Abstract—
Using electron microscopy, the effect of pronase on nerve ganglia of a mollusk, leech, and frog was studied for the first time. It was revealed that the effect of pronase causes the retraction and removal of glial membranes, as well as denudation of nerve fibers and neuronal bodies with a simultaneous convergence of neuromembranes of these structures, and leads to the formation of gap junctions. This effect of pronase on the membranes belongs to a number of unusual, unexpected functions, and we obtained the observed effect for the first time. Since we previously registered reverberation of a nerve impulse in the ganglia of a frog and a leech under the same conditions, we believe that the obtained morphological data represent an evidence of the formation of electrical synapses under the action of pronase on the nerve ganglia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Proteolysis (enzymatic hydrolysis of amide bonds in proteins and peptides) is a generally recognized function of proteases; in this regard, proteases are used in the isolation of individual cells to create a tissue culture (Sotnikov and Kostenko, 1981). For a long time, the functions of proteolytic enzymes were associated only with their role in destruction in catabolic processes (Antonov, 1991). However, it was found that the functions of proteases are not limited to this (Kerstein et al., 2017). The regulatory functions of these enzymes also attract attention. Thus, active proteolysis in a chemical synapse was recognized as a key factor in controlling the dynamic changes in the shape and function of dendritic spines (Magnowska et al., 2016). Increasing the intracellular concentration of Ca2+, metalloproteases contribute to the activation of branching (arborization) of neurites (Allen et al., 2016).
Although a wide range of proteolytics exist for experiments in biology and these enzymes have a high specificity, we considered it expedient to use pronase, since it is a complex of several proteases simultaneously. The nerve ganglia of a mollusk and a leech, as well as the sympathetic ganglion of a frog, the morphological and electrophysiological characteristics of which are well known, were selected as objects for studying the effect of pronase.
At present, gap junctions (GJs) are clearly recognized as a vital component in mammalian brain circuitry. Their importance and wide distribution have been noted in reviews of recent years (Alcamí and Pereda, 2019; Ixmatlahua et al., 2020; Thomas et al., 2020). The study of a number of properties and morphological peculiarities of GJs in the brain in vivo is constrained by a number of factors (the main one being that nerve cells that are experimentally difficult to access) and the small amount of GJs in the brain volume. The works were mainly carried out on mice knockout for connexins (Cx36, Cx45, and Cx26) and using their blockers (Wang and Belousov, 2011; Xu et al., 2020; Wang and Wu, 2021; Talukdar et al., 2022). It is more difficult to conduct a morphological study of GJ structures, which are extremely rare in the brain. It is considered that GJ and tight junctions (TJs) between neuromembranes allow their functional role to be determined only indirectly (Kirichenko et al., 2008). Some authors denote partially fused membranes of neurons and fibers as “gap junctions,” not even suspecting that interneuronal perforations (syncytia) have the same function (Fontes et al, 2015; Nakagawa and Hosoya, 2019; Spray et al, 2019). GJs in neuromembranes not only carry out metabolic connection between neurons, but also contribute to the ordering of high-frequency nerve impulses; instead of a single impulse, they can form a series (Sergeeva et al., 2020; Sotnikov, 2021).
The aim of this work was to study the experimentally obtained using pronase GJs, which, according to all morphological criteria, correspond to classical, known structures of ESs at an ultrastructural level.
MATERIALS AND METHODS
Ganglia of a mollusk (Lymnaea stagnalis) (n = 5), ganglia of the brain of a medicinal leech (Hirudo medicinalis L.) (n = 6), and a sympathetic ganglion of a common frog (Rana temporaria) (n = 6) were the object of the study. A 0.4% pronase solution (lyophilized pronase from Streptomyces grisens, Serva, Germany), in which the ganglia were placed for 60 min, was used.
For electron-microscopic study, nerve ganglia were fixed for 1 h in a cooled 2.5% glutaraldehyde solution (Acros Organics, United States) and 4% paraformaldehyde prepared on 0.1 M cacodylate buffer (pH 7.2–7.4) and, then, in 1% solution of chilled osmium tetroxide (Sigma-Aldrich, Germany). After dehydration in ethanol solutions of increasing concentration and absolute acetone, the samples were poured into a mixture of araldites (araldite M, araldite H, araldite B, dibutyl phthalate) (Fluka, Switzerland). Ultrathin sections were prepared on an LKB-5 ultratome (Sweden) and contrasted with a triple contrast method (lead citrate, uranyl acetate, lead citrate) (Sigma-Aldrich, Germany). Viewing and photography were carried out in an FEI Tecnai G2 Spirit BioTWIN electron microscope (Netherlands) at a voltage of 80 kV provided by the Center for Collective Use of the Sechenov Institute of Evolutionary Physiology and Biochemistry, Russian Academy of Sciences. The number of high-quality images was up to 50 copies in each series of experiments.
