Abstract—
The effect of 6-hydroxy-2,2,4-trimethyl-1,2-dihydroquinoline on markers of hepatocyte cytolysis (aspartate transaminase, alanine transaminase and gamma-glutamyl transpeptidase), parameters reflecting the state of oxidative status (intensity of biochemical luminescence and the content of conjugated dienes), and on activity of oxidative metabolism enzymes (aconitate hydratase, glucose-6-phosphate dehydrogenase, NADP-isocitrate dehydrogenase) was studied in rats with CCl4-induced liver injury. The results obtained in the course of this study demonstrate the ability of the test compound to reduce oxidative stress severity and the degree of liver cell damage, as well as to change activity of aconitate hydratase and NADP-generating enzymes towards control values. 6-Hydroxy-2,2,4-trimethyl-1,2-dihydroquinoline was more effective in normalizing CCl4-induced changes of most analyzed parameters than carsil used as a reference compound. The tendency to normalize the state of oxidative status and enzyme activity of oxidative metabolism can be attributed to hepatoprotective and antioxidant properties of the test compound.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Liver inflammatory diseases causing degeneration of this organ represent a serious problem important due to their increased prevalence. There is evidence that complications associated with toxic liver damage are the main causes of mortality among patients with gastrointestinal tract diseases [1].
Carbon tetrachloride (CCl4) is one of the most studied hepatotropic poisons, for which the pathogenesis picture is similar to acute liver injuries induced by various xenobiotics. In this regard, it is widely used in experimental medicine to model toxic hepatitis. During CCl4 metabolism, numerous free radicals (FR) are formed in the liver monooxygenase microsomal system, (particularly, the trichloromethyl radical) causing oxidative stress. The generated reactive molecules induce lipid peroxidation (LPO) in membranes and interact with various proteins with covalent bond formation. This finally leads to impaired functioning of proteins (including impaired functions of enzymatic systems), cell membrane damage causing its increased permeability, the release of liver enzymes into the blood circulation and cell death [2].
Exposure to high levels of carbon tetrachloride may exhaust resources of the antioxidant defense system and this leads to the development of oxidative stress and promotes development of toxic hepatitis. Thus, taking into consideration existing problem of toxic liver injury the search for hepatoprotective agents with pronounced antioxidant properties is a very urgent issue. The possibility of using antioxidants to correct disorders typical for a wide range of hepatic pathologies is being actively discussed. In particular, the possibilities of using compounds of natural origin for treatment of alcoholic and non-alcoholic fatty liver disease are considered [3]; certain attempts have been undertaken to evaluate the protective properties of vitamin C, N-acetylcysteine, and astaxanthin on a model of liver steatosis [4]; the effects of vegetable essential oils are investigated in experimental toxic liver injury induced by cyclophosphamide [5]. In addition to the search and study of natural antioxidants, protectors obtained by chemical synthesis are also actively studied. Good evidence exists in the literature that dihydroquinoline derivatives data exhibit hepatoprotective properties. For example, 6,6-methylene-bis(2,2,4-trimethyl-1,2-dihydroquinoline) and 6,6-methylene-bis(2,2-dimethyl-4-methanesulfonic acid sodium-1,2-dihydroquinoline) were able to prevent acute liver damage induced by galactosamine and CCl4 [6]. Some dihydroquinoline derivatives exhibit antioxidant activity. For example, 6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline, known as ethoxyquin (Santoquin), has a pronounced antioxidant effect, and therefore it is used as a food preservative for farm animals, as well as for prevention of certain poultry diseases [7]. Thus, it seems reasonable to study biological effects of this derivative under conditions accompanied by the development of oxidative stress. Recently, a method for synthesis of 6-hydroxy-2,2,4-trimethyl-1,2-dihydroquinoline (DHQ) has been developed at the Department of Organic Chemistry (Voronezh State University; VSU) and this compound has been synthesized for examination in the present study [8].
We have chosen Carsil as a reference drug for this study. It is widely used as a hepatoprotective agent; its active ingredient is silymarin, which also exhibits antioxidant activity. Carsil is popular as a reference drug for evaluating the effectiveness of potential drugs for treatment of toxic liver injury [9].
