Abstract
The spatial distribution of zooplankton communities in part of the Volga River (the Cheboksary and Kuibyshev reservoirs), including areas with different hydrological conditions, has been analyzed. The zooplankton composition and species structure gradually changes along the river profile. Littoral communities from different parts are more similar between themselves than with pelagic ones. In the river mouth zone of the tributary, the ecotone effect is manifested as fauna mixing, but not as an increase in species richness. Variations in the community structure are mostly determined by depth and water temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Reservoirs combine a set of conditions typical for different types of reservoirs and represent a complex quasi-natural system. In this regard, it is important to study the patterns of distribution of communities in an extremely heterogeneous environment of reservoirs and identify the factors that determine it. The issue of discreteness and continuity, as well as the size of discrete plankton spots in the reservoir, was discussed in the study of Shurganova et al. (2014). However, the spatial organization of discrete communities under conditions of the simultaneous influence of various factors requires additional study.
Changes in the species structure of zooplankton communities in the Cheboksary reservoir have been studied since it was filled (Shurganova and Cherepennikov, 2010). The features of its formation at the present stage of development are presented in the study (Shurganova et al., 2014). In the Volga reach of the Kuibyshev reservoir, the trophic structure of zooplankton was studied in open shallow waters overgrown with macrophytes (Borisovich and Yakovlev, 2011). The zooplankton of the entire part of Volga River from the lower Cheboksary reservoir to the Volga reach of the Kuibyshev reservoir was studied without a detailed description of distribution by Lazareva et al. (2012, 2018).
The aim of this study was to detect the patterns of spatial distribution (along the longitudinal, transverse, and vertical profiles of reservoirs) of zooplankton communities in the areas of the Cheboksary and Kuibyshev reservoirs, characterized by different flow rates and influenced by their tributaries.
MATERIALS AND METHODS
Observations were carried out at the Cheboksary (August–November 2011) and Kuibyshev (August 2011) reservoirs and their tributaries (August 2008–2013) (Fig. 1). Samples were taken with a 10-L planktobatometer and an Apstein net with a mesh size of 120 µm and filtration with 50–100 L of water. The collected material was fixed with 4% formalin. The abundance and biomass of each species of crustaceans and rotifers were determined. At the sampling sites of zooplankton, the water temperature was measured using a thermometer built into a planktobatometer or a Kit 5E thermooximeter.
In the Cheboksary reservoir, the material was collected in the littoral zone below the river mouth of Parat River (Ch1) and in the shallow water of the left bank of Volga River (1–4 km downstream of Cheboksary) (Ch2), as well as in the pelagic zone of the same area from the surface and at the bottom at a depth of 10–12 m (Ch3_1 and Ch3_2). Two sites were investigated in the Kuibyshev reservoir. In the upper river flowing part of the Volga reach with a preserved bed of Volga River, samples were taken 10 km below the Cheboksary HPP (K1_1 and K1_2), 1 km above the river mouth of the Tsivil River (from the surface and at the bottom at a depth of 5 m) (K2), in the area of the confluence of Tsivil River (from the surface and at the bottom at a depth of 3 m) (K3_1 and K3_2), and opposite the town of Mariinsky Posad (from the surface) (K4). On the site of the Volga reach from the river mouth of the Sviyaga River up to Kazan, the surface layer of 0–1 m was examined at 11 stations in the pelagic zone (K5, 7, 8, 10, 12–14, and 16–19) and at 4 stations in the littoral zone (K6, 9, 11, 15). In addition, the material was collected in the estuarine zones of the Parat (left tributary of the lower part of the Cheboksary Reservoir) (Ch_P), Ilet (K_I), and Tsivil (K_Z) rivers (the left and right tributaries of the Volga reach of the Kuibyshev Reservoir, respectively).
The dam site of the Cheboksary reservoir and the Volga reach below the river mouth of Sviyaga River of the Kuibyshev reservoir was considered a low-flow site; the upper river site of the Volga reach was considered a flowing site (Table 1). The influence of reservoir waters on the fauna of the tributary was studied using the example of Tsivil River (length 197 km) from the source to the river mouth. Material was collected in the lower reaches of the river in three areas in more detail: above the zone of contact between the waters of the river and the reservoir (conventionally designated as “downstream”); in the zone of contact (mixing) of waters, where constant countercurrents are observed; and at the river mouth.
