Abstract
The combined effect of melatonin and cytarabin at low concentration on the cells of acute promyelocytic leukemia HL-60 was studied. It has been shown that the combined effect of these substances led to a decrease in the number of cells by 70% and the mitotic activity index by 75%. It was found that the combined effect of melatonin and cytarabin also contributed to a decrease in the expression of mitochondrial proteins. It has been demonstrated that the combined use of melatonin with low concentration of cytarabin (2 nM) reduced the expression of the voltage-dependent anion channel (VDAC1). Melatonin in combination with cytarabin similarly affected the expression of translocator protein (TSPO) and antiapoptotic protein Bcl-2. The synergistic effect of melatonin in the combined action with cytarabin has been revealed, which provides an increase in the effectiveness of its antitumor action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Oncological diseases are one of the main problems of modern medicine; the search for new approaches to the treatment of tumors is important for its solution. To this end, it is necessary to study the combined action of already known drugs and the search for more effective new ones, which will increase the survival of patients and slow down the development of malignant tumors. Melatonin (MEL, N-acetyl-5-methoxytryptamine) is a hormone secreted by the pineal gland and other tissues; it has similarities with derivatives of a biogenic amine serotonin synthesized from tryptophan [1, 2]. The study of MEL and its effects on the human body showed that it has oncostatic, antioxidant and immunoregulatory properties; thus it can be considered as an antitumor agent. MEL is used in combination therapy of tumors. Abnormal levels of MEL in cancer patients indicate its participation in oncogenesis [3, 4]. Significant antitumor effects of MEL upon different types of tumor cells have been reported [5].
Cytarabin (CYT) is an antitumor drug based on pyrimidine nucleoside and arabinose; it is widely used in the treatment of leukemia. CYT is used mainly in acute promyelocytic leukemia (APL), non-Hodgkin lymphoma (NHL) and chronic myelocytic leukemia [6, 7]. CYT can be used alone or in combination with other antitumor agents such as daunorubicin, doxorubicin, thioguanine, and vincristine.
CYT acts on rapidly dividing cells and inhibits DNA synthesis in the S-phase of the cell cycle, as well as prevents the transition of cells from G1-phase to S-phase. The exact mechanism of action of cytarabin triphosphate is not clear; probably, it inhibits DNA polymerase, by competing with deoxycytidine triphosphate [8]. In addition to antitumor activity, CYT causes a toxic side effect.
The formation of a nonspecific pore (mitochondrial permeability transition pore, mPTP) and an increase in the permeability of the inner membrane of mitochondria occurs as a response to oxidative stress and overload of the mitochondrial matrix with calcium. Voltage-dependent anion channel (VDAC), adenine nucleotide translocase, translocator protein (TSPO), and some other proteins are regulators/modulators of mPTP. VDAC is a 32 kDa protein; it is represented in humans by three isoforms (VDAC1, VDAC2, and VDAC3). VDAC1 is the most common isoform [9]. Interaction of VDAC with pro- and anti-apoptotic proteins of the mitochondrial outer membrane can both promote and prevent cell death. Thus, mitochondrial VDAC may be a promising therapeutic target in various types of tumors. TSPO is not only a partner, but also a VDAC modulator. [10] In addition, some researchers believe that TSPO plays a key role in tumor cell growth [11–13]. TSPO expression has also been shown to increase in various types of tumors, including brain tumors and gliomas. It has been found that TSPO participates in cell proliferation and regulation of apoptosis in some types of tumor cells [13–15]. TSPO located near VDAC forms a strong complex with this protein; thereby it regulates VDAC permeability and mitochondrial activity [16]. We have shown previously that the combination of MEL (1 µM) with trans-retinoic acid (ATRA, 10 nM) increased its cytotoxicity against HL-60 cells and inhibited the expression of antiapoptotic protein Bcl-2, as well as VDAC1 and TSPO [17].
In this study, we investigated the combined effect of MEL (1 mM) and CYT at low concentrations (2 nM) on the proliferative activity of APL cells, using HL-60 cells as a model, and analyzed changes in the level of mitochondrial proteins Bcl-2, VDAC1, and TSPO.
MATERIALS AND METHODS
Cell culture. Human APL cells HL-60 (CCL-240) were purchased derived from ATCC (Manassas, USA). The cells were grown in RPMI 1640 medium (Sigma–Aldrich, USA) with the addition of 20% inactivated fetal bovine serum (Gibco, USA) and 40 µg/mL of gentamicin sulfate (Sigma–Aldrich, USA) at 37°C, 95% air humidity and 5% CO2. Cell population doubling time was about 24 h.