It is known that steatosis is one of the key pathogenetic factors in the development of non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, and progression of fibrosis [10]. Fat accumulation in hepatocytes is also characteristic of acute toxic liver injury. One of the mechanisms of hepatotropic action of CCl4 includes inhibition of β-oxidation and activation of biosynthesis of fatty acids and cholesterol, which occurs due to an increase in the acetate supply of liver cells [11]. Thus, under conditions of CCl4-induced development of oxidative stress and exposure to compounds that can limit the severity of FR-mediated damage, it is of interest to study the functioning of oxidative metabolism enzymes involved in lipid metabolism and the work of antioxidant cell systems. Among these enzymes, aconitate hydratase (AH, aconitase, EC 4.2.1.3), which catalyzes the reaction of reversible isomerization of citrate to isocitrate is especially interesting. Two isoforms of AH are known: cytoplasmic and mitochondrial. These isoforms differ in physicochemical and structural properties and perform different physiological functions, determining their involvement in both catabolic and anabolic processes. The active center of this enzyme contains an iron-sulfur cluster, which is easily damaged by FR [12]. Inhibition of the mitochondrial form of AH under conditions of intensified free radical oxidation is associated with decreased activity the tricarboxylic acid cycle and, correspondingly, with a decrease in the level of electron flow through the respiratory chain [12]. The cytoplasmic form of the enzyme is primarily responsible for regulation of accumulation and utilization of citrate [12], which plays the role of a substrate precursor for fatty acids biosynthesis, acetyl CoA, which is formed in the ATP-citrate lyase reaction. On the other hand, citrate can chelate prooxidant transition metal ions. Glucose-6-phosphate dehydrogenase (G6PDH, EC 1.1.1.49) is a rate-limiting enzyme of the pentose phosphate pathway, which produces ribose-5-phosphate (necessary for nucleic acid biosynthesis) and NADPH. NADP-isocitrate dehydrogenase (NADP-IDH, EC 1.1.1.42) catalyzes the reaction of oxidative decarboxylation of isocitrate to 2-oxoglutarate and is localized mainly in the cytoplasm; a small pool of enzyme activity is also detected in mitochondria. Generating NADPH, G6PDH and NADP-IDH are involved in the regulation of pathways associated with lipid biosynthesis [13] and protection against oxidative stress by providing reducing equivalents for the functioning of a number of antioxidant protection components [14].
The aim of this study was to investigate the hepatoprotective activity of DHQ and its effect on the functioning of oxidative metabolism enzymes during liver injury induced by CCl4. Based on the major goal of this study, following tasks were formulated: (i) to evaluate the hepatocyte cytolysis indices after DHQ administration to animals with Cl4-induced liver injury; (ii) to analyze the effect of DHQ on the intensity of the processes of free radical oxidation during the development of pathology; (iii) to study the activity of AH, NADP-IDH and G6PDH, the enzymes involved in the lipogenesis and maintenance of the redox status of cells after DHQ administration to animals with Cl4-induced liver injury.
Thus, in this study we have evaluated for the first time the hepatoprotective potential of newly synthesized DHQ and its impact on the intensity of free radical oxidation and activity of several enzymes involved in oxidative metabolism.
MATERIALS AND METHODS
Experiments were carried out using male albino Wistar rats (Rattus norvegicus) weighing 200−250 g; animals were obtained from the animal nursery Kro-lInfo, (Moscow region, Russia). Acute toxic liver injury was modeled by a single intragastric administration of CCl4 at a dose of 0.064 mL/100 g (the dose was dissolved in 1 mL of vaseline oil). The experimental animals were subdivided into 6 groups: group 1 (n = 12) included intact animals; group 2 (n = 12) included rats with induced toxic liver injury; group 3 (n = 10) included animals, which received three intragastric administrations of DHQ (50 mg/kg) dissolved in 1 mL of 1% starch; the treatment started 3 h after CCl4 administration with 24 h interval between each DHQ introduction; group 4 (n = 10) included rats with CCl4-induced hepatitis, which were treated with carsil (50 mg/kg) according to the same scheme as DHQ treatment. In terms of the amount of silymarin in this dosage form (22.5 mg per tablet of 500 mg), the animals received 2.25 mg/kg; this is comparable to the therapeutic dose for humans indicated in the drug leaflet. Rats of groups 5 and 6 (n = 8) maintained at the standard vivarium conditions were treated with DHQ or carsil (50 mg/kg, respectively). On day 4 of the experiment, the liver and blood from the heart were collected from anesthetized animals. Liver tissue was homogenized in a 4-fold volume of the isolation medium containing 0.05 M Tris-HCl buffer, pH 7.8, 1 mM EDTA, 1% β-mercaptoethanol, and then centrifuged at 8000 g for 15 min. The supernatant and blood serum were used in the study.