During the observation period, the water temperature in the Cheboksary reservoir was higher than in the Kuibyshev reservoir (Table 1) and exceeded that in the same period of 2008 (which had typical thermal conditions), but did not reach the values of the extremely hot 2010 (Lazareva et al., 2012).
To determine the similarity of the communities of the surveyed areas based on data on the abundance of individual species, as well as an assessment of the influence of factors (temperature, depth, and location in the water area) on the composition of the fauna of planktonic crustaceans, we used the canonical correlation analysis method (CCA) using Canoco for Windows 4.5 (Ter Braak, 1988). The data included in the analysis were previously normalized by a logarithm. The statistical significance of the relationships was assessed by the Monte Carlo permutation test with 4999 permutations.
The significance of a particular species in the community (dominance) was assessed based on data regarding its abundance using the rank distribution function (Fedorov et al., 1977), as well as the indicator of its occurrence (Pesenko, 1982). Ecological groups of organisms according to the modes of locomotion and capture of food were distinguished according to the classification of Chuikov (1981). The species forming the basis of the biomass (>5% of the total biomass) were listed for characterizing the groups.
RESULTS
Fauna composition and occurrence of common species. In the Cheboksary reservoir during the studied period, 47 species of zooplankters were identified: 19 Rotifera, 22 Cladocera, and 6 Copepoda; there were 36 species in the Kuibyshev: 18 Rotifera, 12 Cladocera, and 6 Copepoda. In August, seven species of invertebrates were recorded simultaneously, but with different occurrences, in the zooplankton communities of all three studied areas. Rotifers verticators Keratella cochlearis (Gosse, 1851) prevailed in regards to frequency of occurrence in the low flow sites of both reservoirs and Euchlanis lucksiana Hauer, 1930 prevailed in the Cheboksary Reservoir. Primary filter feeder crustaceans Bosmina longirostris (O.F. Müller, 1776), as well as active catchers Mesocyclops leuckarti (Claus, 1857), dominated the weak-flowing areas of both reservoirs; Daphnia galeata Sars, 1863 dominated in the low flow sites of the Kuibyshev reservoir. The occurrence in >50% of samples throughout the entire investigated area of Volga River was recorded for the Euchlanis dilatata Ehrenberg, 1832, and the secondary filter feeder Chydorus sphaericus (O.F. Müller, 1776).
Spatial distribution along the longitudinal profile. The occurrence of Bosmina longirostris, Keratella cochlearis, Testudinella patina (Herman, 1783), and Trichocerca pusilla (Lauterborn, 1903) was 2–3 times higher in the low flow sites of both reservoirs (near the dam in the Cheboksary reservoir and the Volga reach downstream of the Sviyaga River in the Kuibyshev reservoir). Crustaceans Mesocyclops leuckarti, Diacyclops crassicaudis (Sars, 1863), Daphnia galeata, and Alona quadrangularis (O.F. Müller, 1776) were confined to the river flow site of the Kuibyshev reservoir; e.g., they were revealed only here, or their occurrence was 3–12 times higher than in other areas. Rotifers of the pond complex, indicators of increased saprobity of waters Brachionus diversicornis (Daday, 1883); B. angularis Gosse, 1851 (occurrence of both in ~50% of samples); B. calyciflorus Pallas, 1776; and Synchaeta pectinata Ehrenberg, 1832 (occurrence of both <10% samples) were recorded only in the Volga reach of the Kuibyshev Reservoir.
In the pelagic zone of the dam site of the Cheboksary Reservoir in August, the biomass was dominated by swimming primary filter feeders Daphnia galeata at the water surface and Limnosida frontosa Sars, 1862 in the deep layer (43 and 89% of the community biomass, respectively), creeping–swimming secondary filter feeders Chydorus sphaericus (4–30%), and swimming–creeping verticators Euchlanis lucksiana (5–12%). Downstream in the river site of the Kuibyshev Reservoir, primary filter feeders Daphnia galeata (30–92%) and secondary filter feeders Chydorus sphaericus (6–69%) dominated everywhere. Below the river mouth of the Sviyaga River, swimming primary filter feeders Bosmina longirostris; B. cf. crassicornis Lilljeborg, 1887; and verticators Keratella cochlearis (9–31, 35–46, and 1–13%, respectively) were revealed.
