Abstract
A review is presented of thermally stable polymer polyimide based adhesives capable of long-term operation at temperatures above 200°C. The most frequently applied methods for the synthesis of polyimides and their forepolymers are considered, alongside with the processes occurring in the course of their curing at terminal molding stages. The principal advantages and shortcomings of existing polyimide adhesives are demonstrated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The contemporary trend of development in materials science is in the direction of improving the operational and technological characteristics of existing and new materials for the purpose of using them as efficiently as possible in the composition of units and aggregates of designed equipment. In view of this fact, the development and introduction of a complex of thermally stable PSMs, glues, and polymer honeycomb fillers, which would extend the operational range of equipment significantly, are relevant [1].
Thermally stable and heat-resistant PSMs are customarily considered materials capable of long-term operation at temperatures above 200°C. From the theoretical standpoint, a maximum thermal stability of organic polymers can be attained if the chain of a polymer consists exclusively of aromatic rings linked in the para position. However, such a rigid-chain structure makes processing of a polymer impossible due to the unreachability of the melting and solubility temperatures. The most promising solution for the problem of attaining a balance between the properties of serviceability and thermal stability is the use of polymers with a heterocyclic structure [2]. There exist several approaches to the application of these polymers as thermally stable adhesives, PSM binders, films, and protective coatings, such as polycondensation of monomers and their derivatives in the process of molding, synthesis of recyclized oligomers capable of curing in the course of processing, and synthesis of forepolymers capable of forming a heterocyclic structure. Despite the rather great amount of different heterocyclic polymers that have been synthesized and studied beginning from the 1950s, only some of them were able to find broad industrial application. Examples of heterocyclic polymers that have been successfully introduced include cyanate esters, bismaleinimides, benzoxazines, phthalonitriles, and polyimides. In this case, depending on the requirements to the operational and technological parameters, it is reasonable to use the class of heterocyclic polymers characterized by an optimal combination of serviceability and thermal stability. Naturally, when the requirements for the operational temperature are increased, the possibility of selecting a polymer matrix for the creation of a thermally stable material is restricted and there occur some technological difficulties in processing and, frequently, an overall decrease in physicomechanical properties. Polyimides may be one of the few classes of polymers able to combine high thermal and heat resistance and performance characteristics with the availability of feedstocks.
This review is devoted to existing thermally stable polyimide-based polymer adhesives capable of long-term operation at temperatures above 200°C.
METHODS FOR SYNTHESIS OF POLYIMIDE MATERIALS AND THEIR FOREPOLYMERS
Aromatic polyimides are a class of heterocyclic polymers that are characterized by high thermal and heat resistance and have found broad application in industry worldwide for the creation of products from polymer materials for operation at increased temperatures.
Aromatic polyimides were synthesized for the first time in the beginning of the 20th century, and the first industrial two-stage method of their synthesis was patented and successfully commercialized by DuPont Co. in 1955 for a large-tonnage polyimide material, Kapton thermally stable film [3].
Since that time, many methods for the synthesis of polyimides and their compositions and subclasses have been developed with the purpose of optimizing their technological and operational properties.
The chemical structure of a monomer unit of aromatic polyimides entails the existence of imide and aromatic rings, which provide a high rigidity to the macromolecules of this class of polyheteroarylenes. For this reason, their processing by standard methods is feasible only for a small series of polyimides incorporating flexible asymmetric carded or copolymer moieties, which decrease the softening temperature of a polymer and increase its solubility.
To increase the serviceability of polyimides, approaches associated with the use of forepolymers, oligomers with low molecular masses, and imide-forming mixtures are rather widely used.
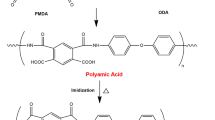
One of the most widely recognized methods for the production of polyimides is by means of the synthesis of a forepolymer, i.e., polyamido acid with further thermal or chemical imidization. This method consists in the reaction of tetracarboxylic-acid anhydrides with di- and polyamines. The first stage consists in acyclization of amine by means of an a hydride group with the formation of a polyamido acid (PAA). In contrast to most cyclized polyimides, polyamido acids are well soluble in polar aprotic solvents, e.g., dimethylformamide, dimethylacetamide, and N-methylpyrrolidone (N-MP). This circumstance significantly broadens the opportunities for the industrial production of different products, such as films, fibers, coatings, etc. After removing the solvent, PAA is subjected to thermal or chemical imidization, which results in the formation of a high-molecular-weight polyimide (Fig. 1).