The activity of marker enzymes of hepatocyte cytolysis, alanine transaminase (ALT), aspartate transaminase AST, and gamma glutamyl transpeptidase (GGT), was determined using kits from Olvex Diagnosticum (Russia).
The intensity of free radical oxidation processes and the total antioxidant activity in a biological sample were determined by using biochemiluminescence (BCL) induced by hydrogen peroxide with iron sulfate. The BCL kinetic curve was recorded for 30 s using a BHL-07 biochemiluminometer (Medozons, Russia), and the following parameters were determined: total biochemiluminescence (S), maximum flash intensity (Imax), tangent of the BCL kinetic curve slope (tan α2). The reaction medium contained 0.4 mL of 0.02 mM potassium phosphate buffer (pH 7.5), 0.4 mL of 0.01 mM FeSO4 and 0.2 mL of a 2% hydrogen peroxide solution added immediately before the measurement. The test material (0.1 mL) was added before hydrogen peroxide.
The intensity of LPO processes was evaluated by the optical method of determination of conjugated dienes (CD) at 233 nm using a Hitachi U1900 spectrophotometer (Japan) [15].
AH activity was determined spectrophotometrically at 235 nm. The assay medium contained 0.15 mM citrate, 50 mM Tris-HCl buffer, pH 7.8. The reaction was initiated by adding the test sample into a spectrophotometric cell. The citrate concentration was estimated by the method of Natelson [16].
G6PDH and NADP-IDH activities were evaluated by increased optical density of the test samples at 340 nm, caused by NADP reduction. The spectrophotometric medium for G6PDH assay contained 50 mM Tris-HCl buffer, pH 7.8, 3.2 mM glucose-6-phosphate, 0.25 mM NADP, 1.0 mM MgCl2. NADP-IDH activity was measured in a spectrophotometric medium containing 50 mM Tris-HCl buffer, pH 7.8, 1.5 mM isocitrate, 2 mM MnCl2, 0.4 mM NADP.
Protein content was determined using the biuret reagent [17].
Results of the study were processed using methods descriptive statistics with determination of the mean value, standard deviation, and standard error of the mean (SEM) [18]. The results of the work were analyzed using the Student’s t-test for multiple comparisons with the Bonferroni correction. The normality of the distribution of values in groups was evaluated using the Kolmogorov−Smirnov criterion. Differences were considered as statistically significant at p < 0.05. In tables and figures data represent mean ± (SEM).
RESULTS AND DISCUSSION
During the first stage, we have compared hepatoprotective activity of DHQ and carsil in rats with induced toxic liver injury. DHQ administration to CCl4-treated rats changed activity indicators of AST, ALT, and GGT activities towards control level and these changes were more pronounced than after administration of carsil. For example, DHQ administration caused a 2.5-, 2.9- and 3.9-fold decrease in serum activity of AST, ALT, and GGT respectively as compared with the values of these parameters determined in animals of group 2 (toxic liver injury induced by CCl4). The introduction of the reference drug was less effective and led to a decrease in these indicators by 2.1, 2.5 and 1.4 times, respectively (Fig. 1). The de Ritis coefficient (AST/ALT activity ratio) for animals with toxic liver injury demonstrated a 1.6-fold decrease versus this parameter in intact rats, thus suggesting a significant role of inflammatory processes in the development of this pathological condition [19]. Administration of DHQ and carsil to rats with CCl4-induced toxic liver injury changed the AST/ALT activity ratio towards control level by 19% and 15%, respectively. All these results indicate that DHQ has a more pronounced hepatoprotective effect compared to carsil. Apparently, the protective activity of the test compound is based on its ability to deactivate FR, because their excessive generation is a key factor in the hepatocyte damage during toxic liver injury. The manifestation of the antiradical activity of DHQ may be attributed to the presence of a hydroxyl group and p-coupling of N and O electrons in the para position of the aromatic cycle [20]. In addition, the protective properties of other derivatives of dihydroquinoline are known. Earlier, the neuroprotective properties of the compound related to the quinoline 1,2-dihydro derivatives, ethoxyquin were reported [21]. In the control groups of rats treated with DHQ and carsil, no significant changes in AST, ALT, and GGT activities were observed.
