator that determines the timing and intensity of root growth. It has been established that a noticeable growth of the roots of tree species begins at a temperature of +5°C (Kalinin, 1975; Bobkova, 1987). The most intensive root growth in the middle taiga was observed in June and early July (Bobkova, 1987).
Measurement of soil temperature showed that the growing season is characterized by a similar dynamics of the temperature of the soil horizons in all studied zones (Fig. 2). The period from May to July is characterized by a significant difference in the temperature of the BF and E soil horizons in the studied zones (Figs. 2b, 2c); this period is shorter and limited to May–June for horizon O (Fig. 2a). In May, the crown zone is warmed up to a greater extent. In the forest litter, the difference in temperature between the trunk and crown zones is more pronounced in comparison with the underlying mineral horizons. In June, the soil of the window zone has a higher temperature compared to the trunk zone both in the O horizon (statistically significant difference) and in the BF horizon (the difference was found at the tendency level). In July, in the forest litter, the temperature difference between the zones is practically not observed; at the same time, in the mineral horizons of the soil, the temperature in the window zone remains higher than in the trunk zone (the difference was found at the level trends) (Figs. 2b, 2c). From August to October, the temperature levels off both between the zones, where the difference becomes less noticeable, and along the soil profile.
In the studied zones of the pine phytogenic field, the decrease in the reserves of NRKS in the forest litter occurred in the series trunk–crown–window. For NKS reserves, differences were found at the level of a statistical trend. In the mineral layer of the soil, the opposite picture was observed: there is a tendency to an increase in the reserves of NRKS in the window zone (Table 5).
The dynamics of the cellulolytic activity of soils in the studied soil horizons has variability during the growing season in different zones of the phytogenic field of the tree (Fig. 3).
The highest rates were observed in the summer period, when favorable conditions for litter destructors are formed. The change in the cellulolytic capacity of the organogenic soil horizon, depending on the location in the zones of the phytogenic field of the tree, can be arranged in descending order: trunk–crown–window. In the mineral horizon, the order of change is somewhat different (crown–trunk–window); however, in general, it corresponds to the general trend of the influence of the crown (increase in indicators) on this process.
DISCUSSION
The spatial influence of a tree on soil characteristics is one of the main reasons for soil heterogeneity in a forest. Trees affect the soil through litter, the death of roots, shading of the soil by crowns, and by changing the chemical composition and distribution of precipitation (Hokkanen et al, 1995; Ipatov et al., 2009). According to the work of A.M. Gorelov (2013a), under the canopy of tree plantations, the illumination can be 1% of the illumination in an open place.
The soil temperature in the zone of the crown space and outside the crown may differ, which affects the intensity of decomposition of organic matter and the growth of roots and their absorption capacity (Voronkov et al., 1979).
In May, the soil of the window zone has the lowest temperature (Fig. 2). As the length of daylight hours increases and the supply of solar energy to the ecosystem increases, the soil warms up quickly and in June the picture changes: the temperature in the window zone is the highest, especially in comparison with the trunk zone. In the period from May to June, the growth of roots is influenced, to a greater extent, by the temperature, and from the middle of July it is influenced by the supply of productive moisture (Lykhmus et al., 1986). This alternation of factors is explained by the peculiarities of the taiga zone, where at the beginning of summer there is always an optimal amount or excess of moisture in the soil (Karpechko, 1986); therefore, the factor limiting plant growth is heat. Low soil temperatures in the trunk zone are explained by the lower input of solar energy (Gorelov, 2013b). In July, the forest litter is almost equally heated in all zones of the tree’s phytogenic field (Fig. 2a), while the temperature in the mineral soil layers in the window zone remains higher than in the trunk zone (Figs. 2b, 2c). Forest litter is most responsive to changes in the amount of incoming solar radiation. This layer has the lowest thermal inertia, so its response to weather changes is faster. From August to October, the soil temperature is practically the same in all zones and horizons. Apparently, due to the decrease in air temperatures, the greater input of solar energy into the window zone can no longer compensate for the accelerated cooling of the soil in it compared to the soil in the trunk zone, where tree crowns impede this process.