RESULTS
We carried out the experiments on the nerve ganglia of a mollusk, the abdominal brain of a medicinal leech, and the sympathetic ganglion of a frog. When analyzing the control material, we found no GJs in our preparations. GJs are known to be extremely rare. After processing the material with a pronase, a nonspecific process of destruction and removal of glial membranes from the ganglion is noted in all types of ganglia. The remaining minor fragments of gliocyte processes are contracted and form varicose extensions (Fig. 1a), which can be seen, in particular, in the gaps left after proteolysis of gliocytes (Fig. 1b). Many nerve fibers lacking gliocytes are adjacent to each other at a usual intercellular distance (20 nm). Other opposing membranes of nerve fibers form GJs or TJs. On some frog-brain preparations, GJs are formed between almost all neighboring cells after the treatment with pronase (Fig. 2). There is a peculiarity of membranes during the treatment with pronase that consists in the fact that, over time, the axolemma proteins, during denaturation, aggregating with the membrane, significantly thicken its surface formations (“fringe”) and mask a light band corresponding to hydrophobic fatty acids of the bilipid membrane and its outer contours.
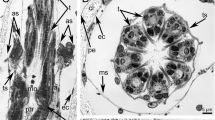
Fragments of nonspecific changes in outer and inner glial membranes of sympathetic ganglia of leech, mollusk, and frog normally and after treatment with pronase. (a) Leech ganglion normally; (b) frog ganglion normally; (c) mass dissociation of glial outer membrane in leech ganglion, varicosity after the effect of pronase; and (d) remains of the glial membrane between two neurons in the frog ganglion; (1) varicose form of a gliocyte fragment after its detachment, before a final lysis; arrow, gap junction; G, glia; A, axon; M, mitochondrion. Electron microscopy. Scale ruler: (a) 1.5, (b) 1.5, (c) 3, and (d) 4 µm.
(a) Glial-free nerve fibers of mollusk ganglion and (b) group of denuded autonomic nerve fibers of the frog ganglion forming membrane contacts after the treatment with pronase. (1) Cavities where the processes of gliocytes were previously located, (2) microtubules, (3) remainder of the “stump” of the glial membrane reduced as a result of proteolysis, (4) neuron, and (5) mitochondria; arrows, interneuronal gap junctions. Electron microscopy. Scale ruler: (a) 2 and (b) 3 µm.
In the case of the membranes of nerve cells and fibers formed by gliocytes, GJs are detected in all preparations. Within the gap, the fringe often masks the layers of bilipid membranes. The expansions of intercellular space are often observed on the sides of GJs (Fig. 3); they are apparently formed due to the accumulation of “free water” formed as a result of the process of proteolysis of membrane proteins. A large number of single local GJs also attract attention (Fig. 4). Their gaps are filled with osmiophilic protein aggregates masking a seven-layer GJ structure. There are also multiple, serial GJs in the form of a chain (Fig. 5) that are located sequentially on presynapses.
Variants of paired gap junctions of leech brain fibers and frog ganglion after the action of pronase. (a) Three GJs on a leech-brain preparation, (b) four GJs on a frog-ganglion preparation, (1) interneuronal gaps expanded due to the accumulation of “free water” after protein denaturation; arrows, electrical synapses; M, mitochondrion; N, neurons. Electron microscopy. Scale ruler: (a)1 and (b) 2 µm.
Multiple gap junctions in the area of chemical synapses that lost chemoreceptor specializations (specific morphological traits of pre- and postsynaptic membranes) after treatment with pronase, in (a, c) mollusk and (b) frog ganglia. N, neuron; arrows, gap and tight neuro-neuronal junctions of different value. Electron microscopy. Scale ruler: (a–c) 1 µm.
At the ultrastructural level, the contacts between mollusk, frog, and leech neurons have a similar structure. There can be as many as six on one neurite. At the same time, attention should be paid again to numerous, almost continuous seven-layer GJs. Along with multiple GJs, chemical synapses, for which the absence of typical presynaptic and postsynaptic chemoreceptor specializations in the area of accumulation of synaptic vesicles is a characteristic feature (which is a consequence of proteolysis), are presented in Fig. 5.
Surprisingly, as a result of the action of pronase, a complex of proteolytic enzymes—intercellular septa—are formed (which are aggregates of interneuronal proteins) (Fig. 6). Sometimes, they are similar in size and alternate, being located at about the same distance from each other. There are also a small number of narrow protein glial–neuronal bridges (Fig. 6). Intercellular protein near-membrane aggregates are localized across the membranes and thicken at their ends. They protrude both inward and outward from both membranes, masking the sizes of the intercellular gap and, apparently, disrupting its isolation function. In cases in which there is an intercellular gap, it can be noted that both membranes are connected by transverse, poorly visible protein bridges. The bridges continue inside the neurolemma and usually have a pyramidal shape. As a result of the action of pronase, a significant variety of structures were obtained.