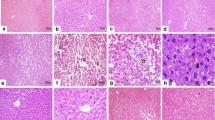
Activity of ALT, AST (a) and GGT (b) in serum of rats with CCl4-induced toxic liver injury and exposured to 6-hydroxy-2,2,4-trimethyl-1,2-dihydroquinoline. Here and in other figures: 1—intact animals; 2—animals with toxic liver injury; 3—rats with toxic liver injury treated with 6-hydroxy-2,2,4-trimethyl-1,2-dihydroquinoline (50 mg/kg); 4—rats with toxic liver injury treated with carsil (50 mg/kg); 5—control rats treated with 6-hydroxy-2,2,4-trimethyl-1,2-dihydroquinoline (50 mg/kg); 6—control rats treated with carsil (50 mg/kg). Statistically significant differences, p < 0.05: * compared with the intact rats, ** compared with rats with CCl4-induced toxic liver injury, # compared with rats with CCl4-induced toxic liver injury treated with carsil.
DHQ had a hepatoprotective effect in rats with toxic liver injury. Since oxidative stress is one of the key factors involved in pathogenesis of CCl4-induced hepatitis, we have evaluated the intensity of FR oxidation in experimental animals for elucidation of mechanisms of the cytoprotective effect of DHQ. It has been previously shown that the development of toxic liver injury is associated with a significant increase in BCL, CD content, as well as with decreased AH activity [22]. The results of this study have shown that DHQ administration to rats with toxic liver injury decreased Imax, S, and tan α2 by 1.6, 2.3, and 1.3 times in the liver and by 2.0, 2.4, and 1.9 times in serum, respectively. Administration of carsil to animals with this pathology was accompanied by less significant changes in the liver BCL parameters. For example, Imax, S, and tan α2 in rats of group 4 decreased by 1.4, 2.1, and 1.2 times versus untreated rats with toxic liver injury (Table 1).
The serum and liver CD content in rats with toxic liver injury treated with DHQ decreased by 1.9 times and 1.5 times, respectively, versus animals of group 2 (CCl4-induced liver injury). Administration of carsil also caused a decrease in this parameter in serum (1.6-fold) and the liver (1.2-fold) (Fig. 2).
It is known that the development of toxic liver injury is accompanied by a decrease of AH activity; this enzyme of oxidative metabolism is a sensitive target of the action of free radicals [22]. A decrease in the activity of this enzyme was also demonstrated in patients with drug-induced hepatitis [23]. Administration of DHQ to rats with CCl4-induced toxic liver injury resulted in a tendency to normalization of AH activity: in the liver and serum this parameter increased by 1.3 and 1.6 times, respectively, versus the values of these parameters in rats of group 2 (Fig. 3a). These changes resulted in a decrease of the citrate content by 1.7 and 1.6 times, respectively (Figs. 4a, 4b). Thus, a decrease in the concentration of the substrate precursor for fatty acids biosynthesis, playing a significant role in the development of steatosis and fibrosis under conditions of its activation, was noted [10]. In the control groups of animals treated with DHQ and carsil, no significant changes in the rates of FR oxidation were detected. The specific activity of AH in the tissues of animals of the experimental groups demonstrated similar changes (Fig. 3b). Probably, the detected changes can be attributed to the antioxidant properties of DHQ during the development of toxic liver injury, characterized by oxidative stress as the main pathogenic factor. It is known that, CCl4 entering the liver can be transformed at a low partial pressure of oxygen to \({\text{CCl}}_{3}^{\centerdot }\) and \({\text{CHCl}}_{2}^{\centerdot }\) radicals [24]. These conditions cause impairments in lipid metabolism and development of liver steatosis or obesity. On the other hand, the high partial pressure of oxygen promotes CCl4 conversion to the CCl3-OO• radical, followed by LPO activation and induction of cell apoptosis. In addition, damage of cell membranes is accompanied by the release of pro-inflammatory chemokines and cytokines, leading to endotoxin shock and inflammatory liver damage [24]. Administration of the reference drug to animals with toxic liver injury had a slightly more pronounced impact on the liver AH activity as compared with the effect of DHQ. It is possible that this effect of carsil may be associated with the ability of the main component of the drug, silymarin, to have a positive effect on metabolism. For example, it was shown that the use of silymarin, either alone or in combination with succinic acid, helped to: (i) restore the cell respiration rate, (ii) increase coupling of oxidation and phosphorylation, (iii) increase the metabolic rate of Krebs cycle intermediates during experimental inhibition of β-oxidation of fatty acids [25]. It should be noted that for other derivatives of dihydroquinoline, the presence of the antioxidant effect was demonstrated. For example, using a virtual screening, compounds of the dihydroquinoline series that showed high antioxidant activity were obtained [26]. It is also known that the derivatives of 8-hydroxyquinoline can reduce the level of β-amyloid, act as FR scavengers, and chelate copper ions [27].