The zooplankton of the pelagic zone of the near-dam part of the Cheboksary and river sites of the Kuibyshev reservoirs was represented by larger specimens (average W 3.36 ± 1.87 and 4.33 ± 1.91 mg × 10–3, respectively) when compared with those in the Volga reach located downstream (W 0.75 ± 0.13 mg × 10–3). This was due to the massive development of Daphnia.
In August, unique species constituted a significant fraction of the fauna (39–50%) at each of the three surveyed sites. However, the statistical method used in the study did not allow identifying the communities of these sites of the Volga River as being reliably isolated. Transitional communities were observed which were equally similar to those in both adjacent sites of the river. The result of the canonical analysis is shown graphically in Fig. 2, where the distance between the points was determined by the degree of community similarity.
Ordination of zooplankton communities: (a) dam part of the Cheboksary reservoir (○) and river flowing site of the Kuibyshev reservoir ( ) and the Volga reach of the Kuibyshev Reservoir (∆), as well as the tributaries of the reservoirs (×); (b) the same, without the Volga reach of the Kuibyshev Reservoir. See text for letter designations.
) and the Volga reach of the Kuibyshev Reservoir (∆), as well as the tributaries of the reservoirs (×); (b) the same, without the Volga reach of the Kuibyshev Reservoir. See text for letter designations.
Cenoses of the shallow coastal area below the confluence of Parat River in the Cheboksary Reservoir were clearly distinguished (Ch 1, Fig. 2b), as well as cenoses of the river mouth areas of the tributaries of the Cheboksary (Parat River, Ch_P) and Kuibyshev reservoir (Ilet River, K_I; Tsivil River, K_Z), where the mixing of river fauna with that of the reservoir fauna was observed (Fig. 2b). This indicated the presence of factors contributing to the differentiation of communities along the transverse profile of Volga River (see below).
Spatial distribution along the transverse profile. In August, the pelagial of the near-dam part of the Cheboksary Reservoir was inhabited by somewhat larger zooplankton specimens (W 3.36 ± 1.87 mg × 10–3) than the coastal part (W 2.58 mg × 10–3). This tendency in the distribution of zooplankton was observed not only in summer, but also in autumn before freeze-up (W 1.78 ± 0.31 and 0.56 mg × 10–3 respectively). In the coastal area of the Cheboksary Reservoir, secondary filter feeders Chydorus sphaericus, Disparalona rostrata (Koch, 1841), Eurycercus lamellatus (O.F. Müller, 1776), and others (in total 34% of the community biomass) were more abundant in comparison with the pelagic zone. In November, verticator rotifers (Keratella quadrata (O.F. Müller, 1786) and Kellicottia longispina (Kellicott, 1879)), as well as primary (Bosmina longirostris) and secondary (Acroperus angustatus Sars, 1863) filter feeders of cladocerans (26, 22, and 5%, respectively), prevailed in the littoral. The proportion of juvenile copepods at this time reached 17% of the plankton biomass in the littoral zone and >90% in the pelagic zone.
The pelagic zone on the river flow site and in the Volga reach of the Kuibyshev Reservoir was inhabited by smaller specimens (W 4.33 ± 1.91 and 0.75 ± 0.13 mg × 10–3, respectively) than the coastal area (W 8.40 and 2.05 ± 1.14 mg × 10–3). In the Volga reach, this was due to the abundance of rotifers (26 ± 4% of the total biomass) in the pelagic zone of the reservoir; in the littoral zone, they formed only 5 ± 2% of the biomass. Large crustaceans filter feeders Daphnia galeata and Eurytemora velox (Lilljeborg, 1853), preferring less dynamic hydrological conditions, developed in the littoral zone of the river flow area.