Cell viability analysis. Cell viability was assessed with resazurin. The cells were seeded into a 96-well plate at a density of 5 × 103 cells per well. After 24 h of incubation, the cells were treated with MEL and CYT at the indicated doses. Resazurin (Sigma–Aldrich, USA) was added to each well at a final concentration of 100 µg/mL 24 h after the treatment, and the cells were incubated for 4 h at 37°C. Fluorescence intensity was measured using an Infinite F200 microplate reader (Tecan, Austria) at an excitation wavelength of 535 nm and an emission wavelength of 595 nm. The data were presented as a percentage of the control (untreated cell samples).
Determination of mitotic index. Mitotic activity was determined as follows. The cells incubated for 96 h under various conditions were centrifuged (250 g, 10 min), resuspended in phosphate buffered saline (PBS) and fixed with 70% ethanol (30 min, room temperature). The fixed cells were stained with bisBenzimide H33342 (Sigma–Aldrich, USA) and the number of mitotic cells was calculated using a DM 6000 fluorescent microscope (Leica, Germany). The mitotic index (MI) was determined by the formula MI = (P + M + A + T)/N, where P + M + A + T was the sum of all cells in the following phases: prophase, metaphase, anaphase, and telophase, respectively, and N was the total number of cells.
Analysis of cell proliferation. Cell growth was assessed by counting cells at different time points after treatment. The cells were collected by centrifugation for 10 min at 250 g and washed with PBS. The cells were stained with 0.4% trypan blue (Sigma–Aldrich, USA) to assess the number and viability of the cells. In each experiment, three samples per group were counted. The experiments were performed at least in triplicate.
Electrophoresis and Western-blot analysis. HL-60 cells (2 × 105 cells/mL) treated with MEL and/or CYT were harvested, washed twice with chilled PBS and lysed in a lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, 1 mM Na3VO4 and 1 mM NaF) with the addition of proteinase/phosphatase inhibitors. The extracts were incubated on ice for 30 min and centrifuged at 13 000 × g for 20 min at 4°C. The supernatants were collected and the protein concentration was quantified by the Bradford method. The supernatants were solubilized with a 4× Laemmli sample buffer (Bio-Rad, USA). To determine the level of mitochondrial proteins, the samples were heated at 95°C for 5 min and applied to the gel. Protein samples were separated by 12.5% denaturing gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane at 300 mA for 1 h. The membrane was blocked in a solution of Roti-block (ROTH, Germany) for 1 h at room temperature, incubated with primary antibodies at 4°C overnight and with secondary antibodies conjugated with horseradish peroxidase (HRP). Polyclonal antibodies to TSPO and VDAC1 were obtained from Abcam (UK), monoclonal antibodies to Bcl-2 were from Santa Cruz, USA. Antibodies to α‑tubulin (Cell Signaling, Danvers, MA, USA) were used as a protein load control. Peroxidase activity was determined by chemiluminescent reagents (Bio-Rad, USA). Quantitative analysis of the intensity of protein bands was carried out using densitometry (Image Lab program, Bio Rad, USA).
Statistical analysis. All data were presented as mean ± SD. Statistical significance was confirmed by Student’s t-test using Sigma plot software (Systat Software Inc., USA).
RESULTS AND DISCUSSION
CYT is widely used in the treatment of leukemia and solid tumors both alone and in combination with other anticancer drugs [18]. Combinations of drugs that have a synergistic effect usually improve the results of therapy [19]. Understanding of the mechanism of action and metabolism of CYT enabled to overcome its low bioavailability and increase stability, but the negative effects of CYT and its toxicity continue to pose a challenge for its use. It is known that MEL has a beneficial effect; it reduces the growth and progression of liver and pancreatic cancer cells [20, 21]. In this study, we investigated the combined effect of MEL and CYT at concentrations below those used in medical practice on the proliferative activity of HL-60 cells and changes in the expression of mitochondrial proteins Bcl-2, VDAC1 and TSPO. First, the effect of different concentrations of MEL and CYT on HL-60 cells was analyzed. APL cells were treated for 96 h with different concentrations of MEL (10–3, 3.3 × 10–4, 1.1 × 10–4, 4 × 10–5, 10–5, 4.1 × 10–6, 1.4 × 10–5, 5 × 10–6, and 2 × 10–6 M) and CYT (4.4 × 10–6, 1.5 × 10–6, 0.5 × 10–6, 5.4 × 10–10, 1.8 × 10–10, 6 × 10–10, 2 × 10–10, and 7 × 10–11 M). We selected the maximal non-toxic concentration of CYT of 2 nM, and the working concentration of MEL equal to 1 mM.
