Abstract
The operation of nuclear power plants routinely leads to the release of waste water flows contaminated with radioactive isotopes, tritium in particular. In order to prevent its entry into the environment, it is proposed to use a catalytic process of chemical isotope exchange in the water–hydrogen system. The novelty of the work lies in the combination of energy production facilities and commercial hydrogen into a single complex, which simultaneously allows the ecological problem of environmental protection to be solved. The proposal corresponds to the concept of atomic–hydrogen energy and is entirely based on the use of domestic developments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
During the operation of any nuclear power plant reactor, tritium is produced in the coolant that is released from nuclear power plants (NPP) in the form of gaseous emissions into the atmosphere or water discharged into the cooling pond. The largest amount of tritium is formed in the moderator of heavy water reactors of the CANDU type: its concentration in the moderator reaches 1.85 × 1012–2.59 × 1012 Bq/kg (50–70 Cu/kg), and in the coolant – 3.7 × 1011–5.55 × 1011 Bq/kg (10–15 Cu/kg) [1]. According to [2], in 2001–2005, heavy water reactors in Canada annually emitted about 4 g of tritium into the environment in the form of water vapor in the air and approximately the same amount in waste water, which corresponds to the release of tritium into the environment in the amount of 1500 TBq/year. Another 400 TBq/year was released into the environment in the form of molecular hydrogen as a result of the operation of the Tritium Recovery Facility for Detritiation of the heavy water moderator of the Canadian reactors at Darlington [3]. In order to reduce emissions of tritium into the environment, over the past 10–15 years, some other countries operating these reactors have been introducing facilities for reducing the tritium concentration in heavy water (D2O) [4, 5].
Discharges of tritium from other types of power reactors are significantly less. For example, for light water reactors of the PWR (pressurized water reactor)/WWER (water–water energy reactor) type, tritium release into the environment is more than an order of magnitude less [1]. Therefore, the operation of light water reactors of all types does not provide for the purification of discharges and emissions from tritium.
During the operation of such reactors, the radioactive hydrogen isotope is produced in the primary coolant. Due to the diffusion of tritium, which is formed as a product of the triple fission of uranium and plutonium isotopes (~4.1 TBq/day of tritium is formed per one GW of electric power [6]) through the fuel-element jacket, and in reactors with boron regulation, it is due to tritium emergence resulting from nuclear reaction 10В(n, 2α)3H.
As is known, WWER is the backbone of our country’s nuclear energy. Currently, the construction of generation 3+ WWER is underway, these are WWER TOI (typical optimized and informatized) (two power units at the Kursk NPP-2) and WWER-1200 (one power unit has been in operation since 2018, one power unit is under construction, and Leningrad NPP) [7]. An increase in the specific capacity of a power unit inevitably leads to a growing rate of accumulation and concentration of tritium in the primary coolant. Thus, according to the WWER TOI project, the annual discharge of tritium can reach the value of 3.3 × 1013 Bq/year. On the other hand, the regulatory documents [8, 9] stipulate that the tritium activity of the water discharged from NPPs should not exceed 105 Bq/L or 108 Bq/m3 and the design value of the volume of discharged water for the WWER TOI reactor is 2880 m3/year [10], which suggests that the annual tritium discharge should not exceed 2.9 × 1011 Bq/year; therefore, the above value is a serious violation of regulatory requirements.
Thus, the operation of generation 3+ nuclear reactors without effective technologies for purifying aqueous media from tritium will inevitably lead to a violation of the requirements of regulatory documents in the field of radiation safety. Tritium removal is required for unbalanced water resulting from the operation of the primary circuit water treatment system (SWP-6) and the water leakage treatment system (SWP-3), which are directly connected to the primary circuit. It should be noted that disregarding the tritium content, the distillate obtained after water treatment systems can meet the regulatory requirements for waste water, and if the water is purified from tritium with a total activity of 3.3 × 1013 Bq/year, the operation of generation 3+ reactors will proceed without violating the law of the Russian Federation. In addition, it should be taken into account that, since the standards for the tritium content in drinking water in the Russian Federation (7600 Bq/L) are significantly higher than in a number of foreign countries (for example, in the EU, they call for 100 Bq/L [11]), the lack of effective tritium purification systems for waste water at domestic NPPs may limit their export competitiveness. Therefore, the purpose of this work is to consider ways to reduce the discharge of tritium during the operation of nuclear power plants with generation 3+ light water reactors.