It is known that NADPH acts as a reducing equivalent needed for fatty acids biosynthesis; it also plays a role in the functioning of the glutathione antioxidant system, protecting against oxidative stress. It was previously demonstrated that the induction of toxic liver injury in rats and drug hepatitis in humans were associated with the mobilization of this protective system, as well as with increased activity of enzymes generating NADPH [22, 23]. It was shown that mice knocked out by the gene of the cytoplasmic form of NADP-IDH were more susceptible to oxidative stress, inflammation, and hepatocyte cytolysis induced by lipopolysaccharide administration [28]. There is evidence that under the conditions of oxidative stress, the activated protein SIRT2 (sirtuin 2) deacetylates the G6PDH molecule at Lys-403 position, and this increases its activity and NADPH generation [29]. Results of this study have shown that the effect of DHQ in toxic liver injury changed G6PDH and NADP-IDH activity towards control values, while the administration of carsil was less effective. For example, in animals with toxic liver injury treated with DHQ activity of NADP-IDH in the liver and serum reached the control level. After administration of carsil to animals with this pathology, NADP-IDH activity decreased by 1.4 and 1.5 times, respectively (Fig. 5a). The use of DHQ as a protector also contributed to a decrease in G6PDH activity in the liver and serum by 1.4 and 1.8 times relative to the values of group 2 (untreated animals with toxic liver injury). Carsil administration had a similar effect on the activity of G6PDH in the liver, while a decrease in serum activity of this enzyme was less pronounced (Fig. 6a). Changes in the specific activity of G6PDH and NADP-IDH had a similar tendency (Figs. 5b, 6b). Obviously, the observed changes in the activity of both enzymes induced by DHQ administration to animals with toxic liver injury are associated with a decrease in the need for NADPH by the antioxidant system of liver cells. Apparently, DHQ administration contributed to a decrease in the intensity of FR oxidation, followed by a compensatory decrease of the adaptive compensatory response to stress [30].
Thus, under the conditions of the toxic liver injury DHQ demonstrated hepatoprotective and antioxidant activity, which was manifested in a decrease in marker indicators of liver cell cytolysis and intensity of FR oxidation intensities; DHQ also changed the activity of oxidative metabolism enzymes towards control level. In most cases DHQ had a more pronounced effect on the analyzed parameters than carsil.
CONCLUSIONS
Administration of DHQ to animals with induced toxic liver injury changed parameters reflecting hepatocyte cytolysis and intensity of FR processes (BCL and CD concentration) towards the control level. There was a decrease in the degree of mobilization of NADPH generating enzymes and restoration of AH activity, which could be a consequence of the antioxidant effect of the protector. The results of this study indicate that the test compound has a more pronounced positive effect on most of the studied parameters then the reference drug, carsil. The data obtained emphasize the feasibility of searching for new hepatoprotective compounds among dihydroquinoline derivatives that can be applicable as therapeutic agents for treatment of acute liver injuries.
Based on the results obtained in the study, the following conclusions can be drawn: (1) DHQ administration under conditions of toxic liver injury decreased in marker parameters of hepatocyte cytolysis; (2) the test compound decreased in the intensity of free radical oxidation in tissues of rats with induced pathology; (3) DHQ had a regulatory effect on the activity of oxidative metabolism enzymes involved in the functioning of the antioxidant system of the liver and the supply of acetyl CoA and NADPH for fatty acid biosynthesis; (4) in most cases the test compound had a more significant effect on the analyzed parameters than carsil.