Chemical structure of melatonin (MEL) (a), and cytarabin (CYT) (b). The concentration dependences of cytotoxic effects of MEL (c) and CYT (d). The cells were seeded into a 96-well plate at a density of 5 × 103 cells per well and treated with MEL and CYT at indicated concentrations for 96 h. The data are presented as mean ± standard deviation (SD) calculated from the results of 10 separate experiments.
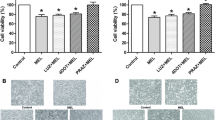
We evaluated the proliferative activity of HL-60 cells treated with CYT and MEL for 96 h (Fig. 2). Figure 2a shows growth of the cells under different conditions relative to the control. It can be seen that the number of cells treated with MEL and CYT decreased by 50 and 20%, respectively, as compared to the control (100%). Thus, MEL (1 mM) and CYT (2 nM) at low concentrations had a cytostatic effect on APL cells and did not affect their viability. The combined action of MEL and CYT resulted in a 70% decrease in the number of cells compared to the control. MEL significantly enhanced the effect of CYT when they were used together.
A combined effect of MEL and CYT on proliferation of the HL-60 cells. The cells were seeded at a density of 2 × 105 cells per dish and treated with MEL and CYT. Control, column 1; CYT, column 2; MEL, column 3; CYT + MEL, column 4. The data are presented as mean ± standard deviation (SD) calculated from 10 separate experiments. * The value differs significantly from the control at p < 0.05; # the value differs significantly from that for cells treated with MEL alone at p < 0.05.
A measure of the proliferation status of a cell population is the mitotic index (MI), which is defined as the ratio of the number of cells in the mitosis phase to the total number of cells. We analyzed MI of the cells under different conditions. Figure 2b shows how MEL and CYT affect the mitotic activity of HL-60 cells. It has been shown that the action of MEL and CYT caused the reduction of MI by ~50 and 25%, respectively. Combined application of these substances reduced MI by 64% as compared to the control value (untreated cells) and by 50%, as compared to the MI value for cells treated by CYT only. These results suggest that MEL may enhance the antitumor effect of CYT in HL-60 cells.
Quintana et al. reported previously that MEL modulates induction of apoptosis in human leukemia cells (U937) exposed to hyperthermia [22]. We determined the change in the level of Bcl-2 protein in our experimental conditions. Western blots of Bcl-2 in lysed APL cells (HL-60) treated with MEL, CYT, or MEL + CYT are presented in the upper panel of Fig. 3. Quantitative changes in the level of Bcl-2 protein are shown in Fig. 3 (bottom panel). The ratio of protein bands was quantified after normalization relative to α‑tubulin. The level of Bcl-2 protein in HL-60 cells in the presence of CYT was twice as high as that in the control conditions. MEL contributed up to ~45% reduction in Bcl-2. A similar result was obtained under the action of MEL + CYT (Bcl-2 level was reduced by ~40%). Interestingly, the combined action of MEL and CYT caused the reduction of elevated Bcl-2 levels, which were observed in the presence of CYT alone. It is possible that the decrease of the Bcl-2 level under the action of MEL + CYT reduced the cellular response to CYT and thereby activated the trigger of the apoptosis signaling cascade.
A combined effect of MEL and CYT on the level of protein Bcl-2 in HL-60 cells. The cells were seeded at a density of 5 × 105 cells per dish and treated with MEL (column 2), CYT (column 3), MEL + CYT (column 4); column 1 represents control (untreated) cells. Immunodetection of α-tubulin was used as a load control. The upper part shows the immunostaining of Bcl-2 and α-tubulin. The lower part shows the diagram of quantitative immunostaining using computer densitometry; protein levels are expressed in arbitrary units. The level of proteins in the lysate of untreated cells is taken as a unit and serves as a control. The data are presented as mean ± standard deviation (SD), calculated from the results of 5 separate experiments (*p < 0.05).
