Abstract
The phase diagram of the supercritical CO2–diethylene glycol monoethyl ether (ethylcarbitol) system is studied on isotherms at 308, 318, and 323 K. The available published data are supplemented by the results of this study. The obtained data are described using the Peng–Robinson equation of state and the Mukhopadhyaya and Rao mixing law. A range of thermodynamic parameters is proposed to effectively remove ethylcarbitol from polymers during their plasticization and to preserve the components of the finished product soluble in supercritical CO2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
One of the promising plasticizers that is replacing the alcohol-ether mixture during plasticization of polymers, including filled masses, is ethylcarbitol (diethylene glycol monoethyl ether) [1]. Since it has a sufficiently high boiling point (475 K [2]) and, as a result, low vapor pressure, it takes a rather long time to remove it from the product by convective drying (drying in air). However, ethylcarbitol is easily soluble in water in any ratio, so it is removed by extraction with water. At the same time, the previously obtained results on the extraction of alcohol [3] from gunpowder for hunting make it possible to assume that a similar process can be implemented for filled masses based on ethylcarbitol. In this case, in addition to the previously solved problem of preserving the chemical stability stabilizer, diphenylamine [3], it is necessary to study the phase diagram of ethylcarbitol–supercritical SC-CO2, based on which it is possible to select the optimal parameters of the extraction process with the preservation of the key components.
The solubility of CO2 in ethylcarbitol at 308 and 318 K isotherms and pressures of up to 5 MPa was studied in [4]; however, these data are insufficient to determine the optimal parameters of the extraction process, and the aim of this work is to study and then describe the phase diagram of the SC-CO2–ethylcarbitol system.
EXPERIMENTAL
The following reagents were used in the work: carbon dioxide of the first or highest grade with the CO2 content of at least 99.5%, and also ethylcarbitol C6H14O3 of the highest CAS 111-90-0 grade with the fraction of the main product of at least 99%.
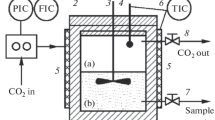
The phase diagram was studied using the setup shown in Fig. 1 and described in detail in [3, 5–9] in accordance with the procedure described in these works.
The setup is based on the R-401 supercritical fluid (SCF) extraction system (Reaction Engineering Inc., South Korea) and allows the solubility studies at temperatures of up to 100°C and pressures of up to 40.0 MPa. To obtain a saturated solution corresponding to the maximum possible equilibrium concentration of the solute in the solvent, the setup was additionally equipped with a mixing device (not shown in the diagram). It was stirred for 60 min, after which the system was settled for 60 min. During the experiments, the samples were taken from the gas phase to determine the solubility of ethylcarbitol in SC-CO2 and from the liquid phase to determine the solubility of SC-CO2 in ethylcarbitol.
RESULTS AND DISCUSSION
The data on the solubility of carbon dioxide in ethylcarbitol on isotherms at 308 K and 318 K presented in [4] were supplemented by the data on the solubility of ethylcarbitol in carbon dioxide on the same isotherms. In addition, the mutual solubility of ethylcarbitol and CO2 on the isotherm at 323 K (Fig. 2) was additionally studied. This isotherm was chosen because at higher temperatures the removal of ethylcarbitol from the filled masses is accompanied by the thermal degradation of the individual components, as a result of which the obtained product does not correspond to the necessary requirements. The maximum error of the solubility measurement (state standard (GOST) 34100-2017) was 5.3%.
Phase diagram of ethylcarbitol-CO2 on different isotherms: (1, 2) taken from [3]; (2, 3) this work; (1, 3) at 308 K; (2, 4) at 318 K; (5) this work, 323 K; (6–8) description of phase equilibrium.