REFERENCES
Polikarpov, A.V. et al., Zabolevaemost’ vsego naseleniya Rossii v 2017 godu. Statisticheskiye materialy, chast 2 (Morbidity of Russian Population in 2017. Statistical Materials, Part 2), Moscow, 2018, p. 142.
Qi, B., Zhang, S., Guo, D., Guo, S., Jiang, X., and Zhu, X., Mol. Med. Rep., 2017, vol. 16, no. 3, pp. 2814−2822.
Han, K.H., Hashimoto, N., and Fukushima, M., World J. Gastroenterol., 2016, vol. 22, no. 1, pp. 37−49. https://doi.org/10.3748/wjg.v22.i1.37
Yang, J.P., Shin, J.H., Seo, S.H., Kim, S.G., Lee, S.H., and Shin, E.H., Int. J. Mol. Sci., 2018, vol. 19, no. 9. https://doi.org/10.3390/ijms19092563
Sheweita, S.A., El-Hosseiny, L.S., and Nashashi-bi, M.A., PLoS One, 2016, vol. 11, no. 11, e0165667. https://doi.org/10.1371/journal.pone.0165667
Feher, J., Bar-Pollak, Z., Sreter, L., Feher, E., and Toncsev, H., Br. J. Exp. Pathol., 1982, vol. 63, no. 4, pp. 394−400.
Bailey, C.A., Srinivasan, L.J., and McGeachin, R.B., Poult. Sci., 1996, vol. 75, no. 9, pp. 1109−1112.
Popova, T.N., Shulgin, K.K., Shikhaliyev, Kh.S., Krylskiy, E.D., Matasova, L.V., Medvedeva, S.M., Popov, S.S., and Verevkin, A.N., Rus. Patent 2 677 883 C1, Byul. Izobret., 2019, no. 3.
Ivanova, V.V., Ligostayea, Yu.V., Poteryaeva, O.N., Russkih, G.S., Grek, O.R., Sharapov, V.I., and Gevorgyan, M.M., Fundamentalniye Issledovaniya, 2013, vol. 3, no. 2, pp. 277−279.
Ipsen, D.H., Lykkesfeldt, J., and Tveden-Nyborg, P., Cell Mol. Life Sci., 2018, vol. 75, no. 18, pp. 3313−3327. https://doi.org/10.1007/s00018-018-2860-6
Weber, L.W., Boll, M., and Stampfl, A., Crit. Rev. Toxicol., 2003, vol. 33, no. 2, pp. 105−136.
Matasova, L.V. and Popova, T.N., Biochemistry (Moscow), 2008, vol. 73, no. 9, pp. 1189−1198.
Koh, H.J., Lee, S.M., Son, B.G., Lee, S.H., Ryoo, Z.Y., Chang, K.T., Park, J.W., Park, D.C., Song, B.J., Veech, R.L., Song, H., and Huh, T.L., J. Biol. Chem., 2004, vol. 279, pp. 39968−39974. https://doi.org/10.1074/jbc.M402260200
Domínguez-Perez, M., Nuno-Lambarri, N., and Clavijo-Cornejo, D., Oxid. Med. Cell Longev., 2016, vol. 2016. https://doi.org/10.1155/2016/7960386
Quiroga, P.L., Soria, E.A., Valentich, M.A., and E-ynard, A.R., Nutr. Cancer, 2018, vol. 14, pp. 1−8. https://doi.org/10.1080/01635581.2018.1497669
Valdez, J.M., Johnstone, A.F.M., Richards, J.E., Schmid, J.E., Royland, J.E., and Kodavanti, P.R.S., Int. J. Mol. Sci., 2018, vol. 20, no. 1. https://doi.org/10.3390/ijms20010011
Menshikova, V.V., Laboratorniye metody issledovaniya v klinike (spravochnik) (Laboratory Methods in Clinical Practice. A Reference Book), Moscow: Meditzina, 1987.
Glantz, S. Mediko-biologicheskaya statistika (Medico-Biological Statistics), Moscow: Praktika, 1999.