The molecular interactions of VDAC with pro- and anti-apoptotic mitochondrial outer membrane proteins are multifaceted, and they can both promote and prevent cell death. Mitochondrial VDAC is the main component that can be considered as a promising target for antitumor therapy. Since VDAC was reported to initiate apoptotic signaling cascades [23] and VDAC1 was used as an antitumor target [24], we determined the content of VDAC1 in our experimental conditions (Fig. 4a). The level of VDAC1 in cells treated with CYT increased by ~70%. Analysis of the results of the action of MEL and MEL + CYT showed a decrease in the level of VDAC1 by ~30 and 35%, respectively, indicating changes in the regulation of metabolic and energy functions of mitochondria and survival of tumor cells. As was mentioned above, VDAC can form a strong complex with TSPO in mitochondria. Thus, TSPO can affect the permeability of the formed VDAC channels and modulate mitochondrial functions. TSPO expression is also known to increase in various tumors, including brain tumors and gliomas [25–27]. With this in mind, we assessed the change in TSPO levels at our experimental conditions. Figure 4 shows that the level of both TSPO and VDAC1 in the presence of CYT increased by ~70%. On the contrary, MEL lowered the level of the proteins studied by ~20%. In the case of MEL + CYT, MEL compensated for the increase in TSPO caused by CYT. Therefore, it can be assumed that MEL contributed to a decrease in the TSPO expression upon combination with CYT and may cause a decrease in oncogenicity.
A combined effect of MEL and CYT on TSPO and VDAC1 levels in the HL-60 cells. The cells were seeded at a density of 5 × 105 cells per dish and treated with MEL (column 2), CYT (column 3), MEL + CYT (column 4); column 1, control untreated cells. Immunodetection of α-tubulin was used as a load control. (a, b) Immunostaining (upper parts) and quantification (lower parts) of the VDAC1 (a) and TSPO (b) levels. Levels of the corresponding proteins are expressed in arbitrary units. The protein level in the cell lysate without any additives is taken as a unit and serves as a control. The data are presented as mean ± standard deviation (SD), calculated from the results of 5 separate experiments (*p < 0.05).
Thus, MEL increased the cytostatic effect of CYT in HL-60 cells and suppressed the expression of the anti-apoptotic protein Bcl-2. The use of MEL enhanced the effect of CYT at low concentrations, which led to significant changes in the proliferative and mitotic activity of HL-60 cells, the model of APL. VDAC1 and TSPO expression decreased under these conditions. Overall, our results show that MEL is able to enhance the effect of other chemotherapeutic agents and can be used in new cancer therapy strategies.
REFERENCES
Carlberg C. 2000. Gene regulation by melatonin. Ann. N. Y. Acad. Sci.917, 387–396.
Stehle J.H., Saade A., Rawashdeh O., Ackermann K., Jilg A., Sebesteny T., Maronde E. 2011. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J. Pineal Res.51 (1), 17–43.
Bizzarri M., Proietti S., Cucina A., Reiter R.J. 2013. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: A review. Expert Opin. Ther. Targets.17 (12), 1483–1496.
Vijayalaxmi, Thomas C.R., Jr., Reiter R.J., Herman T.S. 2002. Melatonin: From basic research to cancer treatment clinics. J. Clin. Oncol.20 (10), 2575–2601.
Shiu S.Y. 2007. Towards rational and evidence-based use of melatonin in prostate cancer prevention and treatment. J. Pineal Res.43 (1), 1–9.
Pinilla-Ibarz J., Bello C. 2008. Modern approaches to treating chronic myelogenous leukemia. Curr. Oncol. Rep.10 (5), 365–371.
Shah M., Agarwal B. 2008. Recent advances in management of acute myeloid leukemia (AML). Indian J. Pediatr.75 (8), 831–837.
Kantarjian H.M., O’Brien S., Smith T.L., Cortes J., Giles F.J., Beran M., Pierce S., Huh Y., Andreeff M., Koller C., Ha C.S., Keating M.J., Murphy S., Freireich E.J. 2000. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J. Clin. Oncol.18 (3), 547–561.
Bayrhuber M., Meins T., Habeck M., Becker S., Giller K., Villinger S., Vonrhein C., Griesinger C., Zweckstetter M., Zeth K. 2008. Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. USA.105 (40), 15370–15375.
McEnery M.W., Snowman A.M., Trifiletti R.R., Snyder S.H. 1992. Isolation of the mitochondrial benzodiazepine receptor: Association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. USA.89 (8), 3170–3174.