METHODS FOR TRITIATED WATER TREATMENT
The simplest method for handling low-level tritiated water flows is, unfortunately, quite widespread at present, and consists in their discharge into receiving water bodies. At the same time, almost all technogenic tritium ends up in the environment. Note that the criterion for classifying tritium as liquid radioactive waste in accordance with [10] is 106 Bq/kg, and up to a concentration of 107 Bq/kg yttrium is classified as low-level waste of class 5. According to [6], the tritium activity in WWER primary water with a total volume of about 300 tons at the end of the planned reactor operation period approaches 5 × 107 Bq/kg. In the presence of constant water exchange between water treatment systems and the presence of leaks (organized and unorganized), significant volume of the produced tritium will be released into unbalanced waters. Thus, these figures confirm the existing problem of tritium in the operation of nuclear power plants with light water reactors.
In order to reduce the tritium release into the cooling ponds at the Kalinin NPP, since 2007, a landfill for deep burial of hazardous industrial waste has been used for unbalanced waters with a concentration of less than 106 Bq/kg, [12–14]. This led to a decrease in the concentration of tritium in cooling ponds from 150–350 Bq/kg in 1996 to 40–45 Bq/kg in 2009 and 20–25 Bq/kg in 2012. However, this method cannot be used for all nuclear power plants in Russia, first, due to the problems of transporting tritiated water from the station to an underground disposal site, and second, due to the negative attitude of the public to this method of handling tritium-containing waste.
Another way to localize tritiated water is its adsorption on molecular sieves with subsequent disposal of solid radioactive waste. Taking into account the large volumes of water flows formed during the operation of nuclear power plants with a low concentration of tritium in them, there is no doubt that this method is inexpedient, both practically and economically.
When handling low-level tritium-containing aqueous media, it is of interest to concentrate and localize tritium in a small volume with simultaneous purification of the main flow to the intervention level. This can be done using hydrogen isotope separation methods. Since the late 1930s, these methods have been used to separate the protium–deuterium isotopic mixture in order to obtain D2O. Table 1 shows a list of hydrogen isotope separation methods used in different countries of the world, the main thermodynamic characteristics of each method (the magnitude of the single separation effect is the separation factor α), the conditions for its implementation (temperature and pressure), and the purpose of the method.
Water electrolysis was the first industrial method used to produce D2O: in the late 1930s and early 1940s, several tons of heavy water was obtained in Norway by this method [15], which after the end of World War II was exported to the United States and used by the developers of atomic weapons. However, this method is extremely energy intensive: it takes several hundred megawatt-hours of electricity to produce 1 kg of D2O.
The second method, chemical isotope exchange (ChIE) between water and hydrogen sulfide, has become the main method for large-scale production of D2O since the 1950s; in a number of countries (China, Iran, India, and Romania), it is still used today. About 90% of all heavy water in the world has been or is currently produced by this method. Its advantage consists in the implementation of a special two-temperature (TT) technological scheme of the separation plant, in which there are no energy-intensive units for the complete transformation of the exchanged substances into one another, and each stage of the plant includes two columns operating at significantly different temperatures, which ensures a change in the transfer direction of the target isotope between water and hydrogen sulfide. Note that the high corrosiveness and toxic properties of hydrogen sulfide forced the largest D2O producers in the USA and Canada to mothball or liquidate their production facilities.
As can be seen from Table 1, three of the presented methods are used to address the issue of detritiation. The technology based on low-temperature hydrogen distillation was first implemented in the USSR in the 1950s for the production of D2O [16]. In 1972, in Grenoble, France, at the Laue-Langevin Institute, an installation was launched for the purification of heavy water simultaneously from protium and tritium by this method in a research reactor [17]. Extraction of protium and tritium from heavy reactor water occurs in a three-stage cascade of apparatus for the catalytic isotopic exchange of its vapors with deuterium. The installation, with the column of the first stage having a diameter of 250 mm and a height of 11 m, provided the extraction of 24 g tritium from the heavy-water moderator per year with an electrical power consumption of about 800 kW.
The last two systems in Table 1 have advantages over hydrogen distillation in terms of the temperature of the process. At the same time, the process of water distillation seems to be the safest of all; considered, however, in terms of the separation factor, this technology is significantly inferior to the process of hydrogen distillation, and even more so to ChIE in the water–hydrogen system.
Table 2 shows an estimated comparison of the main indicators of separation plants using water distillation and ChIE technologies in the water–hydrogen system when solving the same problem of separating tritium from light water. The comparison is based on the general theory of isotope separation [15, 18].
The presented figures demonstrate the clear advantage of ChIE technology in the water–hydrogen system for the detritiation of light water, both in terms of the separation equipment size and with regard to the energy consumption.
