Abstract
Under temperate climatic conditions, temperature is a key abiotic factor that controls the decomposition of coarse woody debris including bark. Using aspen bark (AB), we have carried out a 12-month laboratory experiment at three contrasting temperatures (2, 12, and 22°C) and constant sufficient moisture to quantify the temperature effect on the decomposition rate (DecR) and decay constant (k) of aspen bark. The effect of temperature on the total loss of C–CO2 and changes in the chemical composition of AB throughout the experiment are also estimated. An increase in the incubation temperature from 2 to 12°C and from 12 to 22°C induces a similar increase in the average (over 12 months) value of the DecR: the Q10 values are 1.30 and 1.41 in these temperature intervals, respectively. The most significant effect of temperature on the DecR dynamics is revealed during the 1st and 2nd months of the experiment. The maximum losses of C–CO2 (284 ± 16 mg C g bark–1, or 55.2 ± 3.2% of the initial C content) are observed at 22°C. The decay constants of AB, calculated by the one-component exponential model, significantly increase with the growth of the incubation temperature: from 0.46 ± 0.01 yr–1 at 2°C to 1.02 ± 0.09 yr–1 at 22°C. Changes in the chemical composition of aspen bark due to the processes of biogenic decomposition include a significant reduction in the amount of alcohol-soluble organic compounds and cellulose to 18–32 and 41–57% of their initial amount, respectively. Lignin losses are less significant, being only 9–18% of their content in AB before the incubation. The quantitative changes in the chemical composition of aspen bark are similar at 2 and 12°C and less pronounced than those at 22°C, which may be explained by an increase in the growing activity of organisms, are actively involved in bark decomposition at 22°C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The decomposition of coarse wood debris (CWD) in forest ecosystems leads to the emission of a significant amount of carbon dioxide (CO2) into the atmosphere; the underestimation of carbon dioxide emission can significantly reduce the accuracy of carbon balance estimates, especially in old-growth taiga forests, where the amount of CWD is particularly large (Zamolodchikov, 2009; Liu et al., 2013; Russell et al., 2015). The decomposition of CWD is accompanied by several interrelated processes: physical decay (fragmentation), leaching, and biogenic decomposition (Harmon et al., 1986; Zhou et al., 2007; Russell et al., 2015). It is believed that the vital activity of microflora, which performs the biogenic decay of bark and wood, provides about 76% of carbon losses during CWD decomposition (Chambers et al., 2001). Bark and woody tissues are decomposed mainly by fungi and funguslike organisms (Mukhin, 1993; Kapitsa et al., 2012; Safonov et al., 2013; Kazartsev et al., 2018).
The least studied component of CWD is bark (Shorohova et al., 2012; 2016), which can amount up to 25% of the trunk volume and 13–21% of the dry weight (Ugolev, 2002; Lestander et al., 2012). Tree bark is also an integral part of forest litter. For example, the proportion of bark can reach 6.7% of the total litter weight in middle-taiga spruce forests (Kuznetsov, 2010).
Our knowledge of tree-bark decomposition and its specific features remains rather scarce, since bark and wood are often combined during estimates of the rate of CWD decomposition (Yatskov et al., 2003; Hagemann et al., 2010; Li et al., 2012). However, these components of woody debris differ markedly in their chemical composition and structure (Wetzel and Greenwood, 1989; Franceschi et al., 2005; Martin, 2015); therefore, the study of tree-bark decomposition is of independent interest.
The rate of bark and wood decomposition under natural conditions depends on a combination of biotic and abiotic factors. The key abiotic factor that globally influences the decomposition of wood substrates is climate, while biotic factors are decisive at the local and regional levels (Berglund et al., 2013; Bradford et al., 2014; Fukasawa, 2015), which mainly include substrate quality and wood-decomposing living organisms (Cornwell et al., 2009; Hu et al., 2017). It is well known that the rate of the biogenic (microbial) decomposition of most plant materials, including CWD, in a certain temperature range usually increases with temperature growth, if there is no limitation on moisture content (Harmon et al., 1986; Taylor and Parkinson, 1988; Winckler et al., 1996). The dependence of the rate of CWD decomposition on the moisture level is more complex than its response to temperature changes. For instance, both high and low substrate moisture can limit the activity of organisms living in wood and negatively affect the rate of CWD decomposition (Zhou et al., 2007). Therefore, the effects of abiotic and biotic factors on CWD decomposition are closely interrelated in nature and they can be differentiated only within the framework of laboratory experiments.
One of the most common tree species in the forests of the Northern Hemisphere is European aspen (Populus tremula, L.). Aspen provides habitat and food for a wide variety of mammals, birds, insects, and fungi, thereby acting as a key species for maintaining biodiversity in boreal forests (Latva–Karjanmaa et al., 2007). It is known that over 150 species in the boreal forests of Finland cannot exist without aspen (Kouki et al., 2004). In addition, aspen plays an important role in timber, and pulp and paper industries in European countries, since it is one of the most rapidly growing tree species (Worrell, 1995b). Aspen is becoming increasingly more popular in forestry owing to its high growth rates and ability to regenerate from shoots; as a result, regeneration after felling is much cheaper, since this does not require planting or sowing (Worrell, 1995a). Although aspen is currently not a very promising tree species in Russian forestry, that situation may change in the future.