In August, the zooplankton of the littoral part of the reservoirs (points l, Figs. 3, 4) was more similar to the invertebrate community in the deep layer of the pelagic zone (points b, Figs. 3, 4) and, in November, to what develops on the surface (points s, Figs. 3, 4). The littoral communities from different areas were more similar to each other than to adjacent pelagic ones (Fig. 5). They develop at slightly higher temperatures (20.9 ± 0.5°C) than in the open part of the reservoir (19.4 ± 0.8°C). In this case, the effect of temperature conditions on the composition of communities was insignificant (p > 0.05).
Some aspects of the distribution of zooplankton in the estuarine zones of tributaries. The influence of the Kuibyshev Reservoir on the hydrological regime of Tsivil River was traced at a distance of 22.5 ± 4 km from the river mouth. However, taxa characteristic of the reservoir were found upstream of the river mouth of the Tsivil River only ~4 km in the zone where countercurrents from the side of the reservoir were constantly observed. Here, in summer, the Secchi disk transparency increased (up to 0.45–0.55 m and, in the lower reaches, in general, up to 0.25–0.45 m). Moreover, even in this area, the spatial heterogeneity of zooplankton was noted. This allowed distinguishing the actual river mouth and the marginal zone of mixing of river waters with the waters of the reservoir (the zone of water contact), above which the influence of the fauna of the reservoir has not been traced. In the contact zone, >60% of the abundance and biomass of the community was represented by the rotifer Brachionus calyciflorus. For comparison, in the lower reaches of the river, its abundance was 3.5–7.0% of the total. This species was found mainly in the lower reaches of the Tsivil River (67% of the samples); in the middle reaches its occurrence reached 13%. In addition, in the lower reaches of the river, other representatives of the genus were relatively numerous: B. quadridentatus (13–19% of the abundance) and B. bennini Leissling, 1924 (1–17% of the abundance) (Podshivalina, 2013). They were not found in the zone of water contact.
In the river mouth of the Tsivil River, the representation of typical river forms was relatively low (Fig. 6). Changing hydrological conditions caused by the regime of filling and discharging water from the reservoir probably do not contribute to an increase in the abundance of river representatives of zooplankton entering the area; therefore, mainly relatively large crustaceans (Fig. 6) typical of the reservoir developed at the river mouth. Among them are cladocerans Limnosida frontosa; Leptodora kindtii (Focke, 1844); Daphnia cucullata Sars, 1862; and D. galeata and copepods Eurytemora velox and Megacyclops viridis (Jurine, 1820). Thus, a mixing of the faunas of the tributary and the reservoir was recorded in the river mouth, but there was no increase in the species richness of zooplankton (Fig. 6), which is considered typical for the ecotone of this species (Gidroekologiya …, 2015). This situation was probably due to the pronounced variability of hydrological conditions (drop in water level by 1–2 m during the day and reverse water flow), which is unfavorable for the development of rotifers and small cladocerans.
Simultaneously with the distribution of species from the reservoir to its tributaries, the influence of river waters on the structure of the reservoir zooplankton was revealed, probably due to changes in its habitat conditions. In the upper part of the river site of the Kuibyshev Reservoir (Fig. 3, point 1) rotifers of the genus Euchlanis were abundance-dominant and cladocerans Daphnia galeata were biomass-dominant. In the pelagial of the reservoir opposite the river mouth of Tsivil River (Fig. 3, point 3), the basis of the community was represented by Chydorus sphaericus (more than half of the abundance and biomass). Large crustaceans were noted in the river mouth zone of Tsivil River (Fig. 3, point Z), but in the reservoir itself they were only in the littoral zone above the river mouth of the tributary (Fig. 3, point l). Rotifers of the genus Brachionus typical for the lower reaches of the river have not been found in the river site of the Kuibyshev reservoir. The structure of zooplankton in the reservoir acquired features close to the original (Fig. 3, point 1) 15 km downstream of Volga River (Fig. 3, point 4). Thus, in the reservoir below the river mouth of the tributary (Fig. 3, point 3), the dispersion of the river community in the Volga community was observed.