The water–hydrogen system is the most effective for solving problems related to the purification of water flows from tritium. Thus, at the end of the last century and at the beginning of the present one, installations based on isotope exchange in the water–hydrogen system were operated for isotopic purification of heavy-water moderator from tritium in Japan (Fugen Nuclear Power Station), Korea (Wolsong Tritium Removal Facility), and Canada (Atomic Energy of Canada Ltd., Chalk River Nuclear Laboratories, Chalk River, Ontario). A detailed review of publications on technological schemes and operating modes of these installations, as well as installations using other technologies for the process of detritiation of water and hydrogen, can be found in monographs [19, 20]. A modular system for detritiation of light water Veolia Nuclear Solutions Inc has been developed and is currently being commercially produced in the USA [21].
TRITIUM LOCALIZATION INSTALLATION
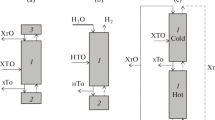
We believe that the relatively low concentration and formation rate of tritium in the first circuit of generation 3+ WWER in comparison with heavy water reactors make it possible to implement the third method (mentioned above) for handling tritiated water. In this case, it is proposed to use catalytic isotope exchange in the water–hydrogen system in a countercurrent column, combined with electrolysis (Combined Electrolysis and Chemical Exchange, CECE process). The installation diagram is shown in Fig. 1.
The main units of the installation are a countercurrent column of chemical isotope exchange (CCIE) filled with a mixture of a hydrophobic catalyst (RCTU-3SM; TU 95 2992, 2013) and a hydrophilic packing, a lower unit for decomposition of the water flow leaving the column to hydrogen and oxygen (electrolyzer) and an intermediate reception container.
The RCTU-3SM catalyst is a nanosystem in which metal platinum is deposited on a hydrophobic support, a copolymer of styrene with divinylbenzene. The granule size of the support is 0.8–1.2 mm, and its main characteristics are a specific surface area of 100 ± 10 m2/g, a porosity of 57 ± 3%, a pore volume of 1.3 ± 0.1 cm3/g, and an average pore diameter of 50 ± 5 nm. The bulk density of the carrier ranges from 0.27 to 0.32 g/cm3. The amount of platinum on the support is from 0.8 to 1.2 wt %.
The operating principle of the installation is as follows. The column is irrigated from above with water of natural isotopic composition with flow L having a background concentration of tritium, and tritium-containing water is fed into an intermediate tank, with flow F having a tritium concentration of XF. The intermediate tank also receives water from the bottom of the CCIE column. Further, the flow of water, equal to the total flow of L and F, enters the electrolyzer, in which its complete decomposition occurs (the water level in the electrolyzer and the intermediate tank remains constant). Tritium-containing hydrogen is fed into the lower section of the column in counterflow to the liquid flow. In the column, tritium is extracted from the gas flow due to the isotopic exchange reaction between water and hydrogen on a hydrophobic catalyst. As a result, the hydrogen flow leaving the column is purified from tritium to background concentrations and sent to the receiver, from which, after drying, hydrogen can be extracted for compression and obtaining a commercial product.
The installation is designed in such a way that the period of tritium accumulation (or the time it reaches a stationary state) is higher than the total operating life of the power unit. That is, all tritium entering the installation is localized in total in a small volume of an intermediate tank and the electrolyzer. As an example (Table 3), we present the results of calculating the ChIE installation in the water–hydrogen system designed to remove tritium from 1500 kg/day of unbalanced water at a nuclear power plant with an initial tritium concentration of 5.3 × 107 Bq/kg.
Over the period of NPP operation (60 years), 1.74 × 1015 Bq tritium is localized in the installation, namely in the volume of the intermediate tank and the electrolyzer. With a total volume of 10 m3 at the time of decommissioning the power unit, the concentration of tritium in the liquid, taking into account the half-life, is 2.61 × 1013 Bq/m3, i.e., intermediate-level waste. The further utilization will make it possible to obtain low-level solid radioactive waste to be buried or removed from the NPP territory.
CONCLUSIONS
Rosenergoatom Concern JSC states that one of the major directions of its activity is development of atomic-hydrogen energy [22]. The authors believe that the technology described in this work, which allows combining the purification of discharges from nuclear power plants and localization of tritium with the production of commercial hydrogen is an option for implementing the concept of atomic hydrogen energy. It follows from Table 3 that the energy consumption for the operation of the tritium localization unit will be approximately 4.8 106 kWh/year (8000 h) or, at current tariffs for industrial enterprises (2 rubles/kWh), 9.6 million rubles.
The considered localization unit, in addition to solving the important environmental problem of purifying tritium from the waste water from nuclear power plants, will make it possible to produce 800 000 m3 of electrolytic hydrogen and 400 000 m3 of oxygen per year. At current prices for technical hydrogen (1000 rubles/cylinder), the turnover may amount to 133 million rubles [22]. Thus, the operation of the tritium removal treatment plant, coupled with the production of industrial gases, can produce market products with an annual turnover of more than 150 million rubles and be cost effective. The creation of an isotope purification unit and the construction of a workshop for the collection and compression of hydrogen and oxygen will require additional capital costs, but they will most likely amount to a fraction of a percent of the total construction cost of a nuclear power plant.