The main objectives of this study were to (1) quantify the effect of temperature on the dynamics and intensity of the biogenic decomposition of aspen bark under sufficient moisture conditions and (2) analyze temperature-related changes in the chemical composition of aspen bark over 12-month incubation. For this purpose, we carried out a laboratory experiment on the long-term incubation of aspen bark at three contrasting temperatures: 2, 12, and 22°C. Since types of bark decomposition, such as physical decay and leaching, are almost completely excluded under laboratory conditions, the main process is biogenic (microbial) decomposition, and its rate is estimated.
MATERIALS AND METHODS
Preparation of substrates. Aspen bark (AB), consisting of phloem and cork, was cut from a recently fallen tree in the southern Moscow region a few days before the beginning of the experiment. The bark at natural moisture (47–53%) was cut into small pieces (less than 2 × 2 cm) using a secateur and placed in 500 mL flasks, followed by the addition of 5 mL soil suspension (soil : water ratio = 1 : 10) prepared from gray forest loamy soil (Moscow oblast, experimental plots of the Institute of Physicochemical and Biological Problems of Soil Science, Russian Academy of Sciences); this allowed us to make the conditions of AB decomposition most similar to natural ones. The suspension was added so that the resulting moisture content of bark substrates corresponded to 70–75% of their water-retention capacity. The flasks with bark were then placed into thermostats and incubated for 12 months at three contrasting temperatures: 2, 12, and 22°C. The choice of temperatures for the experiment was determined by the fact that the average temperature of the upper soil layer (0–5 cm) in the forest cenosis varied from 11 to 13°C (mean 12°C) in the south of Moscow oblast from May to October (long-term monitoring data). The two other temperature values (2 and 22°C) were chosen to estimate the temperature coefficient (Q10) that shows how many times the rate of biogenic decomposition of AB will increase when the temperature increases by 10°C. During the experiment, the bark moisture content was maintained at the constant level by periodically monitoring the weight of the flasks (no less than 2–3 times a month). The loss of the bark weight during decomposition was also taken into account. The experiment was carried out in three replications.
Determination of the rate of AB decomposition. The bark decomposition rate (DecR) was estimated by the intensity of C–CO2 emission, which, in turn, was determined 3–5 times a week during the first two months of the experiment and 1–2 times a week for the rest of the period. On the measurement day, the flasks were ventilated in air for 30 min and sealed with rubber caps. After 2–4 h, the concentration of C–CO2 was determined in the flasks using a LiCor-820 flow-through infrared gas analyzer (Nebraska, United States). The flasks were closed with breathable polythene films between the measurements to significantly restrain evaporation. The DecR (mg C kg bark–1 h–1) was calculated by the formula (Kurganova et al., 2012, 2018)
where dC is the change in CO2 concentration in the flask, volumetric %; Vflask is the flask volume, mL; t is the incubation time, h; and m is the mass of absolutely dry substrate, g.
The total carbon loss over the 12 months of the experiment (TotL-C, mg C/g bark) was determined using the cumulative curves of C–CO2 losses over this period.
Chemical analysis of AB. At the end of the incubation experiment, bark was removed from the flasks, air-dried at room temperature, and grounded to powder. The concentration of carbon (C) and nitrogen (N) in bark samples was determined before and after incubation using an automatic CNHS analyzer (LECO Corp., United States). The pH value was measured in water extraction at a bark : water ratio of 1 : 25 using a potentiometric pH sensor (Hanna, Germany). The content of alcohol-soluble compounds (Eth-Ext, including aromatic and aliphatic carbohydrates; terpenes; carbolic acids; resins; and fatty acids and essential oils, fats, and phytosterols), cellulose (Cel), and lignin (Lig) were measured in the bark samples before and after the 12-month incubation. The concentrations of lignin and cellulose were estimated by Klasson’s and Kurschner’s methods (Dence, 1992), which were adapted at the Analytical Laboratory of the Forest Research Institute, Karelian Research Center, Russian Academy of Sciences (Obolenskaya et al., 1991). Lignin was estimated in the extract residues after removing bituminous substances and treating the residues with 72% sulfuric acid; cellulose was extracted by a mixture of concentrated nitric acid and ethanol (volumetric ratio = 1 : 4). The biochemical analyses and pH measurements were not replicated.
Based on a comparison of the biochemical composition of aspen bark samples prior to the experiment (Table 1) and after the 12-month incubation, we estimated the residual amounts of the main organic substances (Eth-Ext, Cel, and Lig) as proportions of their initial content in aspen bark. Changes in Eth-Ext, Lig, and Cel concentrations in the bark samples over the 12-month incubation experiment were estimated considering the changes in the C concentration in the bark samples over the same period.
Data Processing
The decay constant for aspen bark (k, yr–1) was estimated by fitting a single exponential model (Olson, 1963; Kätterer et al., 1998):
where TotL-C is the cumulative C–CO2 loss as a result of microbial bark decay (g C kg–1 bark), Co is the initial content of total C in bark (g C kg–1 bark), k is the decay constant, yr–1; and t is the incubation time (years). The half-life time required for 50% bark mass loss was estimated as T0.5 = 0.693/k and the turnover time corresponding to the time interval when 95% of organic matter is lost was estimated as T0.95 = 3/k (Grishina et al., 1990; Ul’yanova and Chuprova, 2015; Hu et al., 2017).