However, this method is characterized by a number of serious shortcomings, such as the liberation of volatile compounds in the course of curing, the instability of the forepolymer during storage at room temperature, and the impossibility to prepare highly concentrated PAA solutions.
One rather widely recognized modification of the two-stage polyimide synthesis method is chemical PAA imidization, which results in the formation of polyisoimide (Fig. 2). In this case, dicyclohexyl carbodiimide and trifluoroacetic acid are most frequently used as dehydrating agents.
One clear advantage of this synthesis method is a low softening temperature and viscosity for a melt of polyisoimides as compared to polyimides (in some cases, their viscosities differ from each other by several orders of magnitude at the same temperature) [4]. In addition, polyisoimide-to-polyimide rearrangement is an intramolecular process occurring without the liberation of volatile compounds. In view of these circumstances, polyimide-based adhesives have improved technological properties.
It should be noted that, in addition to polyisoimides, both PAAs subjected to the reaction of imidization in the curing of glue and already cyclized polyimides, which can be softened at increased temperature and, thereby, provide the gluing of a substrate, are used as thermally stable adhesives. Polyesterimides, which are polyheteroarylenes containing a rather high number of ordinary ester bonds in their main chain, can also be classified among cyclized linear polyimides. A distinctive feature of these polymers is the possibility of their processing by standard methods applied for thermoplastic elastomers.
The best-known grades of glues in the form of PAAs, polyisoimides, and polyimides on their basis are adhesives of the series Larc-TPI (LarcTPI, Larc-ODA, Larc-IA, Larc-SO2) developed by Langley Research Center Co. (United States), NR (NR-150A2, NR-150B2, NR-056X, DuPont), and FM 680 glue made by CYTEC Co.
The most widely applied adhesives on the basis of PAAs, polyisoimides, and imidized linear polyimides are listed in Table 1 [5–7].
Despite the great amount of existing thermally stable glues on the basis of forepolymers and cyclized polyimides of linear structure, there exist some difficulties in their application due to long-term regimes of gluing, a great volume of volatile compounds (solvents and low-molecular-weight polycondensation products), high molding temperatures and pressures, and a relatively low operational temperature of glued joints based on them.
THERMOREACTIVE IMIDE-FORMING MIXTURES AND PERTIALLY IMIDIZED OLIGOMERS ON THEIR BASIS
Polyimides synthesized in the process of molding from an imide-forming mixture of components have found broad industrial application. These mixtures are used as a basis for the synthesis of polyimides of both linear and sparsely cross-linked structure and oligomers with unsaturated terminal groups that are able to enter into further chemical reactions.
As for their chemical composition, these thermoreactive compositions represent a mixture of derivatives of polycarboxylyc acids with aromatic diamines; in addition, there are some grades known on the basis of A–B monomers (monomers containing both amine and anhydride groups) [8]. These mixtures are well soluble in low-boiling-point solvents, and, in some cases, such compositions can be applied in the form of a melt for the impregnation of fillers [9]. The process of curing is accompanied by the reaction of polycondensation with the formation of polyimide with a linear or sparsely cross-linked structure, and, if the initial composition contains monomers with unsaturated groups, there further occurs polymerization with the formation of mesh polyimide (this concerns so-called “PRM compositions,” i.e., polymerization of monomeric reactants). The chemical reactions for PMR compositions are schematized in Fig. 3.
One of the first thermoreactive polyimide adhesives, which has found broad application in world industry, is Nolimid 380, which is produced by Rhone Pounlenc Co. This thermoreactive composition was developed on the basis of A–B monomers (Fig. 4).
In addition to a polymeric base, this adhesive also incorporates aluminum powder, an antioxidant, and fiberglass as a reinforced filler. The surface density of Nolimid 380 glue is 380–650 g/m2, and the residual content of volatile compounds is 15 wt % [10]. The strength of glued joints between titanium-alloy specimens is 20 and 16 MPa at 20 and 300°C, respectively.