The distribution of chemical elements over an area within phytocenosis may depend on many factors, including atmospheric precipitation (Karpachevsky et al., 1998; Archegova and Kuznetsova, 2011; Karpechko et al., 2018). This is due to the washing off by rainwater of chemicals that are retained from the atmospheric air by the surface of branches and needles, as well as the interaction of plant parts with aqueous solutions of chemical elements (Karpechko et al., 2015). Passing through the canopy of the stand, sediments, in most cases, are enriched with a set of inorganic and organic compounds (Shiltsova, 2006; Bakhmet et al., 2011, Karpechko et al., 2015; Karpechko et al., 2018; etc.). It was found that, in the studied phytocenosis, the reserves of organic carbon and the main elements of mineral nutrition of plants (NPK) change with distance from the trunk (Table 5). The forest litter and the mineral layer of the soil are characterized by different tendencies of these changes. A slightly higher content of carbon and NPK was noted for the upper soil horizon (O) near the trunk, which is associated with the mineralization of sediments after their passage through the crowns (Karpechko et al., 2015). On the contrary, in the mineral soil layer, NPKC reserves in the window zone are higher when compared to the trunk zone. This can be explained by the leaching of these elements from the upper horizons into the lower ones, since the crowns of trees do not delay precipitation.
It is known that the roots are characterized by chemotropism; i.e., their growth is oriented towards zones with a large number of elements of mineral nutrition (Kalinin, 1983; Bobkova, 1987). The data obtained (Table 2) confirm this pattern: the highest saturation with thin roots is noted precisely in the zone with the highest content of mineral nutrition elements of plants, i.e., in the forest floor in the trunk zone and in the mineral soil layer in the window.
When studying the distribution of roots by area within a community it should also be borne in mind that roots from different trees can concentrate at one point in space (Sennov, 1980; Karpechko, 2009). According to various data, the length of pine roots is 3–4 (Bobkova, 1987) and 4–5 (Sennov, 1980) crown radii. The roots are distributed unevenly over the area and fill the spaces available and the most favorable in terms of the content of mineral nutrients. Thus, studying the root systems, instead of individual trees, the underground part of the stand as a whole is considered.
The seasonal dynamics of the cellulolytic capacity of soils was analyzed: the highest activity was noted in the summer period (with a maximum in August), which does not contradict the studies of other authors (Purtova et al., 2020). In the course of the study, differences were noted in the intensity of decomposition of the cellulose web in the upper organogenic and mineral soil horizons in the zones of the phytogenic field of the tree, which can be arranged in the row trunk–crown–window to reduce the rate of destruction of the web. A decrease in the activity of microorganisms of the cellulolytic complex in the window zone can occur as a result of the inhibitory effect of pine root secretions (Netrusov and Kotova, 2009), the root content of which in the intercrown space is the highest (over the 0–20 cm layer).
It is known that 75% of soil CO2 emissions is produced by the top 0–20 cm soil layer (Jassal et al., 2005). The autotrophic component of soil respiration is associated with soil root saturation. Small roots are characterized by high physiological activity, which is explained by the increased content of respiratory enzymes in them, as well as more active processes of cell division and elongation. The intense respiration of small roots is also related to the fact that only living tissues are present in them (Tsel’niker, 2005). Thus, the lowest intensity of root respiration in the trunk zone is explained by the lower mass of roots in this zone.
Soil temperature affects roots and their distribution over the soil thickness (Björk et al., 2007). In this work, it is shown that the most heated are the intercrown spaces (the window zone). At the same time, in the upper soil horizon (O), there is a slight decrease in the mass of roots in the series trunk–crown–window. However, this does not contradict the statement about the positive influence of temperatures on the roots. In the forest litter, as early as in July, no difference in temperature between the studied zones is observed (Fig. 2) and, in the mineral layer of the soil, the difference in temperature between the zones is expressed for a longer period (including July). An increase in the mass of roots to the window zone is noted here.