The temperature sensitivity of aspen bark DecR, was expressed as a Q10 function (Chen et al., 2000):
where DecR2 and DecR1 are decay rates of bark at temperatures T2 and T1, respectively.
The effect of temperature on the DecR, TotL-C, and k values for the aspen bark substrates was estimated using a one-way ANOVA in STATISTICA 6. All statistical analyzes were performed at significance level α = 0.05.
RESULTS AND DISCUSSION
Chemical composition of aspen bark and its change over the experimental period. The initial AB samples were characterized by an acidic reaction of the water exstraction (pH 5.6), a wide C : N ratio (120 ± 4), a significantly lower C : H ratio (8.6 ± 0.2), and an almost threefold higher content of Lig and Cel (40.8%) than that of Eth-Ext (15%) (Table 1).
Similarly to most plant materials, C is the dominant element in the bark of all tree species (Geles, 2001; Skonieczna et al., 2014). Its concentration varies from 46 to 52% in aspen bark, while the content of nitrogen and phosphorus (P) is only 0.47–0.60% and 0.015–0.07%, respectively (Faustova, 2002). These high ratios (C : N = 77–110 and C : P = 600–3500) can significantly limit the intensity of biogenic decay of plant residues under conditions of the unbalanced (for decomposers) proportion of nutrients (Vedrova, 1997; Semenov and Khodzhaeva, 2006; Palviainenm et al., 2008). It is assumed that the destruction of bark and wood tissues is directly correlated with the activity of enzymes that break down cellulose and lignin and is usually limited by the availability of N and/or P (Sinsabaugh et al., 1993).
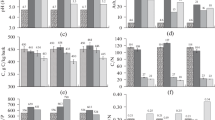
Over the 12 months of incubation, the pH value for bark increased by 2.1–2.5 units and its ash content increased by 1.6–1.8 times, depending on the temperature of incubation (Fig. 1).
We associate this significant change in the pH value (alkalinization) with the prevailing loss of easily hydrolysable organic compounds having low pH values (Fig. 2). The significant increase in the ash content naturally results from the mineralization of organic compounds. The experiment showed a significant decrease in alcohol-soluble organic compounds and cellulose to 18–32 and 41–57% of their initial amount, respectively (Fig. 2). Lignin losses were insignificant, being only 9–18% of their initial amount.
As a consequence, the proportion of organic compounds that are more resistant to mineralization (Cel + Lig) to the alcohol-soluble substances (Eth-Ext) increased 12–17% over the time of the experiment (Fig. 3d). Depending on temperature, the C : N and Cel : N ratios decreased at the end of the experiment by 1.6–2.1 and 1.5–1.8 times, respectively (Figs. 3a, 3c). At the same time, the Lig : N ratio (Fig. 3b) changed little at temperatures of 2 and 12°C and slightly decreased at 22°C over the period of AB incubation.
Therefore, the decomposition of AB was accompanied by a relative enrichment of the substrate with nitrogen, which is considered more favorable for the vital activity of microorganisms decomposing this substrate. The quantitative changes in the chemical composition of aspen bark were very similar at 2 and 12°C and less pronounced than the changes at 22°C (Figs. 2, 3), which may be determined by significant differences in the structure of microbial communities and/or by the trend of the wood-decomposing activity of microorganisms towards the optimum zone at 22°C. The 12-month laboratory experiments on composting the bark of aspen, pine, spruce, and larch showed that the availability of N is a key factor for stimulating the process of bark decay (Ul’yanova and Chuprova, 2015). Our earlier experiments revealed that the addition of nitrogen and phosphorus in the form of mineral fertilizers shifted the C : N and C : P ratios towards more favorable conditions for the microbial community and significantly increased the rate of AB decomposition; they also showed that the combined effect of N and P, added into the mixtures of soil and AB, was higher than the effect of N alone (Kurganova et al., 2018).
Dynamics of C–CO2 emission during the biogenic decomposition of bark. The highest rate of aspen bark decomposition was observed in the first weeks of the experiment, followed by a gradual decrease in the intensity of decomposition processes (Fig. 4).
The period with maximum DecR values (105–115 mg C kg bark–1 h–1 at 22°C) was about 2 weeks. At 12°C, the period with the highest DecR values was significantly shorter (3–4 days), and the intensity of CO2 emission was much lower (<68–81 mg C kg bark–1 h–1) than that at 22°C. At the lowest incubation temperature (2°C), the most intensive decomposition of AB was observed in the first 60 days of the experiment; however, the DecR value did not exceed 30–43 mg C kg bark–1 h–1 during this period (Fig. 4). In the last months of the incubation experiment, the rate of C–CO2 emission changed insignificantly, gradually decreasing to 17–18 mg C kg bark–1 h–1 at 2°C and varying from 11 to 14 mg C kg bark–1 h–1 at 12 and 22°C.
This dynamics of CO2 emission during the decomposition of fresh aspen bark indicates a rapid mineralization of easily decomposed organic compounds, such as aliphatic carbohydrates and carbolic and fatty acids, in the first weeks of incubation, which is very consistent with changes in the chemical composition of AB that were revealed in our study (Fig. 2). In addition, it is quite expected that the rate and completeness of reactions of the decomposition of these compounds increased with growth in the incubation temperature.