In the authors’ opinion, the inclusion of additional equipment into WWER-based NPPs of generation 3+ is highly advisable for the following reasons:
—due to a significant reduction in tritium discharges, the operation of generation 3+ reactors will prevent potential violation of the RF environmental legislation;
—purification of NPP emission from tritium, coupled with the production of hydrogen and oxygen, becomes a source of commercial hydrogen and oxygen and, therefore, is practically cost-effective, realizing the concept of atomic-hydrogen energy production.
REFERENCES
Sources and Effects of Ionizing Radiation, Vol. 1: Sources, Report of United Nations Sci. Committee Effects of Atomic Radiation (United Nations, New York, 2010).
I. Fairlie, Tritium Hazard Report: Pollution and Radiation Risk from Canadian Nuclear Facilities (Greenpeace, London, 2007).
“Evaluation of facilities handling tritium,” Report of Canadian Nuclear Safety Commission (Minister of Public Works and Government Services, Ottava, 2010).
H. A. Boniface, N. V. Gnanapragasam, D. K. Ryland, et al., Fusion Sci. Technol. 67, 258 (2015).
Gh. Ionita, C. Bucur, I. Spiridon, et al., J. Radioanal. Nucl. Chem. 305, 117 (2015).
Yu. N. Zadonskii and N. V. Zadonskaya, in Proceedings of the Conference MNTK-2014, Moscow, p. 167.
http://www.rosenergoatom.ru.
Basic Sanitary Rules for Ensuring Radiation Safety (OSPORB - 99/2010): Resolution of the Chief State Sanitary Doctor of the Russian Federation, SP (Sanitary Rules and Regulations) No. 2.6.1.2612-10 (2010), No. 40.
Radiation safety standards (NRB - 99/2009): Resolution of the Chief State Sanitary Doctor of the Russian Federation, SP (Sanitary Rules and Regulations) SanPiN 2.6.1.2523–09 (2009).
“On the criteria for classifying solid, liquid and gaseous waste as radioactive waste, criteria for classifying radioactive waste as special radioactive waste and for removable radioactive waste, and criteria for the classification of removed radioactive waste,” Resolution No. 1069 of the Government of the Russian Federation of October 19, 2012.
M. B. Rozenkevich and E. P. Magomedbekov, Bezopasn. Okruzh. Sredy, No. 1, 90 (2009).
Assessment of the Environmental Impact of Placement, Construction and Operation. Kursk NPP-2, Vol. 1: Public Report of Atomenergoproekt (Moscow, 2015) [in Russian].
Yu. A. Egorov, Region. Ekol., Nos. 1–2, 13 (2002).
N. N. Del’vig, A. B. Ivanov, and V. A. Krylov, Ekol. Region. At. Stants. 5, 264 (1996).
B. M. Andreev, Ya. D. Zel’venskii, and S. G. Katal’nikov, Heavy Isotopes of Hydrogen in Nuclear Technology (IzdAT, Moscow, 2000) [in Russian].
M. P. Malkov, Extraction of Deuterium from Hydrogen by Deep Cooling (Atomizdat, Moscow, 1960) [in Russian].
G. P. Pautrot, Fusion Sci. Technol. 14, 480 (1960).
A. M. Rozen, Column Isotope Separation Theory (Atoimizdat, Moscow, 1960) [in Russian].
B. M. Andreev, E. P. Magomedbekov, M. B. Rozenke-vich, et al., Separation of Isotopes of Biogenic Elements in Two-Phase Systems (IzdAT, Moscow, 2003) [in Russian].
A. N. Perevezentsev and M. B. Rozenkevich, Tritium Technology for a Fusion Reactor (Intellekt, Dolgoprudnyi, 2019) [in Russian].
Modular Detritiation System MDS, 2020. http://www.nuclearsolutions.veolia.com/en/our-expertise/technologies/tritium-removal-modular-detritiation-system-mds.
V. Bezzubtsev, Rosenergoatom, No. 12, 4 (2019).
Overview of the Hydrogen Market and Equipment for its Production in Russia, InfoMine Review, 3rd ed., (InfoMine, Moscow, 2018) [in Russian]. http://www.infomain.ru.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by I. Pertsovskaya
Rights and permissions
About this article
Cite this article
Rozenkevich, M.B., Pak, Y.S., Bukin, A.N. et al. Atomic–Hydrogen Energy and Tritium Purification of NPP Discharges Based on WWER Generation 3+ Reactors. Nanotechnol Russia 15, 350–355 (2020). https://doi.org/10.1134/S199507802003012X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199507802003012X