Features of the vertical distribution of communities. According to the results of canonical analysis, the composition of zooplankton in the deepwater zone of the Cheboksary reservoir with limnic conditions was different at different depths (Figs. 3, 4) (F = 1.77; р = 0.04). The communities of the surface layer of water in different areas were more similar to each other than to those in the bottom horizon. At a separate point, the zooplankton of the surface and deep layers was closest in August (Fig. 4); the trophic structure of the community did not differ vertically. In autumn, the differences were more noticeable; with a similar species composition, they were manifested as the features of the trophic structure. Thus, in September, in the Cheboksary Reservoir, cladocerans, primary filter feeders, were more abundant in the bottom layer (63–71% of the total biomass) than in the surface layer (25–51%). In the group of active-catcher copepods, the vertical distribution was reversed: 0–5% at the bottom and 25–42% at the surface. The same distribution was also observed for copepods at juvenile stages of development (4–6% at the bottom and 28–23% at the surface). In September, verticator rotifers preferred greater depths (21–30% of the total biomass); in the surface layer they were significantly less abundant (2–4%).
In the flowing river site of the Kuibyshev Reservoir, no significant (р < 0.05) dependence of the composition of zooplankton on depth was revealed. This was probably due to the vertical uniformity of the distribution of zooplankters due to flowage. Waters of a large tributary (Tsivil River) also probably contributed to the formation of the homogeneous vertical structure of the community.
Features of the distribution of zooplankton in different seasons. In August, in the pelagic zone of the dam site of the Cheboksary reservoir, large primary filter feeders Daphnia galeata (at the surface 89%) and Limnosida frontosa (in the deep layer 41%) were biomass-dominant, and the secondary filter feeder Chydorus sphaericus (17–28% at the surface) and verticator rotifer Euchlanis lucksiana (24–35%) were abundance-dominant.
In September, the dominant complex of species included the smaller primary filter feeder Bosmina longirostris (29–48% in terms of abundance and 25–71% in terms of biomass) and predatory and omnivorous catchers represented by adult and juvenile Mesocyclops leuckarti (40% in terms of biomass) and Thermocyclops oithonoides (Sars) (26%), as well as the creeping–swimming verticator Brachionus quadridentatus (11–45% in terms of abundance). Before the freeze-up, the plankton contained mainly cyclops at different juvenile stages of development.
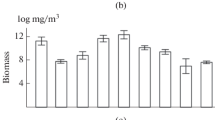
Among the environmental factors changing during the season, the water temperature influenced the composition of zooplankton; according to canonical analysis, it determined 39% of the differences in the composition of communities. For the Cheboksary reservoir, this effect was significant (F = 1.67, р = 0.002). With a decrease in water temperature by autumn, the average W of specimens decreased (Fig. 7) and the contribution to the community of small filter feeders and juveniles of Cyclopoida, characterized by a mixed diet, increased. This reflected the course of the seasonal succession of zooplankton.
DISCUSSION
In the Cheboksary reservoir, the left-bank riverine, right-bank riverine, and transitional and lacustrine zooplanktocenoses were distinguished (Shurganova et al., 2014). The zooplankton composition of the investigated dam part of this reservoir is similar to the described “lake” community (Shurganova et al., 2014). It is characterized by the presence of species common with the parts of the Volga reach of the Kuibyshev reservoir located downstream. There are probably close interrelationships between the communities of the two reservoirs; no signs that would clearly distinguish them from each other have been identified. Flow variations are one of the determining factors in the formation of communities along the longitudinal profile of the investigated site of the Volga River. Changes in the structure of zooplankton were noted: in both reservoirs, in areas with a slow current, swimming and swimming–creeping rotifers–verticators were more diverse and abundant; in relatively more flowing areas, primary and secondary crustacean filter feeders were more abundant. This is typical for lotic objects of various scales (Krylov, 2005; Lazareva, 2010; Chertoprud, 2011).
Border (ecotone) communities are located along the perimeter of reservoirs at the estuaries of the tributaries and littoral. They border on other ecosystems, influenced by a combination of diverse factors and are distinguished by a noticeable originality. Zooplankton in the estuarine zones of the tributaries and in the littoral of both reservoirs is characterized by a significant isolation from the pelagic, which determines the differentiation of communities along the transverse profile. In the littoral, like in the pelagic, there is a gradual change of communities along the longitudinal profile of the Volga River. Thus, parallel continuums are formed in the coastal and pelagic zones. This may be due to drift processes characteristic of lotic systems with the transfer of matter along the longitudinal profile (Bogatov, 2013).