Rates of C–CO2 emission and temperature coefficients at different stages of the experiment. Based on the temporal dynamics of the DecR (Fig. 4), we divided the 12-month period of the experiment into four stages (1–2, 3–4, 5–6, and 7–12 months) and calculated the average DecR values for the entire incubation period and for each separate stage (Fig. 5).
The first two months of the experiment showed the highest average DecR values at all temperatures: 34 ± 2, 42 ± 4, and 68 ± 7 mg C kg bark–1 h–1 at temperatures of 2, 12, and 22°C, respectively. The most gradual decrease in the average DecR values at different stages of the incubation experiment was observed at 2°C, while their highest decrease was observed at 22°C (Fig. 5). The average rates of C–CO2 emission during the second half of the experiment (7–12 months) did not statistically differ at 2 and 22°C (16 ± 1 and 17 ± 1 mg C kg bark–1 h–1, respectively); however, they were significantly lower than those at 12°C (21 ± 2 mg C kg bark–1 h–1). The average rates of AB decomposition throughout the experimental period varied from 23 ± 2 to 42 ± 4 mg C kg bark–1 h–1, depending on the incubation temperature (Fig. 5). The results of the one-way analysis of variance showed that temperature significantly (P < 0.0001) influenced the DecR value at all stages of the experiment.
The Q10 values for the average DecR values throughout the experimental period were similar in the studied temperature intervals (2–12 and 12–22°C): 1.30 and 1.41, respectively. However, the effect of temperature on the DecR value was more pronounced at different stages of the experiment (Table 2). Thus, the increase in the DecR values in the first 2 months and third to fourth months of incubation was more significant in the range of higher temperatures (12–22°C) than in the lower temperature interval (2–12°C): Q10 = 1.62–2.24 vs. 1.06–1.25. The effect of temperature on the DecR was inverse during the 5th–12th months of the experiment: the Q10 values were higher for the lower temperature range of 2–12°C (Q10 = 1.31–1.82) than for the higher temperature range of 12–22°C (Q10 = 0.81–1.17).
In the boreal and temperate zones, temperature is a key abiotic factor that controls the biogenic decomposition of all CWD components, including tree bark (Olajuyigbe et al., 2012; Kahl et al., 2015). An increase in the environmental temperature at a sufficient amount of moisture leads to a significant increase in the rate of CWD decomposition (Wu et al., 2010; Forrester et al., 2012; Ohtsuka et al., 2014). This is also true for other plant substrates, e.g., forest litter (Larionova et al., 2017a, 2017b) or peat-forming plants (Nikonova et al., 2018; Nikonova et al., 2019a, 2019b).
In our laboratory experiment, the gradual increase of temperature (at constant sufficient moisture) induced a significant increase in the DecR values in both temperature intervals (2–12 and 12–22°C) only during the first 2 months of incubation (Fig. 5). During the 3rd to 12th months of the experiment, the increased temperature could induce a significant increase in the average DecR values only in one of the intervals (2–12 or 12–22°C). This can be explained by the fact that the AB at the beginning of incubation contained a sufficient amount of accessible, easily decomposed substrates, which were characterized by high decomposition rates at all temperatures. More stable organic compounds (cellulose and lignin) begin to prevail in the bark in the course of incubation; therefore, the rates of AB decomposition decrease and the effect of temperature on the decomposition process becomes less significant. These mechanisms also control litter decomposition in northern forests. Thus, the concentration of water-soluble substances rapidly decreases at the early stage of forest litter decay (after several months) before reaching relatively stable levels (Berg et al., 1987). In addition, free holocellulose is also decomposed at this stage, while more stable lignin is not yet decomposed or decomposed at a low rate. The rate of forest-litter decomposition at this early stage can also be influenced by the total concentration of main nutrients, such as N, P, and S, which often limit the rate of decomposition of organic substrates (Berg, 2000). Apparently, the amount of nutrients in the phloem tissue, which is decomposed first (during the first 2 months of decay), is sufficient for the active process of biogenic AB decomposition.
Being able to decompose lignin and cellulose, fungi play the dominant role in the decomposition of woody material (xylolysis), including bark (Boddy and Watkinson, 1995; Boddy, 2000). Many fungal species are mesophylls and the optimal temperature range for their growth is 20 to 40°C; the decomposition rates within this temperature range can increase 2–3 times per each 10°C (Käärik, 1974; Zhou et al., 2007). In our study, the Q10 values for the average DecR value during the experiment were almost the same for the temperature ranges of 2–12 and 12–22°C (Table 2). In addition, the DecR of samples incubated at 12–22°C had higher Q10 values than the DecR of samples incubated at 2–12°C at the early stage of the experiment (the first 4 months). At the later stages (5–12 months of incubation), the temperature increase from 12 to 22°C induced a less significant growth in the DecR value than that in the range of 2–12°C (Table 2). Apparently, this is partly due to the fact that the process of the decay of easily decomposed components in AB was more intensive at this temperature and ended during the first 2 months of the experiment, while its period was longer at lower temperatures and the average DecR values decreased less significantly during the 5th to 12th months of incubation.