The results of the research were processed in accordance with the model widely tested in [10]. According to this model, the solubility of low-volatile and incompressible substances in the SCF media is described as follows:
or
where y is the solubility of the substance in the SCF solvent (molar fractions); Pv is the vapor pressure of the solute at temperature T; P is the pressure in the system; Φ is the volatility coefficient of the solute in the fluid; vi is the molar volume of the pure solute; and R is the universal gas constant.
The volatility coefficient of the solute in the fluid phase can be calculated using one of the cubic multiparameter equations of state. In this study, we used the two-parameter Peng–Robinson equation of state, which is widely used to calculate phase equilibria in the substance– SCF systems:
where
where wi is the accentricity factor of molecules of the ith component.
The same equation in the cubic form can be presented in the form of a polynomial:
where
In accordance with the adopted law of mixing and the combination rule of Mukhopadhyaya and Rao [10], quantities a and b are determined for the mixture:
where yi and yj are the mole fractions of the i- and jth components of the mixture in any of the equilibrium phases, and mij is the empirical binary interaction coefficient taking into account the features of the pairwise interaction of dissimilar molecules, which is determined within the combination rule of Mukhopadhyaya and Rao [10].
Then we determine the volatility coefficient of the studied substance in the fluid:
where B1 = \({{{{b}_{i}}} \mathord{\left/ {\vphantom {{{{b}_{i}}} b}} \right. \kern-0em} b}\); A1 = \(\frac{2}{a}{{\sum\nolimits_i {{{y}_{i}}{{a}_{{{{i}_{2}}}}}\left( {{b \mathord{\left/ {\vphantom {b {{{b}_{{{{i}_{2}}}}}}}} \right. \kern-0em} {{{b}_{{{{i}_{2}}}}}}}} \right)} }^{{{{m}_{{{{i}_{2}}}}}}}}\); A2 = \(\frac{1}{a}({{B}_{1}} - 1)\sum\nolimits_i {\sum\nolimits_j {{{y}_{i}}{{y}_{j}}{{a}_{{ij}}}{{m}_{{ij}}}{{{\left( {{b \mathord{\left/ {\vphantom {b {{{b}_{{ij}}}}}} \right. \kern-0em} {{{b}_{{ij}}}}}} \right)}}^{{{{m}_{{ij}}}}}}} } .\)
The fitting empirical binary interaction parameter mij is determined at a fixed temperature by minimizing the standard deviation of the calculated data from the experimental points:
where n is the number of experimental points on the isotherm.
When describing the data on the solubility of carbon dioxide in ethylcarbitol, the vapor pressure was taken equal to the pressure of carbon dioxide. Thus, the first term in Eq. (2) became zero. When describing the solubility of ethylcarbitol in carbon dioxide, the saturated vapor pressure values from [11] were initially used. However, these data did not make it possible to obtain a correct description, and the standard deviation of the calculated values from the experimental ones exceeded 50% (the calculation results are not presented in this work). This is due to the fact that some of the experimental solubility values are at pressures less than 7 MPa, which correspond to the concept of a subcritical fluid. The model we have chosen does not always work correctly in this case.
In order to increase the accuracy of the description, it was proposed to use the saturated vapor pressure as a fitting parameter in addition to the binary interaction coefficient. This made it possible to significantly increase the accuracy of the solubility’s description; however, the saturated vapor pressure obtained in this case differed significantly from the experimental ones. This difference is due both to the fact that the model does not work well in the field of subcritical fluids and to the fact that the proposed technique described in [12] is aimed primarily at describing the solubility and not at determining the saturated vapor pressure of a substance. The critical parameters of carbon dioxide were taken from [13] and those of ethylcarbitol were taken from [14]. The obtained values are presented in Table 1 and the results of the description are given in Fig. 2. The binary interaction coefficients obtained in the description are given in Table 2.