FM-34 and FM-36 glues made by CYTEC Co. are also classified as thermoreactive polyimide compositions containing A–B monomers. These glues are characterized by a rather high shear strength of glued joints for titanium alloy (22 and 13 MPa at 25 and 316°C, respectively) [11]. Depending on the conditions of their application, adhesives containing A–B monomers may be used both with a filler (aluminum powder for FM-34 glue) and in the form of a solution with a partially imidized thermoreactive imide-forming composition (FM-36 glue).
A PMR composition (an imide-forming mixture of compounds with reaction groups of endic anhydride) was applied for the first time as a thermally stable adhesive under trademark LARC-13 with a monomer composition similar to PMR-15, representing one of the most widely used polyimide PMR binders with the exception that 3,3'-isomer was used in it instead of para isomer of diaminodiphenyl methane. The shear strength of glued joints is 22.8 and 19.3 MPa at 25 and 260°C, respectively.
FM-35 glue made by CYTEC is also classified as a glue based on a PMR composition. Aluminum-alloy specimens glued with this adhesive demonstrated the shear strength of 35.8, 21.4, and 21.7 MPa at temperatures of 25, 260, and 288°C. Titanium-alloy specimens glued by FM-35 are characterized by a shear strength of glued joints at a level of 21.7, 17.2, and 13.8 MPa at the same temperatures. It has been established that the brittleness of the polymer matrix rather strongly grows with an increase in the curing temperature to 329°C and, correspondingly, the shear strength of glued joints is decreased.
Renegade Materials Corp. industrially produces the RM-1010 and RM-1014 fiberglass reinforced glues. Despite that the fact that the producer does not disclose the composition of these trademarks, it is specified that these glues are thermoreactive polyimides with reactive terminal groups. The properties of RM-1010 and RM-1014 glues are given in Table 2 [12].
THERMOREACTIVE OLIGOIMIDES WITH REACTIVE TERMINAL GROUPS
Despite the rather broad industrial application of PMR compositions, they are characterized by a number of serious shortcomings, of which the most significant is low thermooxidation stability associated with the presence of aliphatic moieties in the polymer matrix of a cured polymer. A second problem consists in the polymerization mechanism for the terminal groups with the liberation of volatile cyclopentadiene in the process of curing, which necessitates the molding of these compositions at a high specific pressure to avoid the formation of a porous structure.
To solve these problems in the application of high-temperature glues and binders on the basis of thermoreactive oligoimides, some compositions with reactive acetylene and ethynyl groups have been developed. It is a clear advantage of these compositions that there is no liberation of any volatile compounds in the process of curing.
Initially, a series of oligomers with terminal acetylene groups was synthesized for glues based on thermoreactive oligoimides (Fig. 5). The best-known trademarks of these glues are HR-600, Thermid600, and LR-600 (polyamide acid precursor) made by Hughes Aircraft Co. They have proven to be rather good structural adhesives for joints of titanium, copper, and thermally stable composite materials. The shear strength of glued joints for titanium is 22.1, 13.1, and 8.3 MPa at 25, 232, and 260°C, respectively, after thermal aging for 1000 h at these temperatures.
Today, compositions with acetylene terminal groups are almost unused due to a narrow technological gap between the oligomer melting temperature and the initial curing point. Since the 1990s, most industrial thermoreactive oligoimides have been synthesized with the use of phenylethynylphthalic anhydride to improve the serviceability of thermoreactive oligoimides (Fig. 6).
In the foreign literature, these compositions have received the common name PETI (phenylethynyl terminated oligoimides). PETI oligomers are synthesized by a two-stage method: at the first stage, the corresponding oligoamido acid is converted into N-MP, and the forepolymer is thereafter imidized in the presence of acetic anhydride and triethylamine or thermal polycondensation with azeotropic distillation of low-molecular-weight products is performed. To decrease the melting temperature of oligoimides, PETI compositions are developed by using monomers of asymmetric structure, monomers containing “hinge” groups, and mixtures of monomers to decrease main chain regularity.