Landau M., Weizman A., Zoref-Shani E., Beery E., Wasseman L., Landau O., Gavish M., Brenner S., Nordenberg J. 1998. Antiproliferative and differentiating effects of benzodiazepine receptor ligands on B16 melanoma cells. Biochem. Pharmacol.56 (8), 1029–1034.
Veenman L., Gavish M. 2012. The role of 18 kDa mitochondrial translocator protein (TSPO) in programmed cell death, and effects of steroids on TSPO expression. Curr. Mol. Med.12 (4), 398–412.
Wu X., Gallo K.A. 2013. The 18-kDa translocator protein (TSPO) disrupts mammary epithelial morphogenesis and promotes breast cancer cell migration. PLoS One.8 (8), e71258.
Beinlich A., Strohmeier R., Kaufmann M., Kuhl H. 2000. Relation of cell proliferation to expression of peripheral benzodiazepine receptors in human breast cancer cell lines. Biochem. Pharmacol.60 (3), 397–402.
Ikezaki K., Black K.L. 1990. Stimulation of cell growth and DNA synthesis by peripheral benzodiazepine. Cancer Lett.49 (2), 115–120.
McEnery M.W., Dawson T.M., Verma A., Gurley D., Colombini M., Snyder S.H. 1993. Mitochondrial voltage-dependent anion channel. Immunochemical and immunohistochemical characterization in rat brain. J. Biol. Chem.268 (31), 23289–23296.
Krestinina O., Fadeev R., Lomovsky A., Baburina Y., Kobyakova M., Akatov V. 2018. Melatonin can strengthen the effect of retinoic acid in HL-60 Cells. Int. J. Mol. Sci.19 (10), 2873.
Matsumura I. 2009. Standard therapies for acute myeloid leukemia. Rinsho Ketsueki.50 (10), 1389–1400.
Robak T., Wierzbowska A. 2009. Current and emerging therapies for acute myeloid leukemia. Clin. Ther.31Pt 2, 2349–2370.
Fan L.L., Sun G.P., Wei W., Wang Z.G., Ge L., Fu W.Z., Wang H. 2010. Melatonin and doxorubicin synergistically induce cell apoptosis in human hepatoma cell lines. World J. Gastroenterol.16 (12), 1473–1481.
Leja-Szpak A., Jaworek J., Pierzchalski P., Reiter R.J. 2010. Melatonin induces pro-apoptotic signaling pathway in human pancreatic carcinoma cells (PANC-1). J. Pineal. Res.49 (3), 248–255.
Quintana C., Cabrera J., Perdomo J., Estevez F., Loro J.F., Reiter R.J., Quintana J. 2016. Melatonin enhances hyperthermia-induced apoptotic cell death in human leukemia cells. J. Pineal. Res.61 (3), 381–395.
Shimizu S., Narita M., Tsujimoto Y. 1999. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature.399 (6735), 483–487.
Mathupala S.P., Pedersen P.L. 2010. Voltage dependent anion channel-1 (VDAC-1) as an anti-cancer target. Cancer Biol. Ther.9 (12), 1053–1056.
Black K.L., Ikezaki K., Santori E., Becker D.P., Vinters H.V. 1990. Specific high-affinity binding of peripheral benzodiazepine receptor ligands to brain-tumors in rat and man. Cancer.65 (1), 93–97.
Ferrarese C., Appollonio I., Frigo M., Gaini S. M., Piolti R., Frattola L. 1989. Benzodiazepine receptors and diazepam-binding inhibitor in human cerebral-tumors. Ann. Neurol.26 (4), 564–568.
Veenman L., Levin E., Weisinger G., Leschiner S., Spanier I., Snyder S.H., Weizman A., Gavish M. 2004. Peripheral-type benzodiazepine receptor density and in vitro tumorigenicity of glioma cell lines. Biochem. Pharmacol.68 (4), 689–698.
ACKNOWLEDGMENTS
The work was supported by the Russian Foundation for Basic Research (project no 17-04-00747). The work was carried out using the equipment of the Center for the Collective Use of the Research Equipment of the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by E. Puchkov
Rights and permissions
About this article
Cite this article
Lomovsky, A.I., Baburina, Y.L., Kobyakova, M.I. et al. Melatonin Enhances the Chemotherapeutic Effect of Cytarabin in HL-60 Cells. Biochem. Moscow Suppl. Ser. A 14, 140–145 (2020). https://doi.org/10.1134/S1990747819060072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747819060072