The similarity between pelagic and littoral communities is less than between those located along the Volga River and is determined by the flow rate. In the flowing areas, the differences in the communities in the transverse profile are more pronounced. The established picture of the distribution of communities in the investigated site of the Volga River is close to the combined concept of the organization of river ecosystems based on an expanded concept of the river continuum (Bogatov, 2013; Baturina, 2019). The entire community of the watercourse participates in the formation of the river continuum, while the interaction between the separate zones is carried out through the system of interaction of the following local communities confined to specific heterogeneous biotopes (Bogatov, 2013).
It is difficult to clearly define the boundaries of the reservoir communities at the estuaries of the tributaries. These are transitional zones between ecosystems of rivers and reservoirs, which was noted earlier (Krylov, 2005; Gidroekologiya …, 2015). Moreover, the length of these zones is determined by the hydrological regime and can be quite large. Rotifer–verticator Brachionus calyciflorus can serve as indicator of the transition zone at the river mouth of the Tsivil River. Its abundance is significantly higher in the zone of contact (mixing) of waters in comparison with neighboring communities. This can be a manifestation of the action of r-selection under conditions of an unstable environment (Okhapkin and Yulova, 1993).
Along with the species typical for the studied region, an inhabitant of subtropical and tropical latitudes, Keratella tropica (Apstein, 1907) (Kutikova, 1970), as well as the thermophilic Brachionus budapestinensis Daday, 1885, was reordered in the mouth of the Tsivil River. Cladocera Diaphanosoma orghidani Negrea, 1982 was recorded in the river mouth areas of the Ilet and Tsivil rivers and at sites of their tributaries in the backwater of the Kuibyshev reservoir. The northern boundary of the distribution of this species, previously rather rare, runs at ~57° N, (Korovchinsky, 2004). Currently it is distributed along the whole Volga River (Lazareva, 2012; Lazareva et al., 2018). We did not find D. orghidani in the reservoirs, but it is known for the Cheboksary reservoir (Lazareva, 2012; Shurganova et al., 2014). Using the Rybinsk reservoir as an example, it has been shown (Lazareva, 2012) that D. orghidani is concentrated in the most flowing estuarine areas of small tributary rivers. As was noted earlier (Lazareva, 2008), the distribution of southern species to the north was due to the creation of reservoirs, and invaders initially colonize the river mouths of tributaries (Lazareva, 2010).
The influence of the tributaries themselves on the fauna of the reservoir can be traced in a slightly different aspect. The waters of the tributary do not affect the composition, but mainly affect the structure of the community of planktonic invertebrates in the reservoir. This impact consists in changing the conditions of their habitat. First, the physicochemical conditions change: flow rate, the salt composition of the waters, and the content of nutrients (Muraveyskiy, 1960). In the reservoirs of the Volga River, it contributes to the mass development of copepods that actively capture food (up to 24 ± 1% of biomass) and secondary filter feeders from cladocerans (up to 53 ± 11%) with a decrease in the proportion of primary filter feeders to 17 ± 12%.
The depth and temperature of the water equally determine the main part (78%) of variations in the composition of zooplankton, each of the factors explaining 39% of the variations. In each water horizon of the Volga River, the temperature conditions and the nutritional content (phyto-, bacterioplankton and detritus) are relatively uniform (Korneva, 2015). This determines the greater similarity of the structure of communities in one horizon in comparison with those in different vertical layers of water.
Seasonal changes in the structure of zooplankton in the late summer–autumn period are due to the completion of the life cycles of large summer thermophilic species and their replacement by small species that are less demanding on alimental conditions. The changes we revealed in the structure of zooplankton in the studied area of the Volga River are similar to those described for the zooplankton of the open littoral zone of the Kuibyshev reservoir (Borisovich and Yakovlev, 2011) and the pelagic zone of the Rybinsk reservoir (Upper Volga) (Lazareva, 2010).