Total losses of C–CO2 and decay constants of AB throughout the experiment. The growth in temperature led to a significant increase (P < 0.005) in the integral losses of C–CO2 due to the biogenic decomposition of AB throughout the experiment: from 173 ± 3 mg C g bark–1 at 2°C to 284 ± 16 mg C g bark–1 at 22°C. The total losses of C–CO2 over the 12 months of the experiment were 34–55% (depending on temperature) of the initial C stock in AB before incubation (Table 3). The decay constants of AB also significantly increased with growth in incubation temperature. The k values increased more significantly in the temperature interval of 12–22°C than in the range of 2–12°C (Table 3).
In our laboratory study, we estimated the decay constants of AB (k) based on the cumulative curve of C–CO2 losses over the 12 months of incubation using the one-component exponential model. Under field experiments, the k values for bark and wood are usually estimated based on the weight loss in carefully selected CWD chronoseries (Zhou et al., 2007; Shorohova and Kapitsa, 2015, 2016; Dossa et al., 2016). The decay constants of bark as a CWD component, estimated by the method of chronosequences, varied from 0.2 to 0.4 yr–1 for the bark of different tree species (Zhang et al., 2008) and comprised 0.074 yr–1 for the bark of aspen in old-growth forests (Shorohova et al., 2016). The k values that we obtained in our laboratory study at constant sufficient moisture level were 0.46–1.02 yr–1 (depending on incubation temperature), which is significantly higher than the values of the decay constants under natural conditions, at which the temperature and water content significantly vary in the bark throughout a year. Nevertheless, the data of our experiment can be used for the parametrization of models describing the decomposition of CWD.
Depending on the temperature of the experiment, the half-life time for AB varied from 1.5 to 0.7 years, thereby demonstrating the same patterns that were obtained for the decay constant. The turnover time for AB, which corresponds to the time interval during which 95% of organic matter is lost, varied from 6.6 to 3.0 years, depending on temperature. The more significant effect of temperature on the decay constant and decomposition period for AB in the temperature interval of 12–22°C suggests that the intensification of the decomposition of aspen bark will be more pronounced in summer, especially against the background of a very noticeable trend of increased summer temperatures in the south of Moscow region over the last 40 years (Kurganova et al., 2017).
CONCLUSIONS
The temperature that induced the decomposition of aspen bark significantly determined not only the dynamics of C–CO2 emission, but also total carbon losses in the form of CO2, as well as the decay constant and degree of changes in the chemical composition of AB during the long-term incubation experiment. The temperature increase from 2 to 22°C led to an increase in the integral losses of C–CO2 by 1.6 times and an increase in the decay constant of aspen bark by 2.2 times throughout the experiment. Along with almost the same increase in the average (for 12 months) DecR value in both temperature intervals (2–12 and 12–22°C), the most pronounced differences in the temperature-determined dynamics of the DecR were revealed during the first months of the experiment, i.e., when most of the easily hydrolysable organic compounds were decomposed.
The AB decomposition led to a relative enrichment of the substrate with nitrogen, which favorably influenced the vital activity of decomposing microorganisms. All the parameters characterizing the process of the decomposition of aspen bark (dynamics, total losses of C–CO2, decay constants, and quantitative changes in its chemical composition) were similar at 2 and 12°C, being, however, less pronounced than the changes in the same parameters at 22°C. This may be determined by the fact that the activity of organisms involved in bark decomposition processes significantly increases at 22°C.
REFERENCES
Berg, B., Litter decomposition and organic matter turnover in northern forest soils, For. Ecol. Manage., 2000, vol. 133, pp. 13–22.
Berg, B., Staaf, H., and Wessen, B., Decomposition and nutrient release in needle litter from nitrogen-fertilized Scots pine (Pinus silvestris) stands, Scand. J. For. Res., 1987, vol. 2, pp. 399–415.
Berglund, S.L., Ågren, G.I., and Ekblad, A., Carbon and nitrogen transfer in leaf litter mixtures, Soil Biol. Biochem., 2013, vol. 57, pp. 341–348.
Boddy, L., Interspecific combative interactions between wood-decaying basidiomycetes, FEMS Microb. Ecol., 2000, vol. 31, pp. 185–194.
Boddy, L. and Watkinson, S.C., Wood decomposition, higher fungi, and their role in nutrient redistribution, Can. J. Bot., 1995, vol. 73, pp. 1377–1383.
Bradford, M.A., Warren, R.J., Baldrian, P., Crowther, T.W., Maynard, D.S., Oldfield, E.E., Wieder, W.R., Wood, S.A., and King, J.R., Climate fails to predict wood decomposition at regional scales, Nat. Clim. Change, 2014, vol. 4, pp. 625–630.
Chambers, J.Q., Schimel, J.P., and Nobre, A.D., Respiration from coarse wood litter in central Amazon forests, Biogeochemistry, 2001, vol. 52, pp. 115–131.
Chen, H., Harmon, M.E., Griffiths, R.P., and Hicks, W., Effects of temperature and moisture on carbon respired from decomposing woody roots, For. Ecol. Manage., 2000, vol. 138, pp. 51–64.