CONCLUSIONS
The assessment of the spatial heterogeneity of the content of fine roots in the zones trunk–crown–window within the phytocenosis was carried out. The distribution of roots over the area is a complex process influenced by many environmental factors. The hypothesis put forward about the uneven distribution of thin roots over the zones of the phytogenic field of the tree was confirmed. The mass of roots is greater in those zones where there is a tendency for an increase in the content of mineral nutrition elements: in the forest litter in the trunk zone and in the mineral soil layer in the window zone. The redistribution of the light flux and illumination of the soil surface under the canopy of the stand determines, in general, the more favorable temperature regime in the window zone. The temperature factor also affects the distribution of roots within the phytocenosis and is better manifested in the mineral layer of the soil. Temperature is also the leading ecological factor influencing the intensity of the autotrophic (root) respiration of soils. The highest activity of the complex of cellulolytics and microbial transformation of organic matter is observed in the organogenic soil horizon of the near-trunk zone.
REFERENCES
Anderson, J.P.E. and Domsch, K.H., A physiological method for the quantitative measurement of microbial biomass in soils, Soil Biol. Biochem., 1978, vol. 10, no. 3, pp. 215–221.
Archegova, I.B. and Kuznetsova, E.G., Influence of wood plants on chemical composition of atmospheric precipitations during recovery of central-taiga forests, Lesovedenie, 2011, no. 3, pp. 34–43.
Bakhmet, O.N., Fedorets, N.G., and Lastochkina, V.G., Khimicheskii sostav atmosfernykh osadkov i pochvennykh vod Karelii (Chemical Composition of Atmospheric Precipitations and Ground Waters in Karelia), Petrozavodsk: Karel. Nauchn. Tsentr, Ross. Akad. Nauk, 2011.
Björk, R.G., Maidi, H., Klemedtsson, L., Lewis-Jonsson, L., and Molau, U., Long-term warming effects on root morphology, root mass distribution, and microbial activity in two dry tundra plant communities in northern Sweden, New Phytol., 2007, vol. 176, no. 4, pp. 862–873.
Boettcher, S.E. and Kalisz, P.J., Single-tree influence on soil properties in the mountains of eastern Kentucky, Ecology, 1990, vol. 71, no. 4, pp. 1365–1372.
Bobkova, K.S., Biologicheskaya produktivnost’ khvoinykh lesov Evropeiskogo Severo-Vostoka (Biological Productivity of Coniferous Forests of European Northeast), Leningrad: Nauka, 1987.
Chernyaeva, E.V. and Viktorov, V.P., Phytogenic fields: history and current state, Sots.-Ekol. Tekhnol., 2016, no. 1, pp. 89–106.
Demidov, I.N., Lukashov, A.D., and Il’in, V.A., Relief of the Kivach Nature Reserve and geological development of northwestern Onega region in the Quaternary, Tr. Karel. Nauchn. Tsentra, Ross. Akad. Nauk, 2006, no. 10, pp. 22–33.
Ehrenfeld, J.G., Parsons, W.F.J., Han, X., Parmelee, R.W., and Zhu, W., Live and dead roots in forest soil horizons: contrasting effects on nitrogen dynamics, Ecology, 1997, vol. 78, no. 2, pp. 348–362.
Eissenstat, D.M. and Yanai, R.D., The ecology of root lifespan, Adv. Ecol. Res., 1997, vol. 27, pp. 1–60.
Fedotov, G.N. and Lysak, L.V., The possible role of microorganisms in humus formation in soils, Dokl. Biol. Sci., 2014, vol. 455, no. 1, pp. 87–90.
Fisher, F.M. and Gosz, J.R., Effects of plants on net mineralization of nitrogen in forest soil microcosms, Biol. Fertil. Soils, 1986, vol. 2, pp. 43–50.
Francis, A.G., The ecological effects of acid deposition. II. acid-rain effects on soil and aquatic microbial processes, Experientia, 1986, vol. 42, pp. 455–465.
Gersper, P.L. and Holowaychuk, N., Some effects of stem flow from forest canopy trees on chemical properties of soils, Ecology, 1970, vol. 52, no. 4, pp. 691–702.
Gorelov, A.M., Phytogenic field and its structure, Vestn. Mosk. Gos. Obl. Univ., 2013a, no. 1.
Gorelov, A.M., Specific light conditions in the intracrown space of wood plants, Izv. Samar. Nauchn. Tsentra, Ross. Akad. Nauk, 2013b, vol. 15, no. 3, pp. 135–140.
Hanson, P.J., Edwards, N.T., Garten, C.T., and Andrews, J.A., Separating root and soil microbial to soil respiration: a review of methods and observations, Biogeochemistry, 2000, vol. 48, pp. 115–146.