Multiple intercellular transmembrane bridges detected after treatment of the preparation with pronase. (a) Septa forming after treatment of frog ganglion with pronase, (b) septa forming after treatment of frog ganglion with pronase, (c) narrow neuron–glial mollusk septa; and (1) continuous neuron–glial contact of the neurolemma and gliolemma of the preserved varicosity of the gliocyte process after the treatment with pronase; arrows, interneuronal, neuron–glial bridges and aggregates of near-membrane proteins of a pyramidal shape; G, glia; N, neuron. Electron microscopy. Scale ruler: (a, b) 20 and (c) 40 nm.
DISCUSSION
The formation of new GJs under the action of pronase is related to a number of unusual, unexpected functions of pronase. The experimental GJs were for the first time obtained using the treatment of ganglia of vertebrates and invertebrates with a 0.4% pronase solution. A destructive effect of pronase on chemical synapses, leading to the loss of pre- and postsynaptic structures, was detected for the first time. New formation of GJs when the membranes approach each other can occur with traumas and inflammations and can lead to a change in electrogenesis (Belousov et al., 2017). As is known, there are four components in chemical synapses: pre- and postsynaptic structures, synaptic gaps, and vesicles with a mediator in the axon terminals. During proteolysis, chemoreceptor near-membrane protein complexes disappear and vesicles surrounded by a lipid membrane remain clearly visi-ble.
Experimental studies carried out on “simple nervous systems” make a certain contribution to different sections of physiology, allowing cellular, molecular, and genetic mechanisms to be studied. It is known that protein aggregates of lipid bilayers (septa) can be electrically permeable (Berkinblit et al., 1981). Our experiments carried out with pronase demonstrated one more variant of the use of the nervous systems of mollusks, leeches, and frogs. Since pronase does not change the amplitude and kinetic characteristics of ionic currents involved in the generation of nerve impulses (Lun’ko et al., 2014), our experimental model can be useful for studying many not yet understood properties and peculiarities of GJs such as ESs. Indeed, it was demonstrated in our previous electrophysiological studies that created de novo ES chains between the membranes of nerve fibers in mollusks, leeches, and frogs have special electrical functions (they form a frequency series (six to eight) of spikes for one irritating impulse, form a reverberation reaction (Sergeeva et al., 2020; Sotnikov, 2021). It is known that classical physiology presumably explains the emergence of excitation reverberation by a circular connection between the chain of natural GJs in the brain. We obtained this neural process on experimental, clearly morphologically established GJs for the first time.
Our work confirms that a large number of GJs are formed in mollusk, leech, and frogs under the influence of pronase at the ultrastructural level is a result of this work. Since we previously proved that electrical synapses appear under the action of pronase in such conditions, it can be assumed that GJs are a morphological equivalent of ES.
REFERENCES
Alcamí, P. and Pereda, A.E., Beyond plasticity: the dynamic impact of electrical synapses on neural circuits, Nat. Rev. Neurosci., 2019, vol. 20, p. 253. https://doi.org/10.1038/s41583-019-0133-5
Allen, M., Ghosh, S., Ahern, G.P., Villapol, S., Maguire-Zeiss, K.A., and Conant, K., Protease induced plasticity: matrix metalloproteinase-1 promotes neurostructural changes through activation of protease activated receptor 1, Sci. Rep., 2016, vol. 6, p. 35497. https://doi.org/10.1038/srep35497
Antonov, V.K., Khimiya proteoliza (Chemistry of Proteolysis), Moscow: Nauka, 1991.
Berkinblit, M.B., Bozhkova, V.P., Boitsova, L.Yu., Mitelman, L.A., Potapova T.V., Chailakhyan L.M., Sharovskaya, Yu.Yu. Vysokopronitsaemye kontaktnye membrany (Highly Permeable Contact Membranes), Moscow: Nauka, 1981.
Fontes, J.D., Ramsey, J., Polk, J.M., Koop, A., Denisova, J.V., and Belousov, A.B., Death of neurons following injury requires conductive neuronal gap junction channels but not a specific connexin, PLoS One, 2015, vol. 10, p. e0125395. https://doi.org/10.1371/journal.pone.0125395
Ixmatlahua, D.J., Vizcarra, B., Gómez-Lira, G., Romero-Maldonado, I., Ortiz, F., Rojas-Piloni, G., and Gutiérrez, R., Neuronal glutamatergic network electrically wired with silent but activatable gap junction, J. Neurosci., 2020, vol. 40, p. 4661. https://doi.org/10.1523/JNEUROSCI.2590-19
Kerstein, P.C., Patel, K.M., and Gomez, T.M., Calpain-mediated proteolysis of TALIN and FAK regulates adhesion dynamics necessary for axon guidance, J. Neurosci., 2017, vol. 37, p. 1568. https://doi.org/1010.1523/JNEUROSCI.2769-16.2016
Kirichenko, E.Y., Povilaitite, P.E., and Sukhov, A.G., Role of gap junctions in local rhythmogenesis in cortical columns, Neurosci. Behav. Physiol., 2009, vol. 39, no. 2, p. 199.