Cornwell, W.K., Cornelissen, J.H.C., Allison, S.D., Bauhus, J., Eggleton, P., Preston, C.M., Scarff, F., Weedon, J.T., Wirth, C., and Zanne, A.E., Plant traits and wood fates across the globe: rotted, burned, or consumed? Global Change Biol., 2009, vol. 15, pp. 2431–2449.
Dence, C.W., The determination of lignin, in Methods of Lignin Chemistry, Lin, S.Y. and Dence, C.W., Eds., Berlin: Springer-Verlag. 1992, pp. 33–61.
Dossa, G.O., Paudel, E., Cao, K., Schaefer, D., and Harrison, R.D., Factors controlling bark decomposition and its role in wood decomposition in five tropical tree species, Sci. Rep., 2016, vol. 6, p. 34153.
Faustova, N.M., Chemical composition of bark and wood of European aspen Populus tremula L., Cand. Sci. (Chem.) Dissertation, St. Petersburg, 2005.
Forrester, J.A., Mladenoff, D.J., Gower, S.T., and Stoffel, J.L., Interactions of temperature and moisture with respiration from coarse woody debris in experimental forest canopy gaps, For. Ecol. Manage., 2012, vol. 265, pp. 124–132.
Franceschi, V.R., Krokene, P., Christiansen, E., and Krekling, T., Anatomical and chemical defenses of conifer bark against bark beetles and other pests, New Phytol., 2005, vol. 167, pp. 353–376.
Fukasawa, Y., The geographical gradient of pine log decomposition in Japan, For. Ecol. Manage., 2015, vol. 349, pp. 29–35.
Geles, I.S., Drevesnaya biomassa i osnovy ekologicheski priemlimykh tekhnologii ee khimiko-mekhaicheskoi pererabotki (Wood Biomass and Fundamentals of Ecology-Friendly Technologies of Its Chemical-Mechanical Processing), Petrozavodsk: Karel. Nauchn. Tsentr, Ross. Akad. Nauk, 2001.
Grishina, L.A., Koptsik, G.N., and Makarov, M.I., Transformatsiya orgnaicheskogo veshchestva pochv: Uchebnoe posobie (Transformation of Soil Organic Matter: Manual), Moscow: Mosk. Gos. Univ., 1990.
Hagemann, U., Moroni, M.T., Gleissner, J., and Makeschin, F., Disturbance history influences downed woody debris and soil respiration, For. Ecol. Manage., 2010, vol. 260, pp. 1762–1772.
Harmon, M.E., Franklin, J.F., Swanson, F.J., Sollins, P., Gregory, S.V., Lattin, N.G., Sedell, J.R., Lienkaemper, G.W., Cromack, K., Jr., and Cummins, K.W., Ecology of coarse woody debris in temperate ecosystems, Adv. Ecol. Res., 1986, vol. 15, pp. 133–302.
Hu, Z., Xu, C., McDowell, N.G., Johnson, D.J., Wang, M., Luo, Y., Zhou, X., and Huang, Z., Linking microbial community composition to C loss rates during wood decomposition, Soil Biol. Biochem., 2017, vol. 104, pp. 108–116.
Käärik, A.A., Decomposition of wood, in Biology of Plant Litter Decomposition, Dickinson, C.H. and Pugh, G.J.F., Eds., London: Academic, 1974, pp. 129–174.
Kahl, T., Arnstadt, T., Baber, K., Bässler, C., Bauhus, J., Borken, W., Buscot, F., Floren, A., Heibl, C., Hessenmöller, D., Hofrichter, M., Hoppe, B., Kellner, H., Krüger, D., Linsenmair, K.E., et al., Wood decay rates of 13 temperate tree species in relation to wood properties, enzyme activities and organismic diversities, For. Ecol. Manage., 2017, vol. 391, pp. 86–95.
Kapitsa, E.A., Trubitsyna, E.A., and Shorohova, E.V., Biogenic xylolysis of trunks, branches, and roots of forest-forming species of dark coniferous northern taiga, Lesovedenie, 2012, no. 3, pp. 51–58.
Kätterer, T., Reichstein, M., Andren, O., and Lomander, A., Temperature dependence of organic matter decomposition: a critical review using literature data analyzed with different model, Biol. Fertil. Soils, 1998, vol. 27, pp. 258–262.
Kazartsev, I., Shorohova, E., Kapitsa, E., and Kushnevskaya, H., Decaying Picea abies log bark hosts diverse fungal communities, Fungal Ecol., 2018, vol. 33, pp. 1–12.
Kuoki, J., Arnold, K., and Martikainen, P., Long-term persistence of aspen—a key host for many threatened species—is endangered in old-growth conservation areas in Finland, J. Nat. Conserv., 2004, vol. 12, pp. 41–52.
Kurganova, I.N., Lopes de Gerenyu, V.O., Gallardo Lancho, J.F., and Oehm, C.T., Evaluation of the rates of soil organic matter mineralization in forest ecosystems of temperate continental, Mediterranean, and tropical monsoon climates, Eurasian Soil Sci., 2012, vol. 45, no. 1, pp. 68–79.
Kurganova, I.N., Lopes de Gerenyu, V.O., Ableeva, V.A., and Bykhovets, S.S., Climate of southern Moscow region: modern trends and assessment of extreme conditions, Fundam. Prikl. Klimatol., 2017, no. 4, pp. 62–78.