Haynes, R.J., Effects of liming on phosphate availability in acid soils—a critical review, Plant Soil, 1982, vol. 68, pp. 289–308.
Helmisaari, H.S., Derome, J., Nöjd, P., and Kukkola, M., Fine root biomass in relation to site and stand characteristics in Norway spruce and Scots pine stands, Tree Physiol., 2007, vol. 27, pp. 1493–1504.
Helmisaari, H.S., Saarsalmi, A., and Kukkola, M., Effects of wood ash and nitrogen fertilization on fine root biomass and soil and foliage nutrients in a Norway spruce stand in Finland, Plant Soil, 2009, vol. 314, pp. 121–132.
Hokkanen, T.J., Järvinen, E., and Kuuluvainen, T., Properties of top soil and the relationship between soil and trees in a boreal Scots pine stands, Silva Fen., 1995, vol. 29, no. 3, pp. 189–203.
Ipatov, V.S., Zhuravleva, E.N., Lebedeva, V.Kh., and Tikhodeeva, M.Yu., Phytogenic field Picea abies, P. obovata (Pinaceae), Bot. Zh., 2009, vol. 94, no. 4, pp. 558–568.
Jassal, R., Black, A., Novak, M., Morgenstern, K., Nesic, Z., and Gaumont-Guay, D., Relationship between soil CO2 concentrations and forest-floor CO2 effluxes, Agric. For. Meteorol., 2005, vol. 130, nos. 3–4, pp. 176–192.
Kalinin, M.I., Kornevye sistemy derev’ev i povyshenie produktivnosti lesa (Tree Root Systems and Increase of Forest Productivity), Lvov: Vishcha Shkola, 1975.
Kalinin, M.I., Formirovanie kornevoi sistemy derev’ev (Development of Tree Root System), Moscow: Lesnaya Prom-st’, 1983.
Karpachevskii, L.O., Zubkova, T.A., Proisler, T., Kennel, M., Gitl, G., Gorcharuk, N.Yu., and Minaeva, T.Yu., Effect of complex spruce canopy on the chemical composition of sediments, Lesovedenie, 1998, no. 1, pp. 50–59.
Karpechko, A.Yu., Influence of forest thinning on root density in soil of spruce stands in southern Karelia, Izv. Vyssh. Uchebn. Zaved., Lesn. Zh., 2009, no. 3, pp. 19–25.
Karpechko, Yu.V., The water balance of small watersheds of the Korzina lowland, in Pochvenno-meliorativnye issledovaniya v Karelii (Soil Reclamation Studies in Karelia), Petrozavodsk: Karel. Fil., Akad. Nauk SSSR, 1986, pp. 23–43.
Karpechko, Yu.V., Lozovik, P.A., and Potapova, I.Yu., Transformation of chemical composition of atmospheric precipitations by forest vegetation, Trudy IV Vserossiiskoi nauchnoi konferentsii “Fundamental’nye problemy vody i vodnykh resursov” (Proc. IV All-Russ. Sci. Conf. “Fundamental Problems of Water and Water Resources”), Moscow: Inst. Vodn. Probl., Ross. Akad. Nauk, 2015, pp. 155–158.
Karpechko, Yu.V., Lozovik, P.A., and Fedorets, N.G., Balance of chemicals in pine and spruce forests of Karelia, Contemp. Probl. Ecol., 2018, vol. 11, no. 7, pp. 762–770.
Kaviya, N., Upadhayay, V.K., Singh, J., Khan, A., Panwar, M., and Singh, A.V., Role of microorganisms in soil genesis and functions, in Mycorrhizosphere and Pedogenesis, Varma, A. and Choudhary, D., Eds., Singapore: Springer-Verlag, 2019, pp. 25–52.
Kurachev, V.M. and Baturina, V.B., Rates of decomposition of plant residues in soils of technogenic landscapes, Sib. Ekol. Zh., 2005, no. 5, pp. 789–793.
Kuzyakov, Ya.V. and Larionova, A.A., Contribution of rhizomicrobial and root respiration to the CO2 emission from soil (a review), Eurasian Soil Sci., 2006, vol. 39, no. 7, pp. 753–764.