Lun’ko, O.O., Isaiev, D.S., Maxymiuk, O.P., Kryshtal’, O.O., and Isaieva, O.V., The effect of enzymatic treatment using proteases on properties of persistent sodium current in CA1 pyramidal neurons of rat hippocampus, Fiziol. Zhiv., 2014, vol. 60, p. 75.
Magnowska, M., Gorkiewicz, T., Suska, A., Wawr-zyniak, M., Rutkowska-Wlodarczyk, I., Kaczmarek, L., and Wlodarczyk, J., Transient ECM protease activity promotes synaptic plasticity, Sci. Rep., 2016, vol. 6, p. 27757. https://doi.org/10.1038/srep27757
Nakagawa, N. and Hosoya, T., Slow dynamics in microcolumnar gap junction network of developing neocortical pyramidal neurons, Neuroscience, 2019, vol. 406, p. 554. https://doi.org/10.1016/j.neuroscience.2019.02.013
Sergeeva, S.S., Sotnikov, O.S., and Paramonova, N.M., A method for creating a neurophysiological model of a simple nervous system with reverberation, Fiziol. Zh. im. I.M. Sechenova, 2020, vol. 106. № 9, p. 1163. https://doi.org/10.31857/S0869813920080075
Sotnikov, O.S., A series of experimental electrical synapses and reverberation of a nerve impulse, Tekhnol. Zhivykh Sist., 2021, vol. 18, no. 3, p. 52. https://doi.org/10.18127/j20700997-202103-05
Sotnikov, O.S. and Kostenko, M.A., Reactive changes of living nerve endings in the culture of isolated neurons deprived of glia, Arkh. AGE, 1981 vol. 80, no. 6, p. 17.
Spray, D.C., Iglesias, R., Shraer, N., Suadicani, S.O., Belzer, V., Hanstein, R., and Hanani, M., Gap junction mediated signaling between satellite glia and neurons in trigeminal ganglia, Glia, 2019, vol. 67, p. 791. https://doi.org/10.1002/glia.23554
Talukdar, S., Emdad, L., Das, S.K., and Fisher, P.B., GAP junctions: multifaceted regulators of neuronal differentiation, Tissue Barriers, 2022, vol. 10, p. 1982349. https://doi.org/10.1080/21688370.2021.1982349
Thomas, D., Senecal, J.M., Lynn, B.D., Traub, R.D., and Nagy, J.I., Connexin 36 localization along axon initial segments in the mammalian CNS, Int. J. Physiol. Pathophysiol. Pharmacol., 2020, vol. 12, p. 153.
Wang, Y. and Belousov, A.B., Deletion of neuronal gap junction protein connexin 36 impairs hippocampal LTP, Neurosci. Lett., 2011, vol. 502, p. 30. https://doi.org/10.1016/j.neulet.2011.07.018
Wang, G. and Wu, X., The potential antiepileptogenic effect of neuronal Cx36 gap junction channel blockage, Transl. Neurosci., 2021, vol. 12, p. 46. https://doi.org/10.1515/tnsci-2021-0008
Xu, Y., Shen, F.Y., Liu, Y.Z., Wang, L., Wang, Y.W., and Wang, Z., Dependence of generation of hippocampal CA1 slow oscillations on electrical synapses, Neurosci. Bull., 2020, vol. 36, p. 39. https://doi.org/10.1007/s12264-019-00419-z
Funding
This work was supported by state program 47 SP “Science and Technology Development of the Russian Federation” (2019–2030), topic 0134-2019-0001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. The experiments were carried according to the requirements of the Council of the European Community (86/609/EEC) 1986 and the decision on the use of laboratory animals of the Commission of the Pavlov Institute of Physiology, Russian Academy of Sciences, on Humane Treatment of Animals no. 26/12 dated December 26, 2019.
Additional information
Translated by A. Barkhash
Abbreviations: GJ—gap junction; TJ—tight junction; ES—electrical synapse.
Rights and permissions
About this article
Cite this article
Sotnikov, O., Sergeeva, S. & Paramonova, N. The Effect of Pronase on Mollusk, Leech, and Frog Nerve Ganglia Causes the Formation of Neuron–Neuronal Gap Junctions. Cell Tiss. Biol. 17, 197–202 (2023). https://doi.org/10.1134/S1990519X23020128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X23020128