CONCLUSIONS
The results of an analysis of the distribution of zooplankton along the longitudinal profile of the investigated site of the Volga River, which includes zones with different hydrological regimes, indicated a gradual change in the composition and species structure of the community, which corresponds to the expanded model of the continuum as a sequential unidirectional accumulation of differences from the upper areas to areas located downstream. Communities of different parts of the littoral zone were more similar to each other than to neighboring pelagic communities. In the river mouth zone of a small tributary river of the Kuibyshev reservoir, a mixing of faunas was revealed; however, an increased level of species richness of planktonic invertebrates typical for the ecotone was not detected. This was probably due to a hydrological regime unfavorable for a number of species. The depth and temperature of the water to the greatest extent affect the change in the structure of communities along the vertical. At the end of the growing season, with a decrease in water temperature, a decrease in the average W of zooplankton specimens was observed.
REFERENCES
Baturina, N.S., Functional structure of river ecosystems: retrospective of the development of contemporary concepts (review), Inland Water Biol., 2019, vol. 12, no. 1, p. 1. https://doi.org/10.1134/S1995082919010048
Bogatov, V.V., Ekologiya rechnykh soobshchestv Rossiiskogo Dal’nego Vostoka (Ecology of River Communities in the Russian Far East), Vladivostok: Dal’nauka, 1994.
Bogatov, V.V., Patterns of the functioning of river ecosystems in the light of basic scientific concepts, Vestn. Sev.-Vost. Tsentra Dal’nevost. Otd. Ross. Akad. Nauk, Gidrobiolog., 2013, no. 4, p. 90.
Borisovich, M.G. and Yakovlev, V.A., Trophic structure of zooplankton of different types of shallow waters of the Volga and Volga–Kama reaches of the Kuibyshev reservoir, Uch. Zap. Kazan. Univ., Ser. Estestv. Nauki, 2011, vol. 153, p. 214.
Chertoprud, M.V., Diversity and classification of rheophilic communities of macrobenthos in the middle zone of European Russia, Zh. Obshch. Biol., 2011, vol. 72, no. 1, p. 51.
Chuikov, Yu.S., Methods for ecological analysis of the composition and structure of aquatic animal communities. Ecological classification of invertebrates found in freshwater plankton, Ekologiya, 1981, no. 3, p. 71.
Edel’shtein, K.K., Vodokhranilishcha Rossii: ekologicheskie problemy i puti ikh resheniya (Reservoirs in Russia: Environmental Problems and Ways to Solve Them), Moscow: GEOS, 1998.
Fedorov, E.D., Kondrin, E.K., and Levich, A.P., Rank distribution of phytoplankton in the White Sea, Dokl. Akad. Nauk SSSR, 1977, vol. 236, no. 1, p. 264.
Gidroekologiya ust’evykh oblastei pritokov ravninnogo vodokhranilishcha (Hydroecology of Estuarine Areas of Tributaries of a Lowland Reservoir), Yaroslavl: Filigran, 2015.
Korovchinskii, N.M., Vetvistousye rakoobraznye otryada Ctenopoda mirovoi fauny (morfologiya, sistematika, ekologiya, zoogeografiya) (Cladocerans of the Order Ctenopoda of the World Fauna (Morphology, Taxonomy, Ecology, and Zoogeography)), Moscow: KMK, 2004.
Korneva, L.G., Fitoplankton vodokhranilishch basseina Volgi (Phytoplankton of Reservoirs in the Volga River Basin), Kostroma: Kostr. Pechatn. Dom, 2015.
Krylov, A.V., Zooplankton ravninnykh malykh rek (Zooplankton of Small Lowland Rivers), Moscow: Nauka, 2005.
Kuibyshevskoe vodokhranilishche (nauchno-informatsionnyi spravochnik) (Kuibyshev Reservoir (Scientific and Informational Reference Book)), Tolyatti: Inst. Ekol. Volzhsk. Basseina Ross. Akad. Nauk, 2008.
Kutikova, L.A., Kolovratki fauny SSSR (Rotifers of the Fauna of the USSR), Leningrad: Nauka, 1970.
Lazareva, V.I., Distribution and special traits of naturalization of new and rare zooplankton species in waterbodies of the Upper Volga Basin, Inland Water Biol., 2008, vol. 1, no. 1, p. 76. https://doi.org/10.1007/s12212-008-1012-3
Lazareva, V.I., Struktura i dinamika zooplanktona Rybinskogo vodokhranilishcha (The Structure and Dynamics of Zooplankton of the Rybinsk Reservoir), Moscow: KMK, 2010.