Kurganova, I., Lopes de Gerenyu, V., Galibina, N., Kapitsa, E., and Shorohova, E., Coupled effect of temperature and mineral additions facilitates decay of aspen bark, Geoderma, 2018, vol. 316, pp. 27–37.
Kuznetsov, M.A., Influence of decomposition conditions and litter composition on the characteristics and reserves of litter in the middle taiga blueberry-sphagnum spruce forest, Lesovedenie, 2010, no. 6, pp. 54–60.
Larionova, A.A., Kvitkina, A.K., Bykhovets, S.S., Lopes de Gerenyu, V.O., Kolyagin, Yu.G., and Kaganov, V.V., Influence of nitrogen on mineralization and humification of forest litter in a model experiment, Lesovedenie, 2017a, no. 2, pp. 128–139.
Larionova, A.A., Maltseva, A.N., Lopes de Gerenyu, V.O., Kvitkina, A.K., Bykhovets, S.S., Zolotareva, B.N., and Kudeyarov, V.N., Effect of temperature and moisture on the mineralization and humification of leaf litter in a model incubation experiment, Eurasian Soil Sci., 2017b, vol. 50, no. 4, pp. 422–431.
Latva-Karjanmaa, T., Penttil, R., and Siitonen, J., The demographic structure of European aspen (Populus tremula) populations in managed and old-growth boreal forests in eastern Finland, Can. J. For. Res., 2007, vol. 37, no. 6, pp. 1070–1081.
Lestander, T.A., Holmberg, C., Stenberg, L., and Lehtonen, R., Towards multivariate statistical process control in the wood pellet industry, Biom. Bioeng., 2012, vol. 45, pp. 152–158.
Li, Q., Chen, J., and Moorhead, D.L., Respiratory carbon losses in a managed oak forest ecosystem, For. Ecol. Manage., 2012, vol. 279, pp. 1–10.
Liu W., Schaefer D., Qiao L., and Liu X., What controls the variability of wood-decay rates? For. Ecol. Manage., 2013, vol. 310, pp. 623–631.
Martin, A.R., Gezahegn, S., and Thomas, S.C., Variation in carbon and nitrogen concentration among major woody tissue types intemperate trees, Can. J. For. Res., 2015, vol. 45, pp. 744–757.
Mukhin, V.A., Biota ksilotrofnykh bazidiomitsetov Zapadno-Sibirskoi rabniny (Biota of Xylotrophic Basidiomycetes of West Siberian Plain), Yekaterinburg: Nauka, 1993.
Nikonova, L.G., Kurganova, I.N., Lopes de Gerenyu, V.O., Zhmurin, V.A., and Golovatskaya, E.A., Temperature effect on the decomposition rate of peat-forming plants: results of a model experiment, IOP Conf. Ser.: Earth Environ. Sci., 2018, vol. 211, art. ID 012037. https://doi.org/10.1088/1755-1315/211/1/012037
Nikonova, L.G., Kurganova, I.N., Lopes de Gerenyu, V.O., Zhmurin, V.A., and Golovatskaya, E.A., Influence of abiotic factors on the decomposition of litter of peat-forming plants in an incubation experiment, Vestn. Tomsk. Gos. Univ., Biol., 2019a, no. 46, pp. 148–170.
Nikonova, L.G., Golovatskaya, E.A., Kur’ina, I.V., and Kurganova, I.N., Decomposition rate of peat-forming plants in oligotrophic bogs of the southern taiga subzone of Western Siberia: assessment of the effect of water table level and peat deposit temperature, Eurasian Soil Sci., 2019b, vol. 52, no. 9, pp. 1101–1111
Obolenskaya, A.V., El’nitskaya, Z.P., and Leonovich, A.A., Laboratornye raboty po khimii drevesiny i tsellyulozy (Practical Manual on Chemistry of Wood and Cellulose), Moscow: Ekologiya, 1991.
Ohtsuka, T., Shizu, Y., Hirota, M., Yashiro, Y., Shugang, J., Iimura, Y., and Koizumi, H., Role of coarse woody debris in the carbon cycle of Takayama forest, central Japan, Ecol. Res., 2014, vol. 29, pp. 91–101.
Olajuyigbe, S., Tobin, B., and Nieuwenhuis, M., Temperature and moisture effects on respiration rate of decomposing logs in a Sitka spruce plantation in Ireland, Forestry, 2012, vol. 85, no. 4, pp. 485-496.
Olson, J., Energy storage and the balance of producers and decomposers in ecological systems, Ecology, 1963, vol. 44, no. 2, pp. 322–331.
Palviainenm, M., Laiho, R., Mäkinen, H., and Finér, L., Do decomposing Scots pine, Norway spruce, and silver birch stems retain nitrogen? Can. J. For. Res., 2008, vol. 38, no. 12, pp. 3047–3055.
Russell, M.B., Fraver, S., Aakala, T., Gove, J.H., Woodall, C.W., D’Amato, A.W., and Ducey, M.J.T., Quantifying carbon stores and decomposition in dead wood: a review, For. Ecol. Manage., 2015, vol. 350, pp. 107–128.
Safronov, M.A., Bulgakov, E.A., Ostapenko, A.V., and Tyapukhin, P.V., The influence of wood destruction on the temperature and humidity regime in forest biogeocenoses of the southern Urals, Vestn. Orenb. Gos. Univ., 2013, no. 10 (159), pp. 333–335.