Larionova, A.A., Yevdokimov, I.V., Kurganova, I.N., Sapronov, D.V., Lopes de Gerenju, V.O., and Kuznetsova, L.G., Root respiration and its contribution to the CO2 emission from soil, Eurasian Soil Sci., 2003, vol. 36, no. 2, pp. 173–184.
Lawrence, D.J., Luckai, N., Meyer, W.L., Shahi, C., Fazekas, A.J., Kesanakurti, P., and Newmaster, S., Distribution of white spruce lateral fine roots as affected by the presence of trembling aspen: root mapping using simple sequence repeat DNA profiling, Can. J. For. Res., 2012, vol. 42, pp. 1566–1576.
Lehtonen, A., Palviainen, M., Ojanen, P., Kalliokoski, T., Nöjd, P., Kukkola, M., Penttilä, T., Mäkipää, R., Leppälammi-Kujansuu, J., and Helmisaari, H.-S., Modeling fine root biomass of boreal tree stands using site and stand variables, For. Ecol. Manage., 2016, vol. 359, pp. 361–369.
Leuschner, C. and Hertel, D., Fine root biomass of temperate forests in relation to soil acidity and fertility, climate, age and species, in Progress in Botany: Genetics Physiology Systematics Ecology, Berlin: Springer-Verlag, 2003, pp. 405–438.
Liski, J., Variation in soil organic carbon and thickness of soil horizons within a boreal forest stand—effect of trees and implications for sampling, Silva Fen., 1995, vol. 29, no. 4, pp. 255–266.
Lukina, N.V. and Orlova, M.A., Nutrient regime of soils in old forests of the Kola Peninsula, Lesovedenie, 2008, no. 1, pp. 11–22.
Lykhmus, K.N., Lasn, R.R., and Oya, T.A., The root growth of European spruce depending on soil conditions, Pochvovedenie, 1986, no. 6, pp. 89–97.
Metody pochvennoi mikrobiologii i biokhimii (Methods of Soil Microbiology and Biochemistry), Moscow: Mosk. Gos. Univ., 1991.
Nauchno-prikladnoi spravochnik po klimatu SSSR. Seriya 3. Mnogoletnie dannye (Scientific-Applied Handbook on Climate in the USSR, Series 3: Long-Term Observations), Leningrad, 1988, parts 1–6.
Netrusova, A.I. and Kotova, I.B., Mikrobiologiya: uchebnik dlya vuzov (Microbiology: Manual for Higher Education Institutions), Moscow: Akademiya, 2012.
Ostonen, I., Helmisaari, H.-S., Borken, W., Tedersoo, L., Kukumägi, M., Bahram, M., Lindroos, A.-J., Nöjd, P., Uri, V., Merilä, P., Asi, E., and Lõhmus, K., Fine root foraging strategies in Norway spruce forests across a European climate gradient, Global Change Biol., 2011, vol. 17, no. 12, pp. 3620–3632.
Podvezennaya, M.A. and Ryzhova, I.M., The relationships between the variability of soil carbon reserves and the spatial structure of the plant cover in forest biogeocenoses, Moscow Univ. Soil Sci. Bull., 2010, vol. 65, no. 4, pp. 139–144.
Purtova, L.N., Orekhova, T.P., and Kiseleva, I.V., The physicochemical parameters and catalase activity of soils in the range of phytogenic fields of Korean pine plus trees (Pinus koraiensis Sieb. et Succ.) at Upper Ussuri forest stationer, Vopr. Lesn. Nauki, 2020, vol. 3, no. 2, pp. 1–15.
Romanov, A.A., O climate Karelii (Climate of Karelia), Petrozavodsk: Gosizdat Karel. ASSR, 1961.
Semenyuk, O.V., Telesnina, V.M., Bogatyrev, L.G., Benediktova, A.I., and Kuznetsova, Ya.D., Assessment of intra-biogeocenotic variability of forest litters and dwarf shrub–herbaceous vegetation in spruce stands, Eurasian Soil Sci., 2020, vol. 53, no. 1, pp. 27–38.
Sennov, S.N., Forest thinning and intraspecific competition, in Vosstanovlenie i melioratsiya lesov severo-zapada RSFSR (Restoration and Melioration of Forests in the Northwest of the RSFSR), Leningrad: Leningr. Naucchno-Issled. Inst. Lesn. Khoz., 1980, pp. 17–27.