At the same time, it was established earlier in [3] that one of the key components of filled plasticized polymers, the stabilizer of chemical resistance, diphenylamine, is easily soluble in supercritical carbon dioxide. However, with the increase in the solvent temperature at pressures of up to 15 MPa, its solubility decreases significantly (up to 300%), which is explained by the so-called crossover effect and is observed for any substances soluble in SCFs. Thus, in accordance with the phase diagram of the binary CO2–ethylcarbitol mixture and the results of the previous study of the solubility of diphenylamine in SC-CO2, it is possible to conclude that the optimum range of the mode parameters for removing ethylcarbitol from polymers during their plasticization is 323 K and the pressure ranges from 9 to 12 MPa. The obtained results were used in the studies carried out by the State Scientific-Research Institute of Chemical Products, Kazan.
CONCLUSIONS
The optimal temperature of 323 K and pressure ranging from 9 to 12 MPa of the operating parameters for the removal of ethylcarbitol from polymers during their plasticization were determined based on the study of the phase diagram of ethylcarbitol–CO2. On the one hand, this range makes it possible to efficiently remove ethylcarbitol from plasticized polymers, and on the other hand, it makes it possible to keep the components soluble in supercritical carbon dioxide in the finished product.
REFERENCES
V. I. Konovalov, N. M. Lyapin, E. F. Korobkova, A. I. Khatsrinov, L. A. Krasnoperova, R. F. Gatina, and Yu. M. Mikhailov, Vestn. Kazan. Tekhnol. Univ., No. 8, 209 (2010).
O. N. Dyment, K. S. Kazanskii, and A. M. Miroshnikov, Glycols and other Derivatives of Ethylene and Propylene Oxides (Khimiya, Moscow, 1976) [in Russian].
T. R. Bilalov, F. M. Gumerov, and R. F. Gatina, Sverkhkrit. Flyuidy: Teor. Prakt. 13 (1), 40 (2018).
W. Wang, Z. Yun, Z. Tang, and X. Gui, Chin. J. Chem. Eng. 24, 373 (2016).
A. A. Jaddoa, T. Bilalov, F. Gumerov, F. Gabitov, and B. le Neindre, Int. J. Anal. Mass Spectr. Chromatogr., No. 3, 37 (2015).
A. A. Zakharov, T. R. Bilalov, and F. M. Gumerov, Russ. J. Phys. Chem. B 10, 1092 (2016).
T. R. Bilalov, F. M. Gumerov, and R. F. Gatina, Sverkhkrit. Flyuidy: Teor. Prakt. 11 (4), 17 (2016).
T. R. Bilalov, A. A. Zakharov, A. A. Jaddoa, F. M. Gumerov, and B. le Neindre, J. Supercrit. Fluids 130, 47 (2017).
T. R. Bilalov, F. M. Gumerov, and F. R. Gabitov, Russ. J. Phys. Chem. B 12, 1287 (2018).
M. Mukhopadhyay and G. V. R. Rao, Ind. Eng. Chem. Res., No. 32, 922 (1993).
https://webbook.nist.gov/cgi/cbook.cgi?ID=C111900&Units=SI&Mask=7&Type=ANTOINE&Plot=on#ANTOINE.
T. R. Bilalov and F. M. Gumerov, Russ. J. Phys. Chem. B 13, 1290 (2019).
N. B. Vargaftik, Handbook of Thermophysical Properties of Gases and Liquids (Fizmatgiz, Moscow, 1963) [in Russian].
L. C. Wilson, H. L. Wilson, W. V. Wilding, and G. M. Wilson, J. Chem. Eng. Data 41, 1252 (1996).
Funding
This work was supported by Russian Science Foundation (project no. 19-73-10029) and state task no. 13.5112.2017/8/9 of the Ministry of Education and Science of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Mosina
Rights and permissions
About this article
Cite this article
Bilalov, T.R., Zavjalova, N.B. & Gumerov, F.M. Phase Diagram of the Supercritical Carbon Dioxide–Ethylcarbitol System. Russ. J. Phys. Chem. B 14, 1148–1152 (2020). https://doi.org/10.1134/S1990793120070039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793120070039