Parmar, K.S., Singh, G.K., Gupta, G.P., Pathak, T., and Nayak, E., Int. J. Med. Sci. Public Health, 2016, vol. 5, no. 9, pp. 1783−1788.
Kasaikina, O.T., Izv. Akad. Nauk SSSR,Khim. Ser., 1983, vol. 10, p. 2214.
Zhu, J., Carozzi, V.A., Reed, N., Mi, R., Marmiroli, P., Cavaletti, G., and Hoke A., Int. J. Sci. Rep., 2016, vol. 6, 28861. https://doi.org/10.1038/srep28861
Popova, T.N., Matasova, L.V., and Makeeva, A.V., Biomed. Khim., 2007, vol. 53, no. 2, pp. 181−189.
Popov, S.S., Shulgin, K.K., Popova, T.N., Agarkov, A.A., Pashkov, A.N., and Carvalho, D.E., J. Biochem. Mol. Toxicol., 2015, vol. 29, no. 10, pp. 449−457. https://doi.org/10.1002/jbt.21705
Dutta, S., Chakraborty, A.K., Dey, P., Kar, P., Guha, P., Sen, S., Kumar, A., Sen, A., and Chaudhuri, T.K., PLoS One, 2018, vol. 13, no. 4. https://doi.org/10.1371/journal.pone.0196411
Vengerovskiy, A.I. and Khazanov, V.A., Eksper. Klin. Faramakol., 2007, vol. 70, no. 2, pp. 51−55.
El Bakkali, M., Ismaili, L., Tomassoli, I., Nicod, L., Pudlo, M., and Refouvelet, B., Int. J. Med. Chem., 2011, vol. 2011, 592879. https://doi.org/10.1155/2011/592879
Fernández-Bachiller, M.I., Pérez, C., González-Muñoz, G.C., Conde, S., López, M.G., Villar-roya, M., García, A.G., and Rodríguez-Franco, M.I., J. Med. Chem., 2010, vol. 53, no. 13, pp. 4927−4937. https://doi.org/10.1021/jm100329q
Itsumi, M., Inoue, S., Elia, A.J., Murakami, K., Sasaki, M., Lind, E.F., Brenner, D., Harris, I.S., Chio, I.I., Afzal, S., Cairns, R.A., Cescon, D.W., Elford, A.R., Ye, J., Lang, P.A., Li, W.Y., Wakeham, A., Duncan, G.S, Haight, J., You-Ten, A., Snow, B., Yamamoto, K., Ohashi, P.S., and Mak, T.W., Cell Death Differ., 2015, vol. 22, no. 11, pp. 1837−1845. https://doi.org/10.1038/cdd
Wang, Y.P., Zhou, L.S., Zhao, Y.Z., Wang, S.W., Chen, L.L., Liu, L.X., Ling, Z.Q., Hu, F., Sun, Y.P., Zhang, J.Y., Yang, C., Yang, Y., Xiong, Y., Guan, K.L., and Ye, D., EMBO J., 2014, vol. 3, no. 12, pp. 1304−1320. https://doi.org/10.1002/embj.201387224
Gobbo, M.G., Costa, C.F., Silva, D.G., de Almeida, E.A., and Goes, R.M., Oxid. Med. Cell Longev., 2015, vol. 2015, 614579. https://doi.org/10.1155/2015/614579
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All manipulations were performed in accordance with Directive 2010/63/EU of the European Parliament and of the Council of September 22, 2010 on the protection of animals used for scientific purposes, sanitary standards of vivariums (GOST 33 216-2014). They were also approved by the Ethics Committee for the examination of biomedical VSU research (protocol no. 42-01 of January 10, 2019).
The authors declare that they have no conflict of interest.
Additional information
Translated by A. Medvedev
Rights and permissions
About this article
Cite this article
Brazhnikova, D.A., Popova, T.N., Kryl’skii, E.D. et al. The Effect of 6-Hydroxy-2,2,4-Trimethyl-1,2-Dihydroquinoline on the Intensity of Free Radical Processes and Activity of Oxidative Metabolism Enzymes in Rats with Toxic Liver Injury. Biochem. Moscow Suppl. Ser. B 14, 70–77 (2020). https://doi.org/10.1134/S1990750820010060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990750820010060