One of the best-studied PETI adhesives is FM-5 (LARC, NASA, United States), which consists in PETI-5 fiberglass reinforced binder. The strength given in [13] for FM-5 glued joints is 58.3, 52.4, and 38.3 MPa at temperatures of –54, +25, and 177°C, respectively. Hence, FM-5 glue has a relatively low maximum temperature of operation. For this reason, triamines are frequently introduced into the main chain of an oligomer (PETI glue of trademark MPEI) to increase the operational temperature of PETI compositions [14] and a small amount of oligoimides (up to 20%) with a low molecular mass is added to improve the serviceability of a glue (to decrease the viscosity of a melt), as with trademark LV-121 [15]. Thus, the vitrification point of MPEI under curing at 370°C for 1 h is 300°C versus 265°C for PETI-5.
A rather widely used approach to increasing the vitrification temperature of the cured matric of PETI compositions is the use of monomers providing an increased chain rigidity. Thus, the PETI-330 cured composition based on m-phenyllenediamine and dianhydride of 2,3,3',4'-biphenyltetracarboxylic acid is characterized by a vitrification temperature of 330°C.
The properties of the polyimide binder of trademark MHT-R (Nexam Chemical) are considered in [16]. A specific feature of this RETI binder is that its ethynyl groups are located not only at the ends of oligomers, but also in the main chain. This is attained by using difunctional monomers containing an ethynyl group (Fig. 7). Due to this fact, a cured polymer matrix of MHT-R has a vitrification temperature of 420°C. Thus, it seems probable that some trademarks of glues based on this binder will appear in the near future.
REFERENCES
D. S. Lavrishchev and Yu. A. Mikhailin, “Polyimide adhesives,” Klei. Germetiki. Tekhnol., No. 8, 13–18 (2006).
M. I. Valueva, I. V. Zelenina, M. A. Zharinov, and K. R. Akhmadieva, “World market of high-temperature polyimide carbon plastics (a review),” Tr. Vseross. Inst. Aviats. Mater., No. 12, (2019). https://doi.org/10.18577/2307-6046-2019-0-12-67-79
C. E. Sroog, “History of the invention and development of the polyimides,” in Polyimides Fundamentals and Applications, Ed. by M. K. Ghosh and K. L. Mittal (CRC Press, Boca Raton, FL, 1996).
A. W. Chow, et al., “Structure-property relations in processing high-performance polyisoimide-imide resins,” J. Rheol. 36 (8), 1651–1668 (1992).
K. P. Subrahmanian, “High-temperature polymers and adhesives,” in Structural Adhesives: Chemistry and Technology, Ed. by S. R. Hartshorn (Springer, Boston, MA, 1986).
P. S. Blatz, “NR-150 polyimide precursor adhesive solution developed,” Adhes. Age 21 (9), 39–447 (1978).
https://www.solvay.com/en/product/fm-680.
G. Rabilloud, “Heat-resistant adhesives,” in Handbook of Adhesives and Surface Preparation: Technology, Applications and Manufacturing (Elsevier, Amsterdam, 2011).
E. N. Kablov, M. A. Zharinov, K. R. Akhmadieva, and R. R. Mukhametov, RF Patent No. 2666734 (2018).
F. P. Darmory, “Nolimid A 380 extreme high-temperature polyimide adhesive,” in Proceedings of the 5th National SAMPE Technical Conf. “Materials and Processes for the 70’s: Cost Effectiveness and Reliability” (New York, 1973), pp. 415–422.
S. Ebnesajjad, Handbook of Adhesives and Surface Preparation: Technology, Applications and Manufacturing (Elsevier, Amsterdam, 2011).
https://www.renegadematerials.com/products/adhesives.
H. Parvatareddy, PhD Thesis (Virginia Polytechnic Inst., Blacksburg, VA, 1997).
A. C. Chang and B. J. Jensen, “Adhesive properties of cured phenylethynyl-containing imides,” J. Adhes. 72 (2), 209–217 (2000).
R. J. Cano and B. J. Jensen, “Effect of molecular weight on processing and adhesive properties of the phenylethynyl-terminated polyimide LARC™–PETI-5,” J. Adhes. 60 (1–4), 113–123 (1997).
P. Fernberg, et al., “Development of novel high Tg polyimide-based composites. Part I: RTM processing properties,” J. Compos. Mater. 52 (2), 253–260 (2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Zharinov, M.A., Petrova, A.P., Babchuk, I.V. et al. Thermally Stable Polyimide Structural Adhesives. Polym. Sci. Ser. D 15, 143–149 (2022). https://doi.org/10.1134/S1995421222020332
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995421222020332