Lazareva, V.I., The distribution of species of the genus Diaphanosoma (Ctustacea, Ckadocera) of the reservoirs of the Volga and Sheksna rivers: impact of environmental factors, Inland Water Biol., 2012, vol. 5, no. 3, p. 257. https://doi.org/10.1134/S199508291203008X
Lazareva, V.I., Mineeva, N.M., and Zhdanova, S.M., Spatial distribution of plankton in the reservoirs of the Upper and Middle Volga in years with different thermal conditions, Povolzhsk. Ekol. Zh., 2012, no. 4, p. 394.
Lazareva, V.I., Sabitova, R.Z., and Sokolova, E.A., Features of the structure and distribution of late summer (August) zooplankton in the Volga reservoirs, Tr. Inst. Biol. Vnutr. Vod Ross. Akad. Nauk, 2018, no. 82 (85), p. 28.
Litvinov, A.S. and Zakonnova, A.V., Water balance, water exchange, and level regime of the Cheboksary Reservoir in the first years of filling, Vodn. Resur., 1986, no. 3, p. 69.
Muraveyskiy, S.D., Zooplankton of the Kerzhenets River, in Reki i ozera (Rivers and Lakes), Moscow: Gos. Izd. Geogr. Lit., 1960, p. 308.
Okhapkin, A.G. and Yulova, G.A., Analysis of dynamic interactions between the reservoir and the eutrophied inflow according to the indicators of the species structure of phytoplankton, in Ekologicheskie problemy basseinov krupnykh rek (Environmental Problems of Large River Basins), Tolyatti: Inst. Ekol. Volzhsk. Basseina Ross. Akad. Nauk, 1993, p. 112.
Pesenko, Yu.A., Printsipy i metody kolichestvennogo analiza v faunisticheskikh issledovaniyakh (Principles and Methods of Quantitative Analysis in Faunal Research), Moscow: Nauka, 1982.
Podshivalina, V.N., Distribution of zooplankton along the longitudinal profile of a small river under conditions of high anthropogenic load (as exemplified by the Tsivil River, Middle Volga region), Izv. Samar. Nauchn. Tsentra Ross. Akad. Nauk, 2013, vol. 15, no. 1 (3), p. 503.
Shurganova, G.V. and Cherepennikov, V.V., Dynamics of the species structure of zooplanktocenoses of two Volga reservoirs in the process of their formation and development, Zh. Sib. Fed. Univ., Ser. Biol., 2010, vol. 3, no. 3, p. 267.
Shurganova, G.V., Cherepennikov, V.V., Kudrin, I.A., and Il’in, M.Yu., Characteristics of the current state of the species structure and spatial distribution of zooplankton communities of the Cheboksary reservoir, Povolzh. Ekol. Zh., 2014, no. 3, p. 417.
Ter BraakC.J.F., CANOCO—A FORTRAN Program for Canonical Community Ordination, New York: Power Microcomputer, 1988.
ACKNOWLEDGMENTS
I thank V.A. Yakovlev, E.V. Osmelkin, L.V. Egorov, S.S. Maksimov, and the staff of the Chuvash Center for Hydrometeorology and Environmental Monitoring for their assistance in collecting material; A.V. Krylov and N.G. Sheveleva for our discussion of the results; and an anonymous referee for valuable advice and comments.
Funding
This study was carried out with partial financial support from the Russian Foundation for Basic Research and the Government of the Chuvash Republic, project no. 13-04-97158.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by V. Mittova
Abbreviations: W, individual weight.
Rights and permissions
About this article
Cite this article
Podshivalina, V.N. Zooplankton Distribution in the Middle Volga River Reservoirs in Areas Under the Influence of Tributaries. Inland Water Biol 14, 546–554 (2021). https://doi.org/10.1134/S1995082921050114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082921050114








 ). Numerical designations are ordinal numbers of stations with distance from the headwaters.
). Numerical designations are ordinal numbers of stations with distance from the headwaters.