Sennov, S.N., Baneva, N.A., and Smirnov, A.P., Root density and parcel structure in a high-performance spruce forest, Lesovedenie, 1994, no. 2, pp. 78–80.
Shil’tsova, G.V., The role of pine biogeocenoses of the Kivach Nature Reserve in acidity and composition of natural waters, Tr. Karel. Nauchn. Tsentra, Ross. Akad. Nauk, 2006, no. 10, pp. 173–179.
Shishov, L.L., Tonkonogov, V.D., Lebedeva, I.I., and Gerasimova, M.I., Klassifikatsiya i diagnostika pochv Rossii (Classification and Diagnostic System of Russian Soils), Smolensk: Oikumena, 2004.
Shubin, V.I., Mikotrofnost’ drevesnykh porod, ee znachenie pri razvedenii lesa v taezhnoi zone (Mycotrophy of Tree Species: Its Role in Forest Cultivation in the Taiga Zone), Leningrad: Nauka, 1973.
Sun, T., Dong, L.L., Mao, Z.J., and Li, Y.Y., Fine root dynamics of trees and understory vegetation in a chronosequence of Betula platyphylla stands, For. Ecol. Manage., 2015, vol. 346, pp. 1–9.
Taylor, A.F., Martin, F., and Read, D.J., Fungal diversity in ectomyccorhizal communities of Norway spruce (Picea abies (L.) Karst) and beech (Fagus sylvatica L.) along north-south transects in Europe, Ecol. Stud., 2000, vol. 142, pp. 343–365.
Tsel’niker, Yu.L., Root respiration and its role in the carbon balance of the forest stand, Lesovedenie, 2005, no. 6, pp. 11–18.
Uranov, A.A., Phytogenic field, in Problemy sovremennoi botaniki (Problems of Modern Botany), Moscow: Nauka, 1965, pp. 251–254.
Usol’tsev, V.A., What do we know about primary production of tree roots, Eko-Potentsial, 2018, no. 4 (24), pp. 25–79.
Vancura, V., Plant metabolites in soil, in Soil Microbial Associations: Control of Structures and Functions, Vancura, V. and Kunc, F., Eds., Amsterdam: Elsevier, 1988, pp. 57–144
Vanninen, P. and Mäkelä, A., Fine root biomass of Scots pine stands differing in age and soil fertility in southern Finland, Tree Physiol., 1999, vol. 19, pp. 823–830.
Vorob’eva, L.A., Khimicheskii analiz pochv (Chemical Analysis of Soils), Moscow: Mosk. Gos. Univ., 1998.
Voronkov, N.A., Kozhevnikov, S.A., and Shompolova, V.A., Temperature regime of soils under forest and fallow land in Moscow region, Pochvovedenie, 1979, no. 6, pp. 90–99.
Whipps, J.M., Carbon economy, in The Rhizosphere, Lynch, J.M., Ed., Chichester: Wiley, 1990, pp. 59–97.
Yang, L., Li, T., Li, F. Lemcoff, J.H., and Cohen, S., Fertilization regulates soil activity and fertility dynamics cucumber field, Sci. Hort. (Amsterdam), 2008, vol. 116, no. 1, pp. 21–26.
Yevdokimov, I.V., Larionova, A.A., Lopes de Gerenyu, V.O., Schmitt, M., and Bahn, M., Experimental assessment of the contribution of plant root respiration to the emission of carbon dioxide from the soil, Eurasian Soil Sci., 2010, vol. 43, no. 12, pp. 1373–1381.
Yuan, Z.Y. and Chen, Y.H., Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: literature review and meta-analyses, Crit. Rev. Plant Sci., 2010, vol. 29, pp. 204–221.
Funding
This work was supported by the federal budget for fulfilling the state assignment of the Karelian Research Center (Forest Institute, Karelian Research Center, Russian Academy of Sciences).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karpechko, A.Y., Moshkina, E.V., Medvedeva, M.V. et al. Patterns in the Spatial Distribution of Fine Roots in Pinus sylvestris L. (Using the Example of a Mid-Taiga Cowberry-Type Pine Forest). Contemp. Probl. Ecol. 15, 129–138 (2022). https://doi.org/10.1134/S1995425522020032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995425522020032