Semenov, V.M. and Khodzhaeva, A.K., Agroecological functions of plant remains in soil, Agrokhimiya, 2006, no. 7, pp. 63–81.
Shorohova, E. and Kapitsa, E., Stand and landscape scale variability in the amount and diversity of coarse woody debris in primeval European boreal forests, For. Ecol. Manage., 2015, vol. 315, pp. 185–190.
Shorohova, E. and Kapitsa, E., The decomposition rate of non-stem components of coarse woody debris (CWD) in European boreal forests mainly depends on site moisture and tree species, Eur. J. For. Res., 2016, vol. 135, pp. 593–606.
Shorohova, E., Ignatyeva, O., Kapitsa, E., Kauhanen, H., Kuznetsov, A., and Vanha-Majamaa, I., Stump decomposition rates after clear-felling with and without prescribed burning in southern and northern boreal forests in Finland, For. Ecol. Manage., 2012, vol. 263, pp. 74–84.
Shorohova, E., Kapitsa, E., Kazartsev, I., Romashkin, I., Polevoi, A., and Kushnevskaya, A., Tree species traits are the predominant control on the decomposition rate of tree log bark in a mesic old-growth boreal forest, For. Ecol. Manage., 2016, vol. 377, pp. 36–45.
Sinsabaugh, R.L., Antibus, R.K., Linkins, A.E., McClaugherty, C.A., Rayburn, L., Repert, D., and Weiland, T., Wood decomposition: nitrogen and phosphorus dynamics in relation to extracellular enzyme activity, Ecology, 1993, vol. 74, pp. 1586–1593.
Skonieczna, J., Małek, S., Polowy, K., and Węgiel, A., Elements content of Scots pine (Pinus sylvestris L.) stands of different densities, Drewno Pr. Nauk. Doniesienia Komunikaty, 2014, vol. 57, no. 192, pp. 77–87.
Taylor, B.R. and Parkinson, D., Aspen and pine leaf litter decomposition in laboratory microcosms. II. Interactions of temperature and moisture level, Can. J. Bot., 1988, vol. 66, pp. 1966–1973.
Ugolev, B.N., Drevesinovedenie i lesnoe tovarovedenie. Uchebnoe posobie (Wood Science and Forest Commodity Research: Manual), Moscow: Mosk. Gos. Univ. Lesa, 2002.
Ul’yanova, O.A. and Chuprova, V.V., Mineralization of the bark of various of tree species and fertilizer compositions based on it, Agrokhimiya, 2015, no. 2, pp. 33–45.
Vedrova, E.F., Organic matter decomposition in forest litters, Eurasian Soil Sci., 1997, vol. 30, no. 2, pp. 181–188.
Wetzel, S. and Greenwood, J.S., Proteins as a potential nitrogen storage compound in bark and leaves of several softwoods, Trees, 1989, vol. 3, pp. 149–153.
Winckler, J.P., Cherry, R.S., and Schlesinger, W.H., The Q 10 relationship of microbial respiration in a temperate forest soil, Soil Biol. Biochem., 1996, vol. 28, no. 8, pp. 1067–1072.
Worrell, R., European aspen (Populus tremula): a review with particular reference to Scotland 1: Distribution, ecology and genetic variation, Forestry, 1995a, vol. 68, pp. 94–105.
Worrell, R., European aspen (Populus tremula): a review with particular reference to Scotland, 2: Values, silviculture and utilization, Forestry, 1995b, vol. 68, pp. 231–244.
Wu, J., Zhang, X., Wang, H., Sun, J., and Guan, D., Respiration of downed logs in an old-growth temperate forest in north-eastern China, Scand. J. For. Res., 2010, vol. 25, pp. 500–506.
Yatskov, M., Harmon, M.E., and Krankina, O.N., A chronosequence of wood decomposition in the boreal forests on Russia, Can. J. For. Res., 2003, vol. 33, pp. 1211–1226.
Zamolodchikov, D.G., Evaluation of carbon pool of large wood remains in Russian forests taking into account the impact of wild fires and logging, Lesovedenie, 2009, no. 4, pp. 3–15.
Zhang, D., Hui, D., Luo, Y., and Zhou, G., Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors, J. Plant Ecol., 2008, vol. 1, pp. 85–93.
Zhou, L., Dai, L., Gu, H., and Zhong, L., Review on the decomposition and influence factors of coarse woody debris in forest ecosystem, J. For. Res., 2007, vol. 18, no. 1, pp. 48–54.
Funding
This study was performed under the State Assignment of the Puschino Biological Research Center, Russian Academy of Sciences, project no. AAAA-A18-118013190177-9, and State Assignment of the Karelian Research Center, Russian Academy of Sciences, and supported by the Russian Foundation for Basic Research, project no. 19-04-01282a.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Translated by D. Zabolotny
Rights and permissions
About this article
Cite this article
Kurganova, I.N., Lopes de Gerenyu, V.O., Galibina, N.A. et al. Effect of Temperature on the Intensity of the Biogenic Decomposition of Aspen Bark. Contemp. Probl. Ecol. 13, 663–673 (2020). https://doi.org/10.1134/S1995425520060086